Abstract
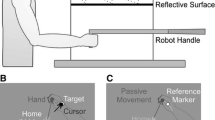
The adaptation of reaching movements has typically been investigated by either distorting visual feedback of the reaching limb or by distorting the forces acting upon the reaching limb. Here, we investigate reach adaptation when error is created by systematically perturbing the target of the reach rather than the limb itself (Magescas and Prablanc in J Cogn Neurosci 18: 75–83, 2006). Specifically, we investigate how adaptation is affected by (1) the timing of the perturbation with respect to the movement of the eye and the hand and (2) participant awareness of the perturbation. In Experiment 1, participants looked and pointed to a target that disappeared either at the onset of their eye movement or shortly after their eye movement and then reappeared, displaced to the right, at the completion of the reach. In Experiment 2, we made the target displacement more explicit by leaving the target at its initial location until the end of the reach, at which point it was displaced to the right. In Experiment 3, we extinguished the target at the onset of the eye movement but also informed participants about the presence and magnitude of the perturbation. In the no-feedback post-test phase, participants for whom the target disappeared during the reach demonstrated much stronger aftereffects of the perturbation, misreaching to the right, whereas participants for whom the target stayed on until reach completion demonstrated rapid extinction of rightward misreaching. Furthermore, participants who were informed about the target perturbation exhibited faster de-adaptation than those who were not. Our results suggest that adaptation to a target displacement is contingent on the explicitness of the target perturbation, whether this is achieved by manipulating stimulus timing or instruction.





Similar content being viewed by others
Notes
However, also see Bekkering et al. (1995). These authors show reach adaptation when pointing to an unseen target jump that occurs online, but they do not control for the effects of saccadic adaptation and they argue, in fact, that their results indicate transfer of saccadic adaptation to the hand. The Magescas et al. (2009) study, which deliberately prevented saccadic adaptation, can, therefore, be viewed as a purer test of reach adaptation to an unseen online target perturbation.
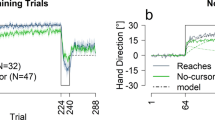
Violation of the assumption of homogeneity of variance (HOV) among groups on the pretest (Bartlett, P = .0075), along with unequal ns among the groups, dictates that a non-parametric test should be applied. When a Kruskal–Wallis test is applied to the groups, the result is not significant, H(3, N = 32) = 6.49, P = .09, However, if one overlooks violation of HOV and applies a one-way ANOVA instead, a significant difference does emerge, F(3,28) = 2.96, P = .049. Subsequent post-hoc testing with Newman-Keuls reveals that the SacEnd and Informed groups do differ significantly in the pretest, P = .04. All other pairwise contrasts are not significant (P > .10).
Based on the finding that peak velocity linearly increased as the perturbation size increased across acquisition trials (r = .86), F(1,48) = 133.62, P < .001.
This ‘bottom-up’ explanation does not preclude the possibility that top–down factors (such as instruction in the Informed group) may initially induce visibility of a target displacement. If visibility were being induced in this way, we would still consider the bottom-up explanation to account for the reduced adaptation.
References
Aivar MP, Brenner E, Smeets JBJ (2008) Avoiding moving obstacles. Exp Brain Res 190:251–264
Baizer JS, Kralj-Hans I, Glickstein M (1999) Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol 81:1960–1965
Bard C, Turrell Y, Fleury M, Teasdale N, Lamarre Y, Martin O (1999) Deafferentation and pointing with visual double-step perturbations. Exp Brain Res 125:410–416
Bekkering H, Abrams RA, Pratt J (1995) Transfer of saccadic adaptation to the manual motor system. Hum Mov Sci 14:155–164
Clower DM, Hoffman JM, Votaw JR, Faber TL, Woods RP, Alexander GE (1996) Role of posterior parietal cortex in the recalibration of visually guided reaching. Nature 383:618–621
Coren S, Ward LM, Enns JT (1999) Sensation and perception, 5th edn. Harcourt Brace, Fort Worth, pp 310–312
Danckert J, Ferber S, Goodale MA (2008) Direct effects of prismatic lenses on visuomotor control: an event-related functional MRI study. Eur J Neurosci 28:1696–1704
Desmurget M, Epstein CM, Turner RS, Prablanc C, Alexander GE, Grafton ST (1999) Role of the posterior parietal cortex in updating reaching movements to a visual target. Nat Neurosci 2:563–567
Deubel H, Schneider WX, Bridgeman B (1996) Postsaccadic target blanking prevents saccadic suppression of image displacement. Vis Res 36:985–996
Diedrichsen J, Hashambhoy Y, Rane T, Shadmehr R (2005) Neural correlates of reach errors. J Neurosci 25:9919–9931
Goodale MA, Pelisson D, Prablanc C (1986) Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320:748–750
Jakobson LS, Goodale MA (1989) Trajectories of reaches to prismatically-displaced targets: evidence for “automatic” visuomotor recalibration. Exp Brain Res 78:575–587
Luaute J, Schwartz S, Rossetti Y, Spiridon M, Rode G, Boisson D, Vuilleumier P (2009) Dynamic changes in brain activity during prism adaptation. J Neurosci 29:169–178
Magescas F, Prablanc C (2006) Automatic drive of limb motor plasticity. J Cogn Neurosci 18:75–83
Magescas F, Urquizar C, Prablanc C (2009) Two modes of error processing in reaching. Exp Brain Res 193:337–350
Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645
Miall RC, Wolpert DM (1996) Forward models for physiological motor control. Neural Netw 9:1265–1279
Michel C, Pisella L, Prablanc C, Rode G, Rossetti Y (2007) Enhancing visuomotor adaptation by reducing error signals: single-step (aware) versus multiple-step (unaware) exposure to wedge prisms. J Cogn Neurosci 19:341–350
Pelisson D, Prablanc C, Goodale MA, Jeannerod M (1986) Visual control of reaching movements without vision of the limb II. Evidence of fast unconscious processes correcting the trajectory of the hand to the final position of a double-step stimulus. Exp Brain Res 62:303–311
Pisella L, Michel C, Grea H, Tilikete C, Vighetto A, Rossetti Y (2004) Preserved prism adaptation in bilateral optic ataxia: strategic versus adaptive reaction to prisms. Exp Brain Res 156:399–408
Ramnani N (2006) The primate cortico-cerebellar system: anatomy and function. Nat Rev Neurosci 7:511–522
Redding GM, Rossetti Y, Wallace B (2005) Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav R 29:431–444
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning of a motor task. J Neurosci 14:3208–3224
Smith MA, Gazizadeh A, Shadmehr R (2006) Interacting adaptive processes with different timescales underlie short-term motor learning. PLOS Biol 4:1035–1043
Tseng Y, Diedrichsen J, Krakauer JW, Shadmehr R, Bastian AJ (2007) Sensory prediction errors drive cerebellum-dependent adaptation of reaching. J Neurophysiol 98:54–62
Wei K, Kording K (2009) Relevance of error: what drives motor adaptation? J Neurophysiol 101:655–664
Weiner MJ, Hallett M, Funkenstein HH (1983) Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33:766–772
Welch RB (1986) Adaptation of space perception. In: Boff KR, Kaufman L, Thomas JP (eds) Handbook of perception and human performance. Wiley, New York, pp 24.1–24.45
Wolpert DM, Ghahramani Z, Flanagan JR (2001) Perspectives and problems in motor learning. Trends Cogn Sci 5:487–494
Acknowledgments
This research was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant awarded to R. Chua. We would also like to thank two anonymous reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cameron, B.D., Franks, I.M., Timothy Inglis, J. et al. Reach adaptation to explicit vs. implicit target error. Exp Brain Res 203, 367–380 (2010). https://doi.org/10.1007/s00221-010-2239-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2239-x