Abstract
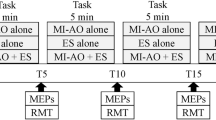
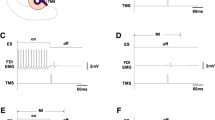
Motor evoked potentials (MEPs) in the right first dorsal interosseous (FDI) muscle elicited by transcranial magnetic stimulation of left motor cortex were assessed in ten healthy subjects during maintenance of a fixed FDI contraction level. Subjects maintained an integrated EMG (IEMG) level with visual feedback and reproduced this level by memory afterwards in the following tasks: stationary FDI muscle contraction at the level of 40 ± 5 % of its maximum voluntary contraction (MVC; 40 % task), at the level of 20 ± 5 % MVC (20 % task), and also when 20 % MVC was preceded by either no contraction (0–20 task), by stronger muscle contraction (40–20 task) or by no contraction with a previous strong contraction (40–0–20 task). The results show that the IEMG level was within the prescribed limits when 20 and 40 % stationary tasks were executed with and without visual feedback. In 0–20, 40–20, and 40–0–20 tasks, 20 % IEMG level was precisely controlled in the presence of visual feedback, but without visual feedback the IEMG and force during 20 % IEMG maintenance were significantly higher in the 40–0–20 task than those in 0–20 and 40–20 tasks. That is, without visual feedback, there were significant variations in muscle activity due to different prehistory of contraction. In stationary tasks, MEP amplitudes in 40 % task were higher than in 20 % task. MEPs did not differ significantly during maintenance of the 20 % level in tasks with different prehistory of muscle contraction with and without visual feedback. Thus, in spite of variations in muscle background activity due to different prehistory of contraction MEPs did not vary significantly. This dissociation suggests that the voluntary maintenance of IEMG level is determined not only by cortical mechanisms, as reflected by corticospinal excitability, but also by lower levels of CNS, where afferent signals and influences from other brain structures and spinal cord are convergent.





Similar content being viewed by others
References
Bagce HF, Saleh S, Adamovich SV, Krakauer JW, Tunik E (2013) Corticospinal excitability is enhanced after visuomotor adaptation and depends on learning rather than performance or error. J Neurophysiol 109:1097–1106
Baker SN, Olivier E, Lemon RN (1995) Task-related variation in corticospinal output evoked by transcranial stimulation in the macaque monkey. J Physiol 488:795–801
Dettmers C, Lemon RN, Stephan KM, Fink GR, Frackowiak RS (1996) Cerebral activation during the exertion of sustained static force in man. NeuroReport 7:2103–2110
Hashimoto R, Rothwell JC (1999) Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res 125:75–81
Hess CW, Mills KR, Murray NMF (1987) Responses in small hand muscles from magnetic stimulation of the human brain. J Physiol 388:397–419
Janssen L, Steenbergen B, Carson RG (2013) Anticipatory planning reveals segmentation of cortical motor output during action observation. Cereb Cortex [Epub ahead of print] PMID: 23960201
Kazennikov OV, Levik YuS (2009) Study of motor cortex excitability in the load holding task. Hum Physiol 35(5):585–591 (in Russian)
Kazennikov OV, Solopova IA, Talis VL, Grishin AA, Ioffe ME (2005) TMS-responses during anticipatory postural adjustment in bimanual unloading in humans. Neurosci Lett 383:246–250
Kazennikov OV, Solopova IA, Talis VL, Ioffe ME (2008) Anticipatory postural adjustment before bimanual unloading reactions: the role of the motor cortex in motor learning. Exp Brain Res 186:215–223
Lemon RN, Johansson RS, Westling G (1995) Corticospinal control during reach, grasp and precision lift in man. J Neurosci 15(9):6145–6156
Loh MN, Kirsch L, Rothwell JC, Lemon RN, Davare M (2010) Information about the weight of grasped objects from vision and internal models interacts within the primary motor cortex. J Neurosci 30(20):6984–6990
Mathis J, Gurfinkel VS, Struppler A (1996) Facilitation of motor evoked potentials by postcontraction response (Kohnstamm phenomenon). Electroencephalogr Clin Neurophysiol 101(4):289–297
Muellbacher W, Ziemann U, Boroojerdi B, Cohen L, Hallett M (2001) Role of the human motor cortex in rapid motor learning. Exp Brain Res 136:431–438
Naito E, Ehrsson HH, Geyer S, Zilles K, Roland PE (1999) Illusory arm movements activate cortical motor areas: a positron emission tomography study. J Neurosci 19:6134–6144
Park W-H, Leonard CT (2008) The effect of intervening forces on finger perception. Neurosci Lett 438:286–289
Porter R, Lemon RN (1993) Corticospinal function and voluntary movement. Oxford University Press, Oxford
Prodoehl J, Corcos DM, Vaillancourt DE (2009) Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev 33(6):900–908
Stuart M, Butler JE, Collins DF, Taylor JL, Gandevia SC (2002) The history of contraction of the wrist flexors can change cortical excitability. J Physiol 545(Pt.3):731–737
Talis VL, Kazennikov OV, Castellote JM, Grishin AA, Ioffe ME (2011) The effect of preceding activity of m. first dorsal interosseous on its voluntary contraction and motor cortex excitability. Soc Neurosci Abstr 41:68
Talis VL, Castellote JM, Kazennikov OV, Grishin AA, Ioffe ME (2012) Control of different electromyogram levels of m. abductor pollicis brevis by means of visual feedback in healthy subjects. Zh Vyssh Nerv Deiat Im I P Pavlova 62(1):12–19
Tinazzi M, Farina S, Tamburin S, Facchini S, Fiaschi A, Restivo D, Berardelli A (2003) Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Exp Brain Res 150:222–229
Turton A, Lemon RN (1999) The contribution of fast corticospinal input to the voluntary activation of proximal muscles in normal subjects and in stroke patients. Exp Brain Res 129:559–572
Acknowledgments
This work was supported by Grants RFBR #1104-01068, #1204-01042 (Russian Foundation of Basic Research) and PR No. 2009-0447 (Spanish Ministry of Education). The authors would like to thank the reviewers for their time to help improve this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Talis, V.L., Kazennikov, O.V., Castellote, J.M. et al. Prior history of FDI muscle contraction: different effect on MEP amplitude and muscle activity. Exp Brain Res 232, 803–810 (2014). https://doi.org/10.1007/s00221-013-3789-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3789-5