Abstract
Efficient flexible behavior requires continuous monitoring of performance for possible deviations from the intended goal of an action. This also holds for joint action. When jointly performing a task, one needs to not only know the other’s goals and intentions but also generate behavioral adjustments that are dependent on the other person’s task. Previous studies have shown that in joint action people not only represent their own task but also the task of their co-actor. The current study investigated whether these so-called shared representations affect error monitoring as reflected in the response-locked error-related negativity (Ne/ERN) following own errors. Sixteen pairs of participants performed a social go/no-go task, while EEG and behavioral data were obtained. Responses were compatible or incompatible relative to the go/no-go action of the co-actor. Erroneous responses on no-go stimuli were examined. The results demonstrated increased Ne/ERN amplitudes and longer reaction times following errors on compatible compared to incompatible no-go stimuli. Thus, Ne/ERNs were larger after errors on trials that did not require a response from the co-actor either compared to errors on trials that did require a response from the co-actor. As the task of the other person is the only difference between these two types of errors, these findings show that people also represent their co-actor’s task during error monitoring in joint action. An extension of existing models on performance monitoring in individual action is put forward to explain the current findings in joint action. Importantly, we propose that inclusion of a co-actor’s task in performance monitoring may facilitate adaptive behavior in social interactions enabling fast anticipatory and corrective actions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When performing a task with or against another person, knowledge about the goals and intentions of the person one is interacting with is crucial. Recent studies have demonstrated that in joint action people not only represent their own task, but also the task of their co-actors. These so-called shared representations have also been shown to affect one’s own motor behavior, as reflected in slower response times (Sebanz et al. 2003, 2005) or in effects on EEG measures of response inhibition (De Bruijn et al. 2008; Sebanz et al. 2006a, b; Tsai et al. 2006, 2008). Hence, these studies demonstrated that performing a task in a social context affects one’s own action-control processes through the formation of shared representations. However, it is unknown whether the evaluative process of error monitoring is also affected by knowledge of the other’s task.
Importantly, efficient flexible behavior requires continuous monitoring of ongoing behavior for possible deviations from the intended goal of an action. This monitoring is essential, as humans have to perform their actions in an environment that is constantly changing and thus requires flexible adaptations. Notably, this also holds for joint action. When jointly performing a task, one needs to not only incorporate the other’s goals and intentions, but also generate behavioral adjustments that are dependent on the other person’s task. For example, when missing a turn while driving, the type of remedial actions taken might be different depending on the task at hand of the passenger. Was he or she carefully paying attention and keeping track of the road map or staring out of the window absentmindedly? In other words, in joint action, the same performed error may induce different neural and behavioral responses depending on the task of a co-actor.
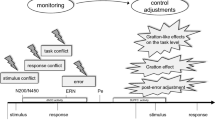
So far, existing theories on error monitoring have focused on individual settings in which participants perform a task on their own. Studies using these tasks have repeatedly demonstrated an event-related potential typically associated with erroneous responses. This component has been termed the error negativity (Ne) or error-related negativity (Ne/ERN) and is evident in the response-locked event-related potential (ERP) as a sharp negative deflection immediately following erroneous responses (Falkenstein et al. 1990; Gehring et al. 1993). In error-monitoring theories, representations of one’s own task (or “goal response”) play a central role. The so-called mismatch theory of the response Ne/ERN states that error detection can take place because a representation of the goal response is compared to a representation of the actual response (see Fig. 1a, upper panel; Coles et al. 2001; Falkenstein et al. 1991). When this comparison process results in a mismatch, the Ne/ERN will be generated in posterior medial frontal brain areas including anterior cingulate cortex or ACC (Ridderinkhof et al. 2004). The larger the mismatch, the larger the Ne/ERN amplitude will be. The central idea is that this error signal is then used as input for remedial actions or error-correction processes.
a Schematic representation of the mismatch hypothesis of the Ne/ERN as proposed by Coles et al. (2001). A representation of the actual response is compared to a representation of the goal response. In the case of an error, this comparison process will result in mismatch, triggering an error signal and generating the Ne/ERN. The larger the mismatch, the larger the Ne/ERN amplitude will be. b Proposed extension of the mismatch hypothesis to joint-action situations. A representation of the other’s goal response is included in the comparison process and may thus affect the magnitude of the mismatch and resulting Ne/ERN amplitude
Along with the mismatch theory, the Ne/ERN has also been extensively described in terms of conflict monitoring (Botvinick et al. 2001; Cohen et al. 2000; Yeung et al. 2004) and reinforcement learning (Holroyd and Coles 2002). Although these theoretical accounts are formulated around different fundamental processes—error detection, conflict monitoring, or reinforcement learning—the similarities between the three seem to be especially relevant to the current study. At the center of the three theories is a signal that carries high predictive information (either mismatch, conflict, or a prediction error) used to improve performance in the future and whose size determines the amplitude of the Ne/ERN and successive behavioral changes.
An often-reported form of remedial actions is so-called post-error slowing (Rabbitt 1966), and it describes the phenomenon that people slow down following errors resulting from premature responding. It is thought to reflect a cautious change of response strategy aimed at minimizing subsequent similar errors. Single-trial Ne/ERN analyses demonstrated that larger Ne/ERN amplitudes resulted in more post-error slowing on the trial following the error, thus demonstrating a direct link between the size of the Ne/ERN and the amount of behavioral adjustments (Debener et al. 2005).
In the current study, we use a social go/no-go task (De Bruijn et al. 2008, 2011) to investigate whether the formation of shared representations affects error monitoring and behavioral adjustments following errors. Or in other words, do people also incorporate other’s task representations into the comparison process enabling error detection (see Fig. 1b, lower panel)? In the social go/no-go task, pairs of participants will have to respond to frequent target stimuli (go) and withhold this response to infrequent distractor stimuli (no-go). It is expected that the frequency distribution and speed instructions will especially result in erroneous responses on no-go stimuli, and therefore these trials will be the main focus of the analyses. All stimuli can either be compatible or incompatible with respect to the task (go or no-go) of the other person (see Fig. 2, left). Importantly, this means that the only difference in a participant on compatible and incompatible no-go stimuli is the task of the other participant (i.e., responding or withholding). More specifically, on compatible no-go trials, both participants have to withhold their response, while on incompatible no-go trials one has to withhold while the other has to respond. Differences in processing of these two types of no-go stimuli will be reflected in the effects of compatibility and will thus only be present when people also represent the task of their co-actor. Through comparison of the brain activity associated with own errors on compatible and incompatible no-go stimuli, we will be able to investigate whether shared representations have an effect on error monitoring as reflected in the amplitude of the Ne/ERN. If participants also incorporate the representation of the goal response of their competitor in the comparison process, a larger mismatch and thus increased Ne/ERN amplitude is expected on erroneous responses to compatible distractor stimuli compared to incompatible distractor stimuli. However, no differences in Ne/ERN amplitude between erroneous responses to compatible and incompatible stimuli are expected when people do not incorporate a representation of the task of the other.
Methods
Participants
Eighteen pairs of participants performed a social go/no-go task. EEG was recorded from one participant in each pair, while behavioral data was collected from both (see Fig. 2, right). All were right-handed and had normal or corrected-to-normal vision. Two pairs were removed from the datasets because of excessive EEG artifacts. Only the data from the EEG participants (12 women; 4 men; mean age = 22.3 years, SD = 2.6 years) will be reported. All participants were paid 8 euro per hour for participation.
Design and procedure
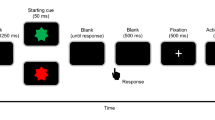
The aim of the task was to respond as quickly and accurately as possible to the presentation of a single letter on a computer screen. Each member of the pair is instructed to respond following presentation of two of the letters (70% “go”) and to withhold their response to the other two letters (30% “no-go”) (see Fig. 2, left). There was only partial overlap between letter-response assignments such that on 70% of the trials (compatible trials), the letter stimuli required the same action in both members of the pair (both should respond or both should withhold their response), while on 30% of the trials (incompatible trials) the required action was different (one should respond while the other should withhold their response). Each stimulus (the letters P, F, E, or T) was associated with one of these four conditions. Stimuli were presented for 100 ms in white against a black background in the center of a computer screen between two gray boxes (see Fig. 2, right). The boxes corresponded to the response buttons of the left and right participant, namely the left gray box colored yellow when the left participant responded—the right gray box colored yellow when the right participant responded. The intertrial interval varied randomly between 2,000 and 3,000 ms. An experimental session consisted of eight blocks of 200 trials. All participants were wearing earplugs to prevent any possible acoustic feedback from response buttons.
Half of the EEG participants were assigned to the left sitting position and half of them to the right. Before the start of the experiment, all participants received the same task instruction describing and emphasizing the stimulus–response mappings for the both of them. As a result, participants were aware of not only their own task but also their competitor’s task. Importantly, they were explicitly instructed to always try to respond faster than their competitor. This speed instruction was especially relevant in the competitive compatible go condition. A previous study using this paradigm in a competitive context demonstrated clear effects of shared representations on response inhibition (De Bruijn et al. 2008). The current competitive context was therefore chosen to ensure the formation of shared representations. Reaction-time feedback averaged over correct responses per participant was presented after each block and at the end of the experiment (total average overall eight blocks). There was a short break between the blocks. The total experiment lasted 2 h, including preparation and breaks.
Electrophysiological recording and data analyses
The electroencephalogram (EEG) was recorded from 27 tin electrodes mounted in an elastic electrode cap (Electrocap International). Electrodes were placed at seven midline (FPz/AFz/Fz/FCz/Cz/Pz/Oz) and twenty lateral (FP1-2/F7-8/F3-4/FC5-6/T3-4/C3-4/CP5-6/T5-6/P3-4/O1-2) locations in accordance with an extended version of the international 10–20 system. All signals were referenced to the left mastoid but were later off-line re-referenced to the average of both mastoids. The vertical electro-oculogram (EOG) was recorded bipolarly from electrodes placed above and below the right eye. The horizontal EOG was recorded bipolarly from electrodes lateral to both eyes. All electrode impedances were kept below 5 kΩ. The EEG and EOG signals were amplified using a time constant of 8 s (high pass .02 Hz) and were filtered off-line low pass at 15 Hz. All signals were digitized with a sampling rate of 500 Hz.
Trials were excluded from both the behavioral and the ERP analyses if one of the two participants had response latency less than 150 ms (1.04% excluded). EOG artifact correction was carried out using the procedure by Gratton et al. (1983). Trials were averaged to ERPs separately for each condition and each subject, relative to a 200 ms pre-response baseline. Ne/ERN amplitude was determined on error trials in separate subject averages by subtracting the most negative peak in the 0–200 ms time window after response onset from the most positive peak in the time window starting 80 ms before and ending 80 ms after response onset at electrode FCz, where the Ne/ERN amplitude was largest (De Bruijn et al. 2007).
For the ERP analyses, average Ne/ERN amplitudes were entered in a 2 × 2 repeated measures general linear model (GLM) with the within subject factors correctness (correct, error) and compatibility (compatible, incompatible). For the behavioral analyses, individual average reaction times were entered in a 2 × 2 repeated measures GLM with the within subjects factor correctness (correct, error) and compatibility (compatible, incompatible). Individual average accuracy rates were entered in a 2 × 2 repeated measures GLM with the within subject factors response type (go, no-go) and compatibility (compatible, incompatible). Finally, adaptive behavior following errors was investigated by entering individual average reaction times for correct responses to compatible go stimuli following correct and incorrect responses in a 2 × 2 repeated measures GLM with the within subject factors post-correctness (post-correct, post-error) and compatibility (compatible, incompatible).
Results
Behavioral results
Reaction times
Figure 3 depicts the reaction times for the different conditions. The reaction time analyses revealed main effects of correctness [F (1, 15) = 106.79, P < .001, η 2 p = .88] and compatibility [F (1, 15) = 10.23, P = .006, η 2 p = .41], revealing faster reaction times for incorrect responses (282 ms; SEM = 3.88) than for correct ones (331 ms; SEM = 5.48) and slower reaction times to incompatible (313 ms; SEM = 4.69) stimuli than to compatible ones (301 ms; SEM = 4.40). However, the significant interaction between correctness and compatibility showed that the main effect of compatibility was primarily caused by the faster reaction times on the more frequent compatible go stimuli than by the responses to the infrequent stimuli [F (1, 15) = 47.87, P < .001, η 2 p = .76].
An additional analysis was conducted to compare the reaction times of the two participants on incorrect incompatible no-go stimuli. The results showed that incorrect responses from the EEG participant on incompatible no-go stimuli were faster (278 ms; SEM = 3.93) than the correct responses on the same trial from the behavioral participant [340 ms; SEM = 11.18; t = 5.60, P < .001].
Accuracy
As expected, the accuracy rate analyses revealed a main effect for response type [F (1, 15) = 105.15, P < .001, η 2 p = .88], indicating better performance on the more frequent go stimuli (99.5%; SEM = .15) than on the infrequent no-go stimuli (77.0%; SEM = 2.22). There was neither a main effect for compatibility [F (1, 15) = 3.04, P = .102, η 2 p = .17] nor a significant interaction between the two [F (1, 15) = 3.73, P = .072, η 2 p = .20].
Post-error slowing
The analyses on adaptive behavior following errors revealed a main effect of post-correctness, indicating slower reaction times on correct trials following errors (332 ms; SEM = 6.71) than following correct responses [318 ms; SEM = 4.34; F (1, 15) = 19.30, P = .001, η 2 p = .56], reflecting post-error slowing. The main effect of compatibility was not significant [F (1, 15) = 3.80, P = .070, η 2 p = .20], but the interaction between the two did reach significance [F (1, 15) = 35.23, P < .001, η 2 p = .70]. This interaction was further investigated by means of two-tailed paired t-tests and showed that reaction times following correct responses were faster for compatible (306 ms; SEM = 4.69) than for incompatible stimuli [329 ms; SEM = 4.34; t = −8.91, P < .001], but the reverse pattern was found for reaction times following errors, indicating slower responses following errors on compatible stimuli (339 ms; SEM = 6.80) than following errors on incompatible ones [326 ms; SEM = 7.41; t = 2.78, P = .014].
ERP results
Figure 4a depicts the response-locked grand average waveforms for correct and incorrect responses. The Ne/ERN peaked around 65 ms after response onset and had a typical frontocentral topographical distribution as can be seen in Fig. 4b. As expected from previous studies, the Ne/ERN amplitude analyses revealed a main effect for correctness [F (1, 15) = 15.43, P = .001, η 2 p = .51], revealing increased Ne/ERN amplitudes for incorrect responses (13.84 μV; SEM = 1.22) compared to correct ones (8.21 μV; SEM = .92). There was no significant main effect for compatibility [F (1, 15) = 2.93, P = .108, η 2 p = .16], but importantly, the interaction between the two was significant [F (1, 15) = 6.37, P = .023, η 2 p = .30]. Follow-up contrast analyses using two-tailed t-tests indicated that Ne/ERN amplitudes on incorrect responses, i.e., on the no-go trials were larger for compatible stimuli (15.01 μV; SEM = 1.39) than for incompatible ones [12.67 μV; SEM = 1.21; t = 2.62, P = .019], but that this was not the case for correct responses [t < 1, P = .601].
a Grand average ERP waveforms relative to response onset (time = 0 ms) for correct (dashed lines) and incorrect (solid lines) responses to compatible (black lines) and incompatible stimuli (gray lines). b Topographical maps showing the typical frontocentral distribution of the Ne/ERN for incompatible and compatible errors and the difference between the two at the time of peak onset (65 ms)
Note that a peak-to-peak measure implies the presence of a negative peak on correct ERP waveforms as well. However, inspection of our individual averages revealed that this was often not the case. Therefore, we conducted additional analyses in which the Ne/ERN was quantified as the mean amplitude in a time window from 40 to 80 ms after response onset.Footnote 1 These analyses yielded similar results. The amplitudes on incorrect responses were more negative (−9.37 μV; SEM = 1.13) than on correct ones [1.73 μV; SEM = .95; F (1, 15) = 104.72, P < .001, η 2 p = .88]. There was no main effect of compatibility [F (1, 15) = 1.66, P = .217, η 2 p = .10], but the interaction between the two was significant [F (1, 15) = 7.56, P = .015, η 2 p = .34]. Follow-up t-tests showed that, similar to the peak-to-peak analyses, the amplitude on incorrect responses was more negative for compatible stimuli (−10.33 μV; SEM = 1.33) compared to incompatible ones [−8.41 μV; SEM = 1.05; t = −2.27, P = .038], but that this was not the case for correct responses [t = 1.56, P = .139].
Finally, to control for possible effects from actual responses of a co-actor, which are present on erroneous responses on incompatible trials but not on compatible ones, additional mean-amplitude analyses were conducted on a subset of participants (N = 10). Incorrect incompatible trials were divided depending on whether the erroneous response from the EEG participant was faster or slower than the correct response from the behavioral participant. Also, compatible no-go trials to which both participants responded incorrectly (double errors) were included. Participants who had less than 10 trials in one of these conditions were excluded from these additional analyses. Ne/ERN amplitudes on the three extra error conditions were compared to the amplitudes on the earlier described incorrect compatible and incompatible trials. In line with the findings from the complete set of participants, a main effect of error type was found [F (1, 6) = 13.81, P = .003 η 2 p = .90]. Follow-up simple contrasts referenced to erroneous incompatible stimuli (7.88 μV; SEM = 1.38) showed that Ne/ERN amplitudes were only significantly increased for errors on compatible stimuli [9.84 μV; SEM = 1.40; F (1, 9) = 14.15, P = .004] but not different for the other three error types [Fast errors: 7.56 μV; SEM = 1.36; Slow errors: 8.12 μV; SEM = 1.63; Double errors: 7.09 μV; SEM = 1.37; all F’s < 1.23, all P’s > .29].
Discussion
In the current study, we investigated whether shared representations have an effect on error monitoring as reflected in the error-related negativity (Ne/ERN). The results demonstrated increased Ne/ERN amplitudes for errors following compatible no-go stimuli compared to erroneous responses to incompatible no-go stimuli. In other words, when you did respond erroneously while not only you but also your co-actor had to withhold, Ne/ERN amplitude is increased compared to the situation in which you did respond but your co-actor had to respond anyway. Hence, the present findings indicate that along with the earlier reported effects on action control and action planning (De Bruijn et al. 2008; Sebanz et al. 2003, 2005, 2006a; Tsai et al. 2006, 2008), shared representations also affect the process of error monitoring. The finding of increased Ne/ERNs following errors to compatible no-go stimuli shows that when performing a task together with another person one also incorporates the task of the other into one’s own error-monitoring process.
Integrating error-monitoring theories and joint action
Interestingly, these error-monitoring findings in social interaction can relatively easy be implemented in existing theories of the Ne/ERN, like the mismatch hypothesis (see Fig. 1b, lower panel). Figure 5 illustrates this schematically. If one assumes that along with the representation of one’s own goal response and one’s own actual response, people also incorporate the representation of the other’s goal response, the mismatch hypothesis would exactly predict the current findings. The mismatch resulting from the comparison process of these different representations is larger when the actual response not only differs from one’s own goal response, but additionally deviates from the other’s goal response. In the current experiment, this holds for incorrect responses to compatible no-go stimuli and not for incompatible no-go stimuli. For the latter type of stimuli, the representation of the other’s goal response actually resembles one’s own actual response and will thus lead to a smaller mismatch and smaller Ne/ERNs.
As mentioned in the introduction, the mismatch hypothesis is not the only existing theory of the Ne/ERN. The Ne/ERN has, for example, also been extensively described in terms of conflict monitoring (Botvinick et al. 2001; Cohen et al. 2000; Yeung et al. 2004) or reinforcement learning (Holroyd and Coles 2002). While we currently chose to focus on the mismatch hypothesis for interpretation purposes, we want to emphasize that a similar line of reasoning can also be applied for conflict monitoring and reinforcement learning. Yeung and colleagues have demonstrated Ne/ERN amplitudes to increase with increasing levels of post-response conflict arising from simultaneous activation of actual- and goal-response tendencies. The level of conflict is directly reflected in the amplitude of the Ne/ERN. Our findings suggest that the co-actor’s goal response is also included in the conflict computation process, resulting in increased conflict on compatible no-go trials compared to incompatible no-go stimuli. Holroyd and Coles have proposed that the Ne/ERN is the result of the arrival of a dopamine-driven prediction error in the ACC where its function is primarily updating of motor behavior. Drawing conclusions on the effects of the current manipulations on dopamine functioning is clearly not possible on the basis of the current study. However, the present findings do suggest that the size of the prediction error and thus the impact of this signal on error-related ACC activity may also be determined by the task of the co-actor. Whether these differences in amplitude actually lead to consequences in, for example the learning rate of errors, remains to be determined in future research. The differences in the amount of direct behavioral adjustments as reflected in the current measures of post-error slowing may be taken as a first hint that this may indeed be the case. To summarize, our results are in accordance with the idea that at the center of the three theories a signal that carries high predictive information (either mismatch, conflict, or a prediction error) is used to improve performance in the future and whose size determines the amplitude of the Ne/ERN and successive behavioral changes.
Indeed, the amplitude of the Ne/ERN has been directly related to the amount of behavioral adjustments on the following trial (Debener et al. 2005), supporting its assumed role in adaptive behavior. Debener and co-workers demonstrated a positive correlation between single-trial Ne/ERN amplitudes and post-error slowing: the larger the Ne/ERN, the slower the response following the error. The current results are also in line with these findings. Increased Ne/ERNs were found on incorrect compatible no-go trials and were followed by slower response times compared to responses following errors on incompatible stimuli.
Although this slowing does not serve an obvious joint function in the current paradigm, the differences in post-error reaction times do suggest differential behavioral adjustments in response to the two types of errors. In daily life, incorporating other’s tasks into one’s own error-monitoring process may be very useful. For example, when missing a turn while driving a car and one knows that the passenger is carefully monitoring the road map waiting for the passenger’s instructions while continuing driving would be an appropriate response. However, when one knows that the passenger is engaged in tasks other than monitoring the route (e.g., staring out of the window absentmindedly), an appropriate response to the error made would be to stop the car and turn around. This example shows that incorporating other’s tasks in error monitoring may facilitate anticipatory and corrective behaviors in joint actions and thus enable social adaptive behavior.
A possible limitation of the current study may be related to the differences in the other’s response on compatible and incompatible trials. One might argue that the difference in Ne/ERN amplitude between the two compatibility conditions may be caused by the presence of a correct go response from the other participant on incompatible trials, which is not present on the compatible ones. However, the additional reaction time and Ne/ERN analyses demonstrate that this is most likely not the case. On incompatible trials, erroneous responses of the EEG participant are significantly faster than the correct responses from the behavioral participant. It seems unlikely that the relatively large difference of 62 ms could affect the fast error-detection process. Importantly, this was supported by the outcomes of the additional Ne/ERN analyses that also included fast and slow error trials as well as double errors. These analyses demonstrated that the increase in Ne/ERN amplitude on incorrect compatible trials is specific for that condition. If the difference were caused by either a fast response from the co-actor or the presence of a response from the other participant in general, differences would also have been observed on these error trials compared to incorrect incompatible ones. Taken together, these findings indicate that not the actual response of a co-actor affects monitoring of own errors but that knowledge about the other’s task—as reflect in shared representations—influences this action-evaluation process.
Conclusions
The current study adds to our understanding of performance monitoring in social contexts, by showing that people incorporate other’s tasks into their own error-monitoring processes and adjust their behavior accordingly. Existing models on performance monitoring in individual action can be extended to explain the current findings in joint action. A possible future research avenue may be to focus on possible individual differences in these processes, for example in psychiatric disorders that are characterized by social deficits like psychopathy, autism, or schizophrenia (De Bruijn and Ullsperger 2011). Importantly, we propose that the inclusion of a co-actor’s task in performance monitoring may facilitate social adaptive behavior in joint action and thus provide a mechanism for fast anticipatory and corrective behaviors in response to other’s actions.
Notes
Please note that additional analyses on the difference waves (incorrect–correct) also showed the similar pattern of increased Ne/ERNs on responses to compatible stimuli (−13.16 μV; SEM = 1.29) compared to responses to incompatible ones [−10.34 μV; SEM = 1.11; F (1,15) = 10.40, p = .006, η 2 p = .41].
References
Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD (2001) Conflict monitoring and cognitive control. Psychol Rev 108:624–652
Cohen JD, Botvinick M, Carter CS (2000) Anterior cingulate and prefrontal cortex: who’s in control. Nat Neurosci 3:421–423
Coles MG, Scheffers MK, Holroyd CB (2001) Why is there an ERN/Ne on correct trials? Response representations, stimulus-related components, and the theory of error-processing. Biol Psychol 56(3):173–189
De Bruijn ERA, Ullsperger M (2011) Pathological changes in performance monitoring. In: Mars RB, Sallet J, Rushworth M, Yeung N (eds) Neural basis of motivational and cognitive control. MIT press, Cambridge (in press)
De Bruijn ERA, Schubotz RI, Ullsperger M (2007) An event-related potential study on the observation of erroneous everyday actions. Cogn Affect Behav Neurosci 7:278–285
De Bruijn ERA, Miedl SF, Bekkering H (2008) Fast responders have blinders on: ERP correlates of response inhibition in competition. Cortex 44:580–586
De Bruijn ERA, Mars RB, Bekkering H, Coles MGH (2011) Your mistake is my mistake… or is it? Behavioral adjustments following own and observed actions in cooperative and competitive contexts. Q J Exp Psychol (in press)
Debener S, Ullsperger M, Siegel M, Fiehler K, von Cramon DY, Engel AK (2005) Trial-by-trial coupling of concurrent electroencephalogram and functional magnetic resonance imaging identifies the dynamics of performance monitoring. J Neurosci 25:11730–11737
Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1990) Effects of errors in choice reaction tasks on the ERP under focused and divided attention. In: Brunia CHM, Gaillard AWK, Kok A (eds) Psychophysiological brain research. Tilburg University Press, Tilburg, pp 192–195
Falkenstein M, Hohnsbein J, Hoormann J, Blanke L (1991) Effects of cross-modal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalogr Clin Neurophysiol 78(6):447–455
Gehring WJ, Goss B, Coles MG, Meyer DE et al (1993) A neural system for error detection and compensation. Psychol Sci 4:385–390
Gratton G, Coles MGH, Donchin E (1983) A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol 55:468–484
Holroyd CB, Coles MGH (2002) The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev 109:679–709
Rabbitt PMA (1966) Errors and error correction in choice-response tasks. J Exp Psychol 71:264–272
Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S (2004) The role of the medial frontal cortex in cognitive control. Science 306:443
Sebanz N, Knoblich G, Prinz W (2003) Representing others’ actions: just like one’s own? Cognition 88:B11–B21
Sebanz N, Knoblich G, Prinz W (2005) How two share a task. J Exp Psychol Hum Percept Perform 31:1234–1246
Sebanz N, Bekkering H, Knoblich G (2006a) Joint actions: bodies and minds moving together. Trends Cogn Sci 10:70–76
Sebanz N, Knoblich G, Prinz W, Wascher E (2006b) Twin peaks: an ERP study of action planning and control in co-acting individuals. J Cogn Neurosci 18:859–870
Tsai CC, Kuo WJ, Jing JT, Hung DL, Tzeng OJL (2006) A common coding framework in self–other interaction: evidence from joint action task. Exp Brain Res 175:353–362
Tsai CC, Kuo WJ, Hung DL, Tzeng OJ (2008) Action co-representation is tuned to other humans. J Cogn Neurosci 20(11):2015–2024
Yeung N, Botvinick MM, Cohen JD (2004) The neural basis of error detection: conflict monitoring and the error-related negativity. Psychol Rev 111(4):931–959
Acknowledgments
EDB was supported by a VENI grant from the Netherlands Organisation for Scientific Research (NWO) (451-07-022) and by the EU-Project “Joint Action Science and Technology” (IST-FP6-003747). For reprints, please contact the corresponding author Ellen de Bruijn (e.debruijn@donders.ru.nl).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
de Bruijn, E.R.A., Miedl, S.F. & Bekkering, H. How a co-actor’s task affects monitoring of own errors: evidence from a social event-related potential study. Exp Brain Res 211, 397–404 (2011). https://doi.org/10.1007/s00221-011-2615-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2615-1