Abstract
Several recent multisensory studies show that sounds can influence visual processing. Some visual judgments can be enhanced for visual stimuli near a sound occurring around the same time. A recent TMS study (Romei et al. 2009) indicates looming sounds might influence visual cortex particularly strongly. But unlike most previous behavioral studies of possible audio–visual exogenous effects, TMS phosphene thresholds rather than judgments of external visual stimuli were measured. Moreover, the visual hemifield assessed relative to the hemifield of the sound was not varied. Here, we compared the impact of looming sounds to receding or “static” sounds, using auditory stimuli adapted from Romei et al. (2009), but now assessing any influence on visual orientation discrimination for Gabor patches (well-known to involve early visual cortex) when appearing in the same hemifield as the sound or on the opposite side. The looming sounds that were effective in Romei et al. (2009) enhanced visual orientation sensitivity (d′) here on the side of the sound, but not for the opposite hemifield. This crossmodal, spatially specific effect was stronger for looming than receding or static sounds. Similarly to Romei et al. (2009), the differential effect for looming sounds was eliminated when using white noise rather than structured sounds. Our new results show that looming structured sounds can specifically benefit visual orientation sensitivity in the hemifield of the sound, even when the sound provides no information about visual orientation itself.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous studies have sought to determine whether the occurrence of a sound at a particular location can affect visual processing. These include classic studies of the so-called ventriloquist effect (e.g., Howard and Templeton 1966; Thurlow and Jack 1973; Bertelson and Radeau 1981), plus studies on other audio-visual psychophysical effects (e.g., McDonald et al. 2000; Vroomen and de Gelder 2000; Teder-Salejarvi et al. 2005; Lippert et al. 2007; Freeman and Driver 2008), including the beep-flash illusion (Shams et al. 2000) or crossmodal exogenous spatial cueing (see Spence et al. 2004, for review). In that paradigm, a sound is presented concurrently with (or shortly prior to) a visual target that can appear at the same location or elsewhere. Several studies have now shown that visual detection latency (e.g., Farah et al. 1989), subjective visual intensity (e.g., Stein et al. 1996), or even visual detection sensitivity d′ (e.g., McDonald et al. 2000; Frassinetti et al. 2002a) can be enhanced at the location of a cue sound, relative to elsewhere. Moreover, several visual discriminations (e.g., upper/lower localization) can show enhanced performance at the lateral location of a sound versus elsewhere (e.g., Spence and Driver 1997; see Spence et al. 2004, for review). Overall these studies show that auditory stimulation can affect visual processing, but the level at which this arises can remain unclear. For example, there has been surprisingly little study of whether visual orientation discrimination—long considered to be a primary function of early visual cortex (cf. Hubel and Wiesel 1968, Hubel et al. 1977; Weiskrantz 1986; Morland et al. 1996; Stoerig and Cowey 1997)—can be enhanced in discriminative sensitivity (d′) at the location of a sound. Here, we studied orientation discrimination for Gabor patches, since this is known to tap into the function of early visual cortex.
A further new issue arises from Romei et al. (2009), who noted that looming sounds (that rapidly increase in amplitude) may provide a particularly salient stimulus (see also Bach et al. 2008, 2009; Neuhoff 1998; Seifritz et al. 2002) with multisensory implications. Humans and other primates show particular responsiveness to looming sounds (e.g., Neuhoff 1998; Seifritz et al. 2002; Ghazanfar et al. 2002; Cappe et al. 2009; Maier and Ghazanfar 2007), possibly as these might indicate potential threat. Romei et al. (2009) suggested that such looming sounds may exert particularly powerful crossmodal influences on visual cortex. Transcranial magnetic stimulation (TMS) revealed that phosphene thresholds (i.e., the intensity of TMS over occipital cortex required to generate an illusory flash) was reduced in the context of harmonically structured looming sounds, relative to “static” or receding (see below) versions of the same sounds. This was interpreted as an increase in excitability of visual cortex due to structured looming sounds. Consistent with some previous research on looming sounds (e.g., Maier et al. 2004; Maier and Ghazanfar 2007), the effect on visual-phosphene threshold disappeared when using white noise rather than sounds with rich harmonic structure, presumably as white noise does not produce an adequate looming percept.
Romei et al. (2009) suggested that the effective (structured) looming sounds have particularly strong crossmodal consequences for visual processing, but no objective psychophysical consequences for judgments of external visual stimuli were documented. Moreover, unlike many of the behavioral audio-visual studies mentioned above (e.g., Spence and Driver 1997; McDonald et al. 2000; Frassinetti et al. 2002a) or a recent TMS study by Bolognini et al. (2010), presentation of sounds to the same or opposite hemifield as that assessed visually was not varied. Accordingly, here we used sounds taken from Romei et al. (2009), but now in combination with a psychophysical measure of visual orientation discrimination for Gabor patches (tapping into the function of early visual cortex), with sounds located in the same or opposite hemifield relative to the external visual target.
Participants had to judge on each trial whether a visual Gabor patch was oriented clockwise or anti-clockwise from vertical. We manipulated whether this visual Gabor patch appeared in the same hemifield as the looming, static, or receding sound, or at the symmetric location in the opposite hemifield (see Fig. 1). Based on Romei et al.’s (2009) finding that looming structured sounds facilitate visual cortical excitability, we predicted better visual orientation discrimination sensitivity (d′) on the side of the looming structured sound.
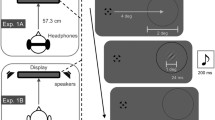
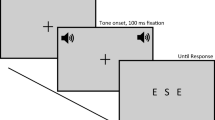
Schematic of successive events within a trial for Experiments 1 and 2. Participants maintained fixation on a central square, as confirmed by eye-tracking for the analyzed trials. A tilted Gabor patch was then presented unpredictably to the left (as shown) or right (equiprobable) visual hemifield, concurrently with a sound on the same or opposite side. Participants reported the perceived orientation of the Gabor patch (tilted clockwise or anti-clockwise from vertical) by button press, then a new trial started. The inset at bottom-left depicts how the intensity profile was manipulated to produce the different sound types. Note that the “static” sound had a constant intensity value that was the mean of the other sound types, while the “looming” and “receding” had the same intensity profile as each other but reversed (i.e., rising or falling, respectively)
Experiment 1
Methods
Participants
Eighteen participants (eight women) had a mean age of 24 years (range 18–33). All reported normal or corrected visual acuity and normal hearing. They gave informed consent in accord with local ethics.
Apparatus
Stimuli were presented on a 21″ CRT display (Sony GDM-F520) at a distance of 66 cm in a dark room. Video mode was 1,600 × 1,200, screen refresh rate 60 Hz. Mid-gray luminance was 65 cd/m². The monitor was calibrated using a Minolta CS-100A colorimeter. Two small stereo PC speakers were placed in front of the monitor on either side, 4.2° of visual angle below each of the two possible visual stimulus locations (see Fig. 1). Eye position was monitored with an infrared CRS 250 Hz High Speed Eye-Tracker (http://www.crsltd.com/). Stimulus control and data recording were implemented on a standard PC, running Psychophysics Toolbox 3 (Brainard 1997) and the CRS Video Eyetracker Toolbox under Matlab r2007b. Manual responses were made using a standard PC keyboard.
Stimuli
Visual target stimuli were Gabor patches composed of a 2D sinusoidal luminance grating with spatial frequency of 3 cycles per degree, within a Gaussian amplitude envelope of standard deviation 0.33°. Visual targets were presented at maximum contrast, for 250 ms randomly on either the left or the right side of a black 0.05° central fixation point, at 9° eccentricity. Auditory stimuli were 400 Hz triangular waveforms of 250 ms duration, sampled at 44.1 kHz (cf. Romei et al. 2009). Triangular waveforms are harmonically rich, containing the fundamental frequency plus all of its odd-numbered harmonics, with their amplitudes falling off in odd-integer ratios. We employed three different types of such sounds (see inset at bottom-left of Fig. 1). Looming sounds had exponentially rising acoustic intensity (as in Romei et al. 2009), from 55 to 75 dB Sound Pressure Level (SPL) as measured with an audiometer at the position of subjects’ ears. Receding sounds had exponentially falling acoustic intensity from 75 to 55 dB SPL. Static sounds had a constant 65 dB SPL (i.e., the average intensity level of looming and receding sounds). All sounds had 5 ms onset and offset ramps to avoid clicks. Auditory stimuli were generated with Cool Edit Pro Software (Syntrillium Software Corp, http://www.syntrillium.com), as in Romei et al. (2009).
Procedure
Visual-orientation threshold estimation session
For each participant, 5 min practice preceded threshold estimation. In order to avoid floor or ceiling effects in visual orientation discrimination, participants then underwent several visual-orientation threshold estimation blocks (range 3–5 blocks) to define for each participant their orientation discrimination threshold (target level of 75% correct) using the method of constant stimuli. Each threshold estimation block comprised 140 trials in which Gabor patches with different orientations (ranging between 3.5º and 0.30º clockwise or anti-clockwise from vertical) were presented in pseudorandom order to one or other visual hemifield, as in the main experiment but without sounds. Participants were required to perform a two-alternative forced choice (2AFC) orientation discrimination task, in which they had to indicate as accurately as possible whether the briefly presented Gabor patch on each trial was tilted anti-clockwise or clockwise from vertical, by pressing, respectively, the left or the right arrow of the keyboard with corresponding index fingers.
To estimate the threshold for 75% correct orientation discrimination separately for the left and right hemifield visual presentations, we used the psignifit toolbox (http://bootstrap-software.org/psignifit/) version 2.5.6 for Matlab, which implemented the maximum-likelihood method described by Wichmann and Hill (2001) for Weibull curve fitting.
Experimental session
Before data collection, the eye-tracker was calibrated using a nine-point grid (23º × 17.5º, 11.5º of horizontal spacing between points, 8.75º of vertical spacing between points). A head stabilization device prevented head movements.
The visual Gabor orientation values (i.e., offset from vertical in clockwise or anti-clockwise direction) for the main experiment were individually set (see above) at the predetermined 75% correct threshold (mean threshold for the left side: 1.36º ± 0.17 Standard Error; right side: 1.53º ± 0.16) for orientation discrimination, according to the preceding threshold estimations. Participants were instructed to maintain fixation on the central fixation point (as confirmed via eye-tracker, see below). Gabor and sound location (left or right hemifield) were chosen randomly and independently on each trial. Thus, sound location did not predict the hemifield of the visual target (nor the orientation of that visual target). Visual targets were coupled equiprobably with a concurrent looming, receding or static sound. All sounds onset synchronously with visual onset and offset synchronously with visual offset.
As in the preceding threshold estimation session, participants were required to perform the 2AFC orientation discrimination visual task, indicating whether the briefly presented Gabor patch was tilted anti-clockwise or clockwise of vertical. Accuracy of this response was stressed, not speed, given our d′ measure (see below), and it was emphasized that all sounds were irrelevant to the task.
Factorial combination of the independent variables sound type (looming, receding, and static) and Hemifield (same vs opposite side of the visual target) generated six possible stimulus conditions, which were sampled with equal frequency and presented in a pseudo-random sequence. Note that our design allows us to compare the impact of different sounds on the same versus opposite side of the Gabor, rather than the less subtle contrast of sound presence versus absence (which we did not compare as any impact of that might just reflect alerting). Participants thus performed a visual orientation discrimination task for which the auditory stimulation was irrelevant (note that the sounds did not provide any information about visual target side nor about visual target orientation). The experiment was subdivided into ten blocks of trials with an overall number of 960 trials for each participant. There were 160 trials for each of the six intermingled conditions.
Data analysis
Performance accuracy was comparable for left and right visual targets overall (proportion correct for the left side was 0.77 ± 0.01 SE; for the right side: 0.77 ± 0.01, t(17) = 0.33, P = 0.73, n.s.). Subsequently, all data were collapsed across visual hemifields.
For each participant, we computed sensitivity (d′) and criterion (c) for each stimulus condition, using standard formulae as in Macmillan and Creelman (1997), namely:
where z(H) stands for the z-transform of the hit rate, while z(F) stands for the z-transform of the false alarm rate. For purposes of scoring hits and false-alarms in the discrimination task, a clockwise tilt was treated as “target-present” arbitrarily, as in any signal-detection analysis of discriminations (MacMillan and Creelman 1997).
Eye traces were recorded during actual stimuli presentations (250 ms) on every trial. Offline analysis showed that participants maintained fixation within a 2.5° square around fixation for >96% of trials. Trials on which participants did not maintain fixation within the 2.5° window were removed from analyses. There were no significant differences between conditions in the proportions of such excluded trials (looming congruent: 3.84%; looming incongruent: 3.76%; receding congruent: 3.65%; receding incongruent: 3.40%; static congruent: 3.85%; static incongruent: 3.83%; F(5,85) = 0.29, P = 0.91, n.s.).
Finally, d′ and criterion data were analyzed using repeated-measure analysis of variance (two-way ANOVAs), with sound type (i.e., looming, receding, static) and hemifield (i.e., same or opposite hemifield) as orthogonal factors; plus Newman-Keuls tests when required.
Results and discussion for Experiment 1
The sensitivity (d′) results are shown in Fig. 2, as group means with standard-error bars. Note the higher sensitivity specifically in the looming congruent condition (leftmost bar). The ANOVA on d′ scores showed no main effects of sound type [F(2,34) = 0.31; P = 0.74, n.s.] and only a marginal effect of hemifield congruency [F(1,17) = 3.41, P = 0.08)], but a significant interaction [F(2,34) = 8.24, P < 0.001]. Sensitivity in the looming spatially congruent condition (d′ = 1.87 ± 0.10 SE) was significantly higher than in the looming spatially incongruent condition (1.58 ± 0.08; P = 0.001). This congruency effect was not present for receding (P = 0.59) nor static sounds (P = 0.98). Thus, visual orientation sensitivity benefited from a looming sound on the same versus opposite side as the visual target, but there was no such spatial effect from the other sounds (which gave a “null” outcome instead). Indeed, the looming spatially congruent condition showed higher sensitivity than any of the other conditions (all at P < 0.05 or better; see asterisks in Fig. 2).
A comparable ANOVA on criterion scores revealed no significant terms [for main effect of sound type, F(2, 34) = 1.88, P = 0.17; for main effect of congruency, F(1,17) = 1.14, P = 0.30; for interaction, F(2,34) = 1.57, P = 0.22, all n.s.].
Thus, Experiment 1 revealed a benefit in visual orientation discrimination sensitivity specifically for visual targets on the same side as a looming sound, even though such sounds provided no information about visual orientation itself. This appears consistent with Romei et al.’s (2009) proposal (based on a very different method incorporating TMS) that structured looming sounds can enhance visual processing. Here, we confirmed this specifically for visual Gabor orientation sensitivity (which taps into the function of early visual cortex) in the hemifield of such a sound. Romei et al. (2009) had not compared the visual hemifield of the sound to the other visual hemifield, so here we go beyond Romei et al. (2009) work not only in extending the impact of looming sounds to objective visual orientation sensitivity but also in showing the spatial specificity of the crossmodal effect.
Another aspect of the Romei et al. (2009) study was that while structured looming sounds modulated excitability of visual cortex (as measured with TMS), ramping up the intensity of white noise instead did not produce the same looming impact on visual cortex excitability as was found for structured looming sounds that underwent the very same intensity manipulation. This is presumably because white-noise stimuli do not produce a sufficiently naturalistic cue for a looming percept (see also Maier et al. 2004; Maier and Ghazanfar 2007). In our second experiment, we too used the white-noise control sounds (which equate the physical intensity manipulations, but not the looming or receding percept) as employed by Romei et al. (2009). We predicted that the advantage in visual orientation sensitivity on the side of the sound with ramped-up intensity should disappear (analogs to the disappearance of the TMS effect in Romei et al.).
In sum, we replaced the structured sounds that were used in Experiment 1 with white noise that had rising, falling, or constant intensity (from 55 to 75 dB, or the reverse; or “static” 65 dB intensity, exactly as before). If the previous advantage for visual orientation discrimination sensitivity (as found in Experiment 1) on the side of the “looming” sound depends merely on ramping up of physical auditory intensity, then it should be replicated in Experiment 2. If instead it depends on the looming percept, then analogous to the Romei et al. (2009) TMS effect, the psychophysical impact on visual orientation sensitivity should now disappear.
Experiment 2
Methods
Subjects
Fifteen participants (only two of whom had already taken part in Experiment 1) took part in Experiment 2 (mean age = 24.9; age range = 19–33; eight men and seven women). All reported normal or corrected visual acuity and normal hearing. All participated with informed consent.
Apparatus
The experimental apparatus was identical to Experiment 1.
Stimuli and procedure
The procedure was as in Experiment 1, except that we now replaced the structured complex sounds used in Experiment 1 with white-noise stimuli (see also Romei et al. 2009), but otherwise using identical rising or falling or static physical-intensity profiles (from 55 to 75 dB, or the reverse, or fixed at 65 dB). Our white-noise stimuli were generated exactly as in Romei et al. (2009).
Data analysis
Data analyses were identical to Experiment 1. Offline eye analyses showed that participants maintained fixation within a 2.5° square around fixation on >96% trials, with no differences between conditions in the percentage or rejected trials with fixation loss (increasing-intensity congruent: 3.16%; increasing-intensity incongruent: 3.91%; decreasing-intensity congruent: 3.52%; decreasing-intensity incongruent: 3.27%; fixed-intensity congruent: 3.76%; fixed-intensity incongruent: 3.10%; F(5,70) = 0.90, P = 0.48, n.s.).
Results and discussion for Experiment 2
Sensitivity (d′) and criterion (c) data were again analyzed using repeated-measure analysis of variances (two-way ANOVAs) with sound type and same or different hemifield as orthogonal factors. ANOVA on d′ showed no significant terms (for main effect of sound type, F(2,28) = 0.09, P = 0.92; for hemifield congruence, F(1,14) = 1.05, P = 0.32; and for the interaction F(2,28) = 0.49, P = 0.62, all terms n.s.); see Fig. 3.
Likewise, the ANOVA on criterion scores showed no significant terms (for main effect of sound type, F(2,280) = 2.74; for hemifield, F(1,14) = 0.25; for the interaction, F(2,28) = 3.11, all P’s > 0.05, n.s.).
Thus, the significant impact of structured looming sounds on visual orientation sensitivity (d′) that had been found in Experiment 1 (for visual Gabors on the same side as the looming sound) was not present for the increasing-intensity white-noise control sounds (as used by Romei et al., 2009) in Experiment 2. To confirm that this difference in outcome was significant, we directly compared the sensitivity results of Experiment 1 and 2, after computing the change in sensitivity between spatially congruent and spatially incongruent conditions (i.e., the difference in d′ between those conditions) for each sound type; see Fig. 4, which plots the Experiment 1 results with dark bars and the Experiment 2 results with light bars.
Mean differences in d′ between spatially congruent and incongruent conditions (SED indicated) for each sound type in Experiment 1 (dark bars) or Experiment 2 (light bars). Note that only the structured looming sounds in Experiment 1 produced a spatial congruency effect on visual orientation discrimination d′. *P < 0.05; **P < 0.01
These d′-difference data were then analyzed using a mixed model ANOVA with group (Experiment 1 vs. Experiment 2) as a between-subjects factor and sound type (i.e., looming/increasing, receding/decreasing, and static/fixed) as a within-subjects factor. For this between-group analysis, we removed those two participants that took part in both the experiments. Analysis of the d′ differences showed not only a main effect of group [F(1,27) = 4.07, P = 0.05] but more importantly a significant interaction between group and sound type [F(2,54) = 5.43, P = 0.007]. This was specifically due to the difference in d′ between looming spatially congruent and incongruent conditions being significantly higher (P = 0.005) in Experiment 1 (structured complex sounds) than in Experiment 2 (white noise with rising intensity). By contrast, there was no difference between the (null) impact of sounds on visual orientation sensitivity in the same-minus-opposite-hemifield for receding structured sounds versus decreasing-intensity white noise (P = 0.79); nor for static structured sounds versus fixed-intensity white noise (P = 0.71).
Discussion
Our two experiments investigated the possible impact of different auditory stimuli on visual sensitivity in Gabor orientation discrimination, which should tap into the function of early visual cortex. Visual orientation discrimination has been strongly associated with early visual cortex (e.g., Hubel and Wiesel 1968, Hubel et al. 1977; Orban et al. 1996; Harrison and Tong 2009), so one basic question here was whether such a visual task could be modulated by sounds, even when audition provides no information about the location or orientation of the visual target. A further new question, triggered by the recent TMS study of Romei et al. (2009), was whether looming sounds in particular might have a differential effect on a visual psychophysics task that taps into early visual cortex.
Our participants had to discriminate whether the single visual Gabor patch presented on each trial was tilted slightly clockwise or anti-clockwise from vertical. This Gabor patch could appear unpredictably in the left or in the right hemifield, with central fixation maintained on the analyzed trials. At the same time as the Gabor patch appeared, a sound was played, also from the left or right, but with its location equally likely to be on the same or opposite side as the visual target (as in so-called exogenous spatial cuing paradigms; Spence and Driver 1996). Following Romei et al. (2009), we used structured looming, or receding, or static sounds in Experiment 1. Romei et al. had found that structured looming sounds in particular can modulate excitability of visual cortex, as assessed with TMS. Here, we found that visual orientation sensitivity (d′) was enhanced by a looming sound on the same side as the visual target, relative to the comparable sound appearing on the opposite side (and indeed, relative to all the other types of sound used in our experiments here). By contrast, the receding or static sounds produced no such “spatial congruence” effect on visual orientation sensitivity in Experiment 1; and neither did any of the control white-noise sounds used in Experiment 2, including even those that underwent the same physical rising-intensity manipulation as for the effective structured looming sounds of Experiment 1 (see also Romei et al. 2009).
Thus, we found a specific benefit in visual orientation discrimination sensitivity on the side of a structured looming sound. This arose even though such a sound provided no information about whether the visual target would appear on the same or opposite side as the structured looming sound and no information about the visual orientation of the Gabor itself. This highly specific crossmodal effect thus joins a growing body of evidence for multisensory influences that can arise even when the influencing modality (here, audition) provides no information about the specific property that has to be judged for the influenced modality (here, vision). See Driver and Noesselt (2008) for a review of other multisensory phenomena that seem analogs in this particular respect.
The specific multisensory influence documented here is consistent with Romei et al.’s (2009) proposal that looming sounds facilitate visual processing by rapidly increasing excitability of visual cortex, presumably as such sounds indicate a potential threat for which it could be useful to obtain further visual information. Here, we go beyond Romei et al.’s (2009) results, extending them psychophysically by showing that visual orientation processing of Gabors (which taps into early visual cortex) can be enhanced by a structured looming sound. Moreover, we find that this multisensory enhancement is specific to the side on which the looming sound appears, demonstrating that it cannot be explained by nonspecific “arousal.” Although concurrent neural measures will be required to document the underlying neural correlates of the particular psychophysical effect, we document, here, the hemifield-specificity of our effect already indicates that it needs to be more (spatially) specific that just a general increase in excitability of visual cortex as a whole. Looming sounds might serve as a particularly salient cue for spatial attention; alternatively they might tap into more specific “threat-related” circuitry for enhancing early visual processing. Applying neural measures to our new paradigm should shed light on this.
Our results are broadly consistent with a wider literature documenting that looming stimuli can be particularly salient and effective (e.g., see Bach et al. 2008, 2009; Ghazanfar et al. 2002; Graziano and Cooke 2006; Maier et al. 2008; Neuhoff 1998; Schiff 1965; Schiff et al. 1962; Seifritz et al. 2002) and produce specific multisensory benefits (Cappe et al. 2009). fMRI data indicate that looming versus receding structured sounds activate a wide network (e.g., Seifritz et al. 2002) including superior temporal sulci, middle temporal gyri, and right temporoparietal junction, plus the amygdala (e.g., Bach et al. 2008). Numerous studies already exist showing that crossmodal and multisensory influences can arise even for early, sensory-specific cortex (e.g., see Driver and Noesselt 2008; Giard and Peronnet 1999; Molholm et al. 2002; Raij et al. 2010; Ramos-Estebanez et al. 2007; Romei et al. 2007; Martuzzi et al. 2007; Noesselt et al. 2007; Kayser et al. 2007; Cappe et al. 2010). But to our knowledge the specific case of influences from looming versus receding sounds upon early visual cortex has yet to be tested with neural measures.
Our new psychophysical results also add to a substantial body of existing behavioral evidence that sounds can influence some visual judgments (e.g., Frassinetti et al. 2002a, b; Vroomen and de Gelder 2000; McDonald et al. 2000; Spence and Driver 1997; Teder-Salejarvi et al. 2005; Stein et al. 1996; Lippert et al. 2007). But again the key difference is that here the visual enhancement was specific to the case of a looming structured sound on the same side as the visual target (in addition to the important fact that here the benefit was found for visual Gabor orientation sensitivity in particular). This specificity for looming structured sounds seem consistent with recent proposals (e.g., Maier et al. 2004; Romei et al. 2009) that such sounds may be particularly effective at engaging other modalities, because they provide salient warning cues. Here, we show that the multisensory benefits of looming sounds can be spatially specific, rather than merely reflecting nonspecific arousal.
The present results suggest several potentially fruitful lines for future research, including the following: (1) assessing the exact spatial specificity by comparing visual performance at different eccentricities within a visual hemifield, as a function of the location of a looming structured sound; (2) testing for spatially specific impacts of looming sounds on visual cortex itself, with neural measures; (3) testing whether looming sounds can influence processing in other modalities also, such as somatosensation, as might be the case for a sound that approaches the body or head and signaling an imminent collision (Hall and Moore 2003; Bach et al. 2008, 2009; Neuhoff 1998; Seifritz et al. 2002); (4) dissociating perceptual versus physical aspects of looming sounds, in terms of the impact on vision.
The present results already demonstrate that (structured) looming sounds can produce multisensory effects that receding, static or white-noise control sounds do not, specifically improving visual orientation sensitivity on the side of the looming structured sound. In many practical situations, looming sounds might thus be particularly useful for enhancing visual processing.
References
Bach DR, Schachinger H, Neuhoff JG, Esposito F, Di Salle F, Lehmann C et al (2008) Rising sound intensity: an intrinsic warning cue activating the amygdala. Cereb Cortex 18(1):145–150
Bach DR, Neuhoff JG, Perrig W, Seifritz E (2009) Looming sounds as warning signals: the function of motion cues. Int J Psychophysiol 74(1):28–33
Bertelson P, Radeau M (1981) Cross-modal bias and perceptual fusion with auditory-visual spatial discordance. Percept Psychophys 29:578–584
Bolognini N, Senna I, Maravita A, Pascual-Leone A, Merabet LB (2010) Auditory enhancement of visual phosphene perception: the effect of temporal and spatial factors and of stimulus intensity. Neurosci Lett 477(3):109–114
Brainard DH (1997) The psychophysics toolbox. Spat Vis 10:433–436
Cappe C, Thut G, Romei V, Murray MM (2009) Selective integration of auditory-visual looming cues by humans. Neuropsychologia 47(4):1045–1052
Cappe C, Thut G, Romei V, Murray MM (2010) Auditory-visual multisensory interactions in humans: timing, topography, directionality, and sources. J Neurosci 30(38):12572–12580
Driver J, Noesselt T (2008) Multisensory interplay reveals crossmodal influences on ‘sensory-specific’ brain regions, neural responses, and judgments. Neuron 57(1):11–23
Farah MJ, Wong AB, Monheit MA, Morrow LA (1989) Parietal lobe mechanisms of spatial attention: modality-specific or supramodal? Neuropsychologia 27:461–470
Frassinetti F, Bolognini N, Làdavas E (2002a) Enhancement of visual perception by crossmodal audio-visual interaction. Exp Brain Res 147:332–342
Frassinetti F, Pavani F, Làdavas E (2002b) Acoustical vision of neglected stimuli: interaction among spatially converging audiovisual inputs in neglect patients. J Cogn Neurosci 14:62–69
Freeman E, Driver J (2008) Direction of visual apparent motion driven solely by timing of a static sound. Curr Biol 18(16):1262–1266
Ghazanfar AA, Neuhoff JG, Logothetis NK (2002) Auditory looming perception in rhesus monkeys. Proc Natl Acad Sci USA 99(24):15755–15757
Giard MH, Peronnet F (1999) Auditory-visual integration during multimodal object recognition in humans: a behavioral and electrophysiological study. J Cogn Neurosci 11(5):473–490
Graziano MS, Cooke DF (2006) Parieto-frontal interactions, personal space, and defensive behavior. Neuropsychologia 44(6):845–859
Hall DA, Moore DR (2003) Auditory neuroscience: the salience of looming sounds. Curr Biol 13(3):R91–R93
Harrison SA, Tong F (2009) Decoding reveals the contents of visual working memory in early visual areas. Nature 458:632–635
Howard IP, Templeton WB (1966) Human spatial orientation. Wiley, London
Hubel DH, Wiesel TN (1968) Receptive fields and functional architecture of monkey striate cortex. J Physiol 195(1):215–243
Hubel DH, Wiesel TN, Stryker MP (1977) Orientation columns in macaque monkey visual cortex demonstrated by the 2-deoxyglucose autoradiographic technique. Nature 269(5626):328–330
Kayser C, Petkov CI, Augath M, Logothetis NK (2007) Functional imaging reveals visual modulation of specific fields in auditory cortex. J Neurosci 27(8):1824–1835
Lippert M, Logothetis NK, Kayser C (2007) Improvement of visual contrast detection by a simultaneous sound. Brain Res 1173:102–109
Macmillan NA, Creelman CD (1997) d′plus: A program to calculate accuracy and bias measures from detection and discrimination data. Spat Vis 11(1):141–143
Maier JX, Ghazanfar AA (2007) Looming biases in monkey auditory cortex. J Neurosci 27(15):4093–4100
Maier JX, Neuhoff JG, Logothetis NK, Ghazanfar AA (2004) Multisensory integration of looming signals by rhesus monkeys. Neuron 43(2):177–181
Maier JX, Chandrasekaran C, Ghazanfar AA (2008) Integration of bimodal looming signals through neuronal coherence in the temporal lobe. Curr Biol 18(13):963–968
Martuzzi R, Murray MM, Michel CM, Thiran JP, Maeder PP, Clarke S, Meuli RA (2007) Multisensory interactions within human primary cortices revealed by BOLD dynamics. Cereb Cortex 17(7):1672–1679
McDonald JJ, Teder-Salejarvi WA, Hillyard SA (2000) Involuntary orienting to sound improves visual perception. Nature 407(6806):906–908
Molholm S, Ritter W, Murray MM, Javitt DC, Schroeder CE, Foxe JJ (2002) Multisensory auditory-visual interactions during early sensory processing in humans: a high-density electrical mapping study. Cogn Brain Res 14(1):115–128
Morland AB, Ogilvie JA, Ruddock KH, Wright JR (1996) Orientation discrimination is impaired in the absence of the striate cortical contribution to human vision. Proc Biol Sci 263(1370):633–640
Neuhoff JG (1998) Perceptual bias for rising tones. Nature 395(6698):123–124
Noesselt T, Rieger JW, Schoenfeld MA, Kanowski M, Hinrichs H, Heinze HJ, Driver J (2007) Audiovisual temporal correspondence modulates human multisensory superior temporal sulcus plus primary sensory cortices. J Neurosci 27(42):11431–11441
Orban GA, Dupont P, Vogels R, De Bruyn B, Bormans G, Mortelmans L (1996) Task dependency of visual processing in the human visual system. Behav Brain Res 76(1–2):215–223
Raij T, Ahveninen J, Lin FH, Witzel T, Jääskeläinen IP, Letham B, Israeli E, Sahyoun C, Vasios C, Stufflebeam S, Hämäläinen M, Belliveau JW (2010) Onset timing of cross-sensory activations and multisensory interactions in auditory and visual sensory cortices. Eur J Neurosci 31(10):1772–1782
Ramos-Estebanez C, Merabet LB, Machii K, Fregni F, Thut G, Wagner TA, Romei V, Amedi A, Pascual-Leone A (2007) Visual phosphene perception modulated by subthreshold crossmodal sensory stimulation. J Neurosci 27(15):4178–4181
Romei V, Murray MM, Merabet LB, Thut G (2007) Occipital transcranial magnetic stimulation has opposing effects on visual and auditory stimulus detection: implications for multisensory interactions. J Neurosci 27(43):11465–11472
Romei V, Murray MM, Cappe C, Thut G (2009) Preperceptual and stimulus-selective enhancement of low-level human visual cortex excitability by sounds. Curr Biol 19(21):1799–1805
Schiff W (1965) Perception of impending collision: a study of visually directed avoidant behavior. Psychol Monogr 79(11):1–26
Schiff W, Caviness JA, Gibson JJ (1962) Persistent fear responses in rhesus monkeys to the optical stimulus of “looming”. Science 136:982–983
Seifritz E, Neuhoff JG, Bilecen D, Scheffler K, Mustovic H, Schachinger H et al (2002) Neural processing of auditory looming in the human brain. Curr Biol 12(24):2147–2151
Shams L, Kamitani Y, Shimojo S (2000) What you see is what you hear. Nature 408(6814):788
Spence C, Driver J (1996) Audiovisual links in endogenous covert spatial attention. J Exp Psychol Hum Percept Perform 22(4):1005–1030
Spence C, Driver J (1997) Audiovisual links in exogenous covert spatial orienting. Percept Psychophys 59(1):1–22
Spence C, McDonald J, Driver J (2004) Exogenous spatial cuing studies of human crossmodal attention and multisensory integration. In: Spence C, Driver J (eds) Crossmodal space and crossmodal attention. Oxford University Press, Oxford, pp 277–320
Stein BE, London N, Wilkinson LK, Price DD (1996) Enhancement of perceived visual intensity by auditory stimuli: a psychophysical analysis. J Cognit Neurosci 8:497–506
Stoerig P, Cowey A (1997) Blindsight in man and monkey. Brain 120(Pt 3):535–559
Teder-Salejarvi WA, Di Russo F, McDonald JJ, Hillyard SA (2005) Effects of spatial congruity on audio-visual multimodal integration. J Cogn Neurosci 17(9):1396–1409
Thurlow WR, Jack CE (1973) Certain determinants of the “ventriloquism effect”. Percept Mot Skills 36:1171–1184
Vroomen J, de Gelder B (2000) Sound enhances visual perception: cross-modal effects of auditory organization on vision. J Exp Psychol Hum Percept Perform 26(5):1583–1590
Weiskrantz L (1986) Blindsight: a case study and implications. Clarendon, Oxford
Wichmann FA, Hill NJ (2001) The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys 63(8):1293–1313
Acknowledgments
FL was funded by a research grant from Polo Scientifico-Didattico di Cesena. JD was funded by the Wellcome Trust and Medical Research Council (UK), and he is a Royal Society Anniversary Research Professor.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Leo, F., Romei, V., Freeman, E. et al. Looming sounds enhance orientation sensitivity for visual stimuli on the same side as such sounds. Exp Brain Res 213, 193–201 (2011). https://doi.org/10.1007/s00221-011-2742-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2742-8