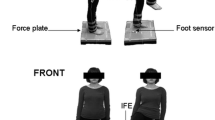
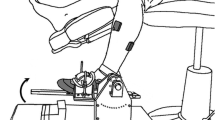
Abstract
Reaction time (RT) is one of the most commonly used measures of neurological function and dysfunction. Despite the extensive studies on it, no study has ever examined the RT in the ankle. Twenty-two subjects were recruited to perform simple, 2- and 4-choice RT tasks by visually guiding a cursor inside a rectangular target with their ankle. RT did not change with spatial accuracy constraints imposed by different target widths in the direction of the movement. RT increased as a linear function of potential target stimuli, as would be predicted by Hick–Hyman law. Although the slopes of the regressions were similar, the intercept in dorsal–plantar (DP) direction was significantly smaller than the intercept in inversion–eversion (IE) direction. To explain this difference, we used a hierarchical Bayesian estimation of the Ratcliff’s (Psychol Rev 85:59, 1978) diffusion model parameters and divided processing time into cognitive components. The model gave a good account of RTs, their distribution and accuracy values, and hence provided a testimony that the non-decision processing time (overlap of posterior distributions between DP and IE < 0.045), the boundary separation (overlap of the posterior distributions < 0.1) and the evidence accumulation rate (overlap of the posterior distributions < 0.01) components of the RT accounted for the intercept difference between DP and IE. The model also proposed that there was no systematic change in non-decision processing time or drift rate when spatial accuracy constraints were altered. The results were in agreement with the memory drum hypothesis and could be further justified neurophysiologically by the larger innervation of the muscles controlling DP movements. This study might contribute to assessing deficits in sensorimotor control of the ankle and enlighten a possible target for correction in the framework of our on-going effort to develop robotic therapeutic interventions to the ankle of children with cerebral palsy.






Similar content being viewed by others
Notes
The results reported here are in agreement with the results of a different method applying the concept of “super-subjects” to the same data set (Michmizos and Krebs 2014b), in terms of the order of the best-fit models and the statistical significance of the differences in the estimated parameters, between DP and IE.
References
Anstey KJ, Mack HA, Christensen H et al (2007) Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia 45:1911–1920. doi:10.1016/j.neuropsychologia.2006.11.020
Baird BJ, Tombaugh TN, Francis M (2007) The effects of practice on speed of information processing using the adjusting-paced serial addition test (adjusting-PSAT) and the computerized tests of information processing (CTIP). Appl Neuropsychol 14:88–100. doi:10.1080/09084280701319912
Bisson E, Contant B, Sveistrup H, Lajoie Y (2007) Functional balance and dual-task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychol Behav 10:16–23. doi:10.1089/cpb.2006.9997
Boff KR, Kaufman L, Thomas JP (1994) Handbook of perception and human performance. Volume 2. Cognitive processes and performance. In: DTIC Document, pp 30–35
Botwinick J (1966) Cautiousness in advanced age. J Gerontol 21:347–353
Brinley JF (1965) Cognitive sets, speed and accuracy of performance in the elderly. In: Welford AT, Birren JE (eds) Behavior, aging and the nervous system. Thomas, Springfield, pp 114–149
Brouwer B, Ashby P (1992) Corticospinal projections to lower limb motoneurons in man. Exp Brain Res 89:649–654
Brown RG, Jahanshahi M, Marsden CD (1993) Response choice in Parkinson’s disease. The effects of uncertainty and stimulus-response compatibility. Brain 116(Pt 4):869–885
Buchanan JJ, Park JH, Ryu YU, Shea CH (2003) Discrete and cyclical units of action in a mixed target pair aiming task. Exp Brain Res 150:473–489. doi:10.1007/s00221-003-1471-z
Buchanan JJ, Park JH, Shea CH (2006) Target width scaling in a repetitive aiming task: switching between cyclical and discrete units of action. Exp Brain Res 175:710–725. doi:10.1007/s00221-006-0589-1
Chang JJ, Wu TI, Wu WL, Su FC (2005) Kinematical measure for spastic reaching in children with cerebral palsy. Clin Biomech (Bristol, Avon) 20:381–388. doi:10.1016/j.clinbiomech.2004.11.015
Ditterich J (2006) Stochastic models of decisions about motion direction: behavior and physiology. Neural Netw 19:981–1012. doi:10.1016/j.neunet.2006.05.042
Donders FC (1969) On the speed of mental processes. Acta Psychol (Amst) 30:412–431
Dutilh G, Krypotos AM, Wagenmakers EJ (2011) Task-related versus stimulus-specific practice. Exp Psychol 58:434–442. doi:10.1027/1618-3169/a000111
Evarts EV, Teravainen H, Calne DB (1981) Reaction time in Parkinson’s disease. Brain 104:167–186
Fasoli SE, Ladenheim B, Mast J, Krebs HI (2012) New horizons for robot-assisted therapy in pediatrics. Am J Phys Med Rehabil 91:S280–S289. doi:10.1097/PHM.0b013e31826bcff4
Fernaeus SE, Ostberg P, Wahlund LO (2013) Late reaction times identify MCI. Scand J Psychol 54:283–285. doi:10.1111/sjop.12053
Fjell AM, Westlye LT, Amlien IK, Walhovd KB (2011) Reduced white matter integrity is related to cognitive instability. J Neurosci 31:18060–18072. doi:10.1523/JNEUROSCI.4735-11.2011
Gamerman D, Lopes HF (2006) Markov chain Monte Carlo: stochastic simulation for Bayesian inference, 2nd edn. Chapman & Hall/CRC Press, Boca Raton
Garry MI, Franks IM (2000) Reaction time differences in spatially constrained bilateral and unilateral movements. Exp Brain Res 131:236–243
Garry MI, Franks IM (2002) Spatially precise bilateral arm movements are controlled by the contralateral hemisphere: evidence from a lateralized visual stimulus paradigm. Exp Brain Res 142:292–296. doi:10.1007/s00221-001-0949-9
Gelman A, Hill J (2007) Data analysis using regression and multilevel/hierarchical models. Cambridge University Press, Cambridge
Gold JI, Shadlen MN (2007) The neural basis of decision making. Annu Rev Neurosci 30:535–574. doi:10.1146/annurev.neuro.29.051605.113038
Goodrich S, Henderson L, Kennard C (1989) On the existence of an attention-demanding process peculiar to simple reaction time: converging evidence from Parkinson’s disease. Cogn Neuropsychol 6:309–331. doi:10.1080/02643298908253422
Gorus E, De Raedt R, Lambert M, Lemper JC, Mets T (2008) Reaction times and performance variability in normal aging, mild cognitive impairment, and Alzheimer’s disease. J Geriatr Psychiatry Neurol 21:204–218. doi:10.1177/0891988708320973
Hanson C, Lofthus GK (1978) Effects of fatigue and laterality on fractionated reaction time. J Mot Behav 10:177–184
Heekeren HR, Marrett S, Bandettini PA, Ungerleider LG (2004) A general mechanism for perceptual decision-making in the human brain. Nature 431:859–862. doi:10.1038/nature02966
Henry FM, Rogers DE (1960) Increased response latency for complicated movements and a “memory drum” theory of neuromotor reaction. Res Q Am Assoc Health, Phys Educ Recreat 31:448–458
Heywood S, Churcher J (1980) Structure of the visual array and saccadic latency: implications for oculomotor control. Q J Exp Psychol 32:335–341. doi:10.1080/14640748008401169
Hick WE (1952) On the rate of gain of information. Q J Exp Psychol 4:11–26. doi:10.1080/17470215208416600
Horgan JS (1980) Reaction-time and movement-time of children with cerebral palsy: under motivational reinforcement conditions. Am J Phys Med 59:22–29
Hyman R (1953) Stimulus information as a determinant of reaction time. J Exp Psychol 45:188–196. doi:10.1037/h0056940
Jahanshahi M, Brown RG, Marsden CD (1993) A comparative study of simple and choice reaction time in Parkinson’s, Huntington’s and cerebellar disease. J Neurol Neurosurg Psychiatry 56:1169–1177
Jepma M, Wagenmakers EJ, Band GP, Nieuwenhuis S (2009) The effects of accessory stimuli on information processing: evidence from electrophysiology and a diffusion model analysis. J Cogn Neurosci 21:847–864. doi:10.1162/jocn.2009.21063
King B, Wood C, Faulkner D (2008) Sensitivity to visual and auditory stimuli in children with developmental dyslexia. Dyslexia 14:116–141. doi:10.1002/dys.349
Klapp ST (2010) Comments on the classic Henry and Rogers (1960) paper on its 50th anniversary: resolving the issue of simple versus choice reaction time. Res Q Exerc Sport 81:108–112
Klauer KC, Voss A, Schmitz F, Teige-Mocigemba S (2007) Process components of the Implicit Association Test: a diffusion-model analysis. J Pers Soc Psychol 93:353–368. doi:10.1037/0022-3514.93.3.353
Krebs HI, Hogan N (2012) Robotic therapy: the tipping point. Am J Phys Med Rehabil 91:S290–S297. doi:10.1097/PHM.0b013e31826bcd80
Krebs HI, Hogan N, Aisen ML, Volpe BT (1998) Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng 6:75–87
Krebs HI, Palazzolo JJ, Dipietro L et al (2003) Rehabilitation robotics: performance-based progressive robot-assisted therapy. Auton Robots 15:7–20. doi:10.1023/A:1024494031121
Krebs HI, Rossi S, Kim SJ, Artemiadis PK, Williams D, Castelli E, Cappa P (2011) Pediatric anklebot. IEEE Int Conf Rehabil Robot 2011:5975410. doi:10.1109/ICORR.2011.5975410
Kruschke JK (2010) What to believe: Bayesian methods for data analysis. Trends Cogn Sci 14(7):293–300
Kveraga K, Boucher L, Hughes HC (2002) Saccades operate in violation of Hick’s law. Exp Brain Res 146:307–314. doi:10.1007/s00221-002-1168-8
Laming DRJ (1968) Information theory of choice-reaction times. Academic Press, London
Lindley DV (1965) Introduction to probability and statistics from a Bayesian viewpoint, Part 2. Cambridge University Press, Cambridge
Leite FP, Ratcliff R (2010) Modeling reaction time and accuracy of multiple-alternative decisions. Atten Percept Psychophys 72:246–273. doi:10.3758/APP.72.1.246
Leth-Steensen C, Elbaz ZK, Douglas VI (2000) Mean response times, variability, and skew in the responding of ADHD children: a response time distributional approach. Acta Psychol (Amst) 104:167–190
Light KE, Reilly MA, Behrman AL, Spirduso WW (1996) Reaction times and movement times: benefits of practice to younger and older adults. J Aging Phys Act 4:27–41
Longstreth LE, el-Zahhar N, Alcorn MB (1985) Exceptions to Hick’s law: explorations with a response duration measure. J Exp Psychol Gen 114:417–434
Luce RD (1986) Response times: their role in inferring elementary mental organization3. Oxford University Press, Oxford
Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA (2004) Maturation of cognitive processes from late childhood to adulthood. Child Dev 75:1357–1372. doi:10.1111/j.1467-8624.2004.00745.x
MacDonald SW, Li SC, Backman L (2009) Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging 24:792–808. doi:10.1037/a0017798
Madden DJ (2001) Speed and timing of behavioral processes. In: Birren JE, Schaie KW (eds) Handbook of the psychology of aging, 5th edn. Academic Press, San Diego, CA, pp 288–312
Matzke D, Dolan CV, Logan GD, Brown SD, Wagenmakers EJ (2013) Bayesian parametric estimation of stop–signal reaction time distributions. J Exp Psychol Gen 142:1047–1073
Marsden CD (1982) The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology 32:514–539
Martelli M, Barban F, Zoccolotti P, Silveri MC (2012) Slowing of information processing in Alzheimer disease: motor as well as cognitive factors. Cogn Behav Neurol 25:175–185. doi:10.1097/WNN.0b013e318274fc44
Merkt J, Singmann H, Bodenburg S, Goossens-Merkt H, Kappes A, Wendt M, Gawrilow C (2013) Flanker performance in female college students with ADHD: a diffusion model analysis. Atten Defic Hyperact Disord. doi:10.1007/s12402-013-0110-1
Michmizos KP, Krebs HI (2012a) Assist-as-needed in lower extremity robotic therapy for children with cerebral palsy. In: 2012 4th IEEE RAS & EMBS international conference on biomedical robotics and biomechatronics (BioRob), pp 1081–1086
Michmizos KP, Krebs HI (2012b) Serious games for the pediatric anklebot. In: 2012 4th IEEE RAS & EMBS international conference on biomedical robotics and biomechatronics (BioRob), pp 1710–1714
Michmizos KP, Krebs HI (2014a) Pointing with the ankle: the speed-accuracy tradeoff. Exp Brain Res 232:647–657. doi:10.1007/s00221-013-3773-0
Michmizos KP, Krebs HI (2014b) Modeling reaction time in the ankle. In: 2014 5th IEEE RAS & EMBS international conference on biomedical robotics and biomechatronics (BioRob), Sao Paulo, Brazil
Miller JO, Low K (2001) Motor processes in simple, go/no-go, and choice reaction time tasks: a psychophysiological analysis. J Exp Psychol Hum Percept Perform 27:266–289
Mirabella G, Iaconelli S, Modugno N, Giannini G, Lena F, Cantore G (2013) Stimulation of subthalamic nuclei restores a near normal planning strategy in Parkinson’s patients. PLoS ONE 8:e62793. doi:10.1371/journal.pone.0062793
Moore BD, Drouin J, Gansneder BM, Shultz SJ (2002) The differential effects of fatigue on reflex response timing and amplitude in males and females. J Electromyogr Kinesiol 12:351–360
Moore KL, Dalley AF, Agur AMR (2010) Lower limb. In: Clinically oriented anatomy, 6th edn. Lippincott Williams and Wilkins, Baltimore, pp 509–669
Morris AF (1977) Effects of fatiguing isometric and isotonic exercise on resisted and unresisted reaction time components. Eur J Appl Physiol Occup Physiol 37:1–11
Moy G, Millet P, Haller S et al (2011) Magnetic resonance imaging determinants of intraindividual variability in the elderly: combined analysis of grey and white matter. Neuroscience 186:88–93. doi:10.1016/j.neuroscience.2011.04.028
Mulder MJ, Wagenmakers EJ, Ratcliff R, Boekel W, Forstmann BU (2012) Bias in the brain: a diffusion model analysis of prior probability and potential payoff. J Neurosci 32:2335–2343. doi:10.1523/JNEUROSCI.4156-11.2012
Naples A, Katz L, Grigorenko EL (2012) Reading and a diffusion model analysis of reaction time. Dev Neuropsychol 37:299–316. doi:10.1080/87565641.2011.614979
Navarro DJ, Fuss IG (2009) Fast and accurate calculations for first-passage times in Wiener diffusion models. J Math Psychol 53:222–230. doi:10.1016/j.jmp.2009.02.003
Perry J (1992) Gait analysis: normal and pathological function. Slack, Thorofare
Philiastides MG, Ratcliff R, Sajda P (2006) Neural representation of task difficulty and decision making during perceptual categorization: a timing diagram. J Neurosci 26:8965–8975. doi:10.1523/JNEUROSCI.1655-06.2006
Ratcliff R (1978) A theory of memory retrieval. Psychol Rev 85:59
Ratcliff R, McKoon G (1981) Automatic and strategic priming in recognition. J Verbal Learn Verbal Behav 20:204–215
Ratcliff R, McKoon G (2008) The diffusion decision model: theory and data for two-choice decision tasks. Neural Comput 20:873–922
Ratcliff R, Tuerlinckx F (2002) Estimating parameters of the diffusion model: approaches to dealing with contaminant reaction times and parameter variability. Psychon Bull Rev 9:438–481
Ratcliff R, Van Dongen HP (2009) Sleep deprivation affects multiple distinct cognitive processes. Psychon Bull Rev 16:742–751
Ratcliff R, Thapar A, McKoon G (2001) The effects of aging on reaction time in a signal detection task. Psychol Aging 16:323
Ratcliff R, Thapar A, Gomez P, McKoon G (2004a) A diffusion model analysis of the effects of aging in the lexical-decision task. Psychol Aging 19:278
Ratcliff R, Thapar A, McKoon G (2004b) A diffusion model analysis of the effects of aging on recognition memory. J Mem Lang 50:408–424
Ratcliff R, Thapar A, McKoon G (2010) Individual differences, aging, and IQ in two-choice tasks. Cogn Psychol 60:127–157
Ratcliff R, Love J, Thompson CA, Opfer JE (2012) Children are not like older adults: a diffusion model analysis of developmental changes in speeded responses. Child Dev 83:367–381
Rikli RE, Edwards DJ (1991) Effects of a three-year exercise program on motor function and cognitive processing speed in older women. Res Q Exerc Sport 62:61–67
Rogers MW, Chan CW (1988) Motor planning is impaired in Parkinson’s disease. Brain Res 438:271–276
Roy A, Krebs HI, Williams DJ, Bever CT, Forrester LW, Macko RM, Hogan N (2009) Robot-aided neurorehabilitation: a novel robot for ankle rehabilitation. In: IEEE transactions on robotics, vol 25, pp 569–582
Salthouse TA, Hedden T (2002) Interpreting reaction time measures in between-group comparisons. J Clin Exp Neuropsychol 24:858–872
Schieppati M, Trompetto C, Abbruzzese G (1996) Selective facilitation of responses to cortical stimulation of proximal and distal arm muscles by precision tasks in man. J Physiol 491:551–562
Schmitz N, Daly E, Murphy D (2007) Frontal anatomy and reaction time in autism. Neurosci Lett 412:12–17
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana
Spencer KM, Coles MG (1999) The lateralized readiness potential: relationship between human data and response activation in a connectionist model. Psychophysiology 36:364–370
Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A (2002) Bayesian measures of model complexity and fit (with discussion). J Royal Stat Soc Ser B 64(4):583–639
Tamnes CK, Fjell AM, Westlye LT, Østby Y, Walhovd KB (2012) Becoming consistent: developmental reductions in intraindividual variability in reaction time are related to white matter integrity. J Neurosci 32:972–982
Teichner WH, Krebs MJ (1974) Visual search for simple targets. Psychol Bull 81:15
Van Maanen L, Brown SD, Eichele T, Wagenmakers E-J, Ho T, Serences J, Forstmann BU (2011) Neural correlates of trial-to-trial fluctuations in response caution. J Neurosci 31:17488–17495
Van Maanen L, Grasman RPPP, Forstmann BU, Keuken MC, Brown SD, Wagenmakers E-J (2012) Similarity and number of alternatives in the random-dot motion paradigm. Atten Percept Psychophys 74:739–753
Voss A, Rothermund K, Voss J (2004) Interpreting the parameters of the diffusion model: an empirical validation. Mem Cogn 32:1206–1220
Wald A (2004) Sequential analysis. Courier Dover Publications, New York
Welford AT (1968) Fundamentals of skill. Methuen, London
White CN, Ratcliff R, Vasey MW, McKoon G (2010a) Anxiety enhances threat processing without competition among multiple inputs: a diffusion model analysis. Emotion 10:662
White CN, Ratcliff R, Vasey MW, McKoon G (2010b) Using diffusion models to understand clinical disorders. J Math Psychol 54:39–52
Wiecki TV, Sofer I, Frank MJ (2013) HDDM: hierarchical Bayesian estimation of the drift-diffusion model in python. Front Neuroinform 7:14. doi:10.3389/fninf.2013.00014
Yeung SS, Au AL, Chow CC (1999) Effects of fatigue on the temporal neuromuscular control of vastus medialis muscle in humans. Eur J Appl Physiol 80:379–385
Zahn TP, Kruesi MJ, Rapoport JL (1991) Reaction time indices of attention deficits in boys with disruptive behavior disorders. J Abnorm Child Psychol 19:233–252
Acknowledgments
We would like to thank Thomas Wiecki for his help with the HDDM toolbox. This work was supported in part by a grant from the Cerebral Palsy International Research Foundation (CPIRF) and the Niarchos Foundation, by a grant from the VA Baltimore Medical Center contract 512-D05015 and by NIH Grant R01HD069776-02. Dr. K. P. Michmizos was partially supported by the Foundation for Education and European Culture. Dr. H. I. Krebs is a co-inventor in the MIT-held patent for the robotic device used in this work. He holds equity positions in Interactive Motion Technologies, the company that manufactures this type of technology under license to MIT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Michmizos, K.P., Krebs, H.I. Reaction time in ankle movements: a diffusion model analysis. Exp Brain Res 232, 3475–3488 (2014). https://doi.org/10.1007/s00221-014-4032-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-4032-8