Abstract
A 2 × 8 button-press task is a sequential hand movement task in which subjects are required to press eight pairs of buttons as accurately and quickly as possible. The 2 × 8 task allows us to examine flexible sequential learning, more aptly called sequence-unselective learning. Sequence-unselective learning is observed after repeated experiences with the task, when subjects have shown good progress in learning, with new sequences as well as previously learned ones. Although cognitive inflexibility has been reported in patients with Parkinson’s disease (PD), there have been few studies investigating their flexibility in sequential learning. We examined PD patients’ ability for sequence-unselective learning through the use of a 2 × 8 button-press task. In the first session, PD patients and subjects from the control group performed a sequential 2 × 8 task until the learning criterion was fulfilled (Session 1). After 1 month, they participated in other sessions: one involving the learned sequence (Session 2) and another involving the new sequence (Session 3). We found that PD patients made more errors than the normal control subjects only when learning the new sequence (Session 3) (P < 0.01). In Session 3, control subjects reached the learning target with fewer errors than in the Session 1 (normal sequence-unselective learning), whereas the PD patients did not exhibit such an improvement. Our results revealed a sequence-unselective deficit in PD patients. The deficit may help to emphasize the cognitive and physical inflexibility of PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hikosaka et al. (2000) developed a sequential hand movement task called the 2 × 5 button-press task, and they have investigated the neural mechanisms of the declarative-to-automatic transition process during learning a sequential behavior. The 2 × 5 task differs from other sequential learning tasks, such as the serial reaction time task (Dominey and Jeannerod 1997; Badgaiyan et al. 2007), on the points that the task structure is hierarchical and the procedure includes a trial and error process. These aspects are contained in the learning of our daily actions, such as riding a bicycle, typing on a keyboard, and so on (Hikosaka et al. 1995). Through repeated experiences of a particular sequence with the 2 × 5 task, monkeys and human subjects become capable of performing the task accurately and quickly, a phenomenon known as sequence-selective learning (for review, Hikosaka et al. 2000). Moreover, in another study involving only monkeys, after more than 1 year of training with multiple sequences, the subjects demonstrated sequence-unselective learning, and showed good progress with the learning of new sequences, as well as with previously learned ones (Hikosaka et al. 1995). Little is known about the neural mechanisms of sequence-unselective learning, although the contribution of multiple brain areas, including the frontal lobe, parietal cortices, basal ganglia and cerebellum, has been reported to be involved in sequence-selective learning in the 2 × 5 task (Hikosaka et al. 2000). The purpose of this study is to examine the neural mechanism for sequence-unselective learning.
Robertson and Flowers (1990) reported that the problem is one of flexibility and not one of learning in patients with Parkinson’s disease (PD). An animal study revealed that, in normal rats, progress in the learning of a second goal was better than that in the learning of the first one, whereas rats with lesions causing dysfunction in the basal ganglia, involving the striatum, did not show this improvement (Furtado and Mazurek 1996). These findings suggest that PD patients, who exhibit basal ganglia dysfunction, may not show an improvement in learning with new sequences (sequence-unselective learning). However, from the study by Robertson and Flowers (1990), the relationship between PD patients and this deficit in sequence-unselective learning is unclear, since even the age-matched healthy participants were unable to learn new sequences better than previous ones. A deficit of cognitive flexibility in PD patients has also been widely reported (for example, Cools et al. 2001; Delazer et al. 2004; Monchi et al. 2004; Shohamy et al. 2006); however, PD patients’ flexibility in sequential learning is still unclear.
We examined PD patients’ ability in sequence-unselective learning through the use of a 2 × 8 button-press task, where subjects were required to press eight pairs of buttons as accurately and quickly as possible. The patients were instructed to execute the 2 × 8 task with one type of sequence in the first session. One month later, in their second and third sessions, respectively, they performed a 2 × 8 task with the same sequence (learned) and with a different sequence (new). We predicted that, in the third session, age-matched healthy participants would demonstrate a decrease in the number of errors, owing to sequence-unselective learning. On the other hand, PD patients were not expected to show such an improvement. We believe that the findings of the present study may provide new insight into both the role of the basal ganglia in visuomotor sequential learning and the cognitive inflexibility of PD patients.
Methods
Patients
This study included ten patients (six women and four men) diagnosed with idiopathic PD. The mean age of the patients was 66.3(±9.3) years and the mean duration of education was 12.6 (±2.9) years. Six patients were on medication (two patients were taking l-dopa and four patients, amantadine). The other four patients were not on medication that affects the striatal dopamine (DA) system. All patients presented with mild to severe akinesia/bradykinesia and mild to moderate tremors. None of the patients had a history of stroke or alcoholism. The results of other physical and neurological examinations were normal. The Mini Mental State Examination (MMSE) revealed that none of the PD patients were demented (mean score 26.8 ± 1.8). We recruited 12 healthy adult volunteers (eight women and four men) who constituted the normal control (NC) group. The mean age of the NC participants was 62.3 (±7.0) years and the mean duration of education was 13.4 (±2.5) years. The t test revealed no significant differences in the mean age [t(20) = 1.11, n.s.] or duration of education [t(28) = 1.38, n.s.] between the two groups. In order to assess their basic neuropsychological ability, the subjects were required to participate in a Rey Complex Figure test, and digit and tapping span tests. Informed consent in accordance with the Declaration of Helsinki (1975) was obtained from all participants.
Task and procedure
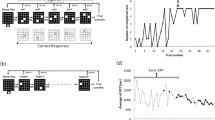
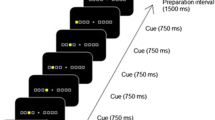
The 2 × 8 task comprised eight pairs of buttons (Fig. 1a). On pressing the home key, 2 of the 16 target buttons illuminated simultaneously (Set 1). Participants were asked to press the two buttons (set) in a random order at first. When they pressed the set in the correct order, the next set was illuminated. However, if the buttons were pressed in an incorrect order, the trial was aborted with a beep sound and participants had to restart from the home key. In the next trial, according to the last error sign, they had to press the set in the reverse order. A total of eight sets were presented in a fixed order for the completion of a trial (hyperset). A hierarchical structure was composed of the set and hyperset in the task. On successfully completing a trial, the same hyperset was repeated from the beginning. The participants continued the procedure until they successfully reached the learning criterion of ten hyperset completions. A computer (PC-9801 NS/R; NEC) controlled the illumination of buttons and recorded the responses of each participant. Before the first session, all participants practiced the task procedure using a 2 × 4 task, where 2 of the 16 target buttons illuminated four times, so as to eliminate any influence of habituation to the test apparatus on their performance.
Two different sequences (types A and B) were used. Both of the sequences contained a hyperset with differing illumination locations (Fig. 1b). There was no general rule to determine the correct order in each sequence. The participants performed the task in three sessions on two separate days. On the first day, each participant performed the 2 × 8 task by trial and error to fulfill the learning criterion for the type A sequence (Session 1). One month later, they were instructed to perform the 2 × 8 task with the same sequence (A) and with a novel sequence (B), in Sessions 2 and 3, respectively. We assessed their declarative knowledge about the sequences in a recall test. In the recall test for the set locations, the participants were asked to point to the maximum possible pairs of buttons (sets) by using the apparatus immediately after they fulfilled the criterion in each session.
Results
Neuropsychological scores
Two individuals from the NC group did not participate in the memory span tests. PD and NC subjects showed nearly equal scores in each of the following Rey Complex Figure tests: copy version (PD: mean 34.4 ± 2.6; NC: mean 35.6 ± 1.0); digit span test, forward version (PD: mean 6.2 ± 1.1; NC: mean 7.0 ± 1.5); and tapping span, forward version (PD: mean 6.2 ± 1.1; NC: mean 7.0 ± 1.5). The t test revealed no significant differences between the two groups in these test scores [Rey Complex Figure, t(20) = 1.51, n.s.; digit span, t(18) = 1.31, n.s., and tapping span, t(18) = 0.88, n.s.].
Task performance
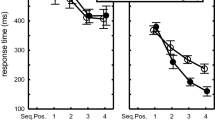
The mean number of errors made before reaching the criterion is shown in Fig. 2a. A two-way analysis of variance (ANOVA) was used to examine factors from the participant group (PD patients and NC) and from each session (1, 2 and 3), and this revealed the significant main effects of the sessions [F(2,40) = 10.51; P < 0.01] and of the interaction between these factors [F(2,40) = 7.79; P < 0.01]. Post hoc comparisons revealed that PD patients made a significantly greater number of errors than NC participants in the third session (P < 0.01), but not in the first and second sessions. In the PD group, the mean number of errors in the second session (learned sequence) was less than that in the first and third sessions (P < 0.05). In the NC group, the mean numbers of errors in the second and third sessions were significantly fewer than in the first session (P < 0.05). NC participants could learn new sequences effectively in the third session, but PD patients could not. The individual error scores of medicated and unmedicated PD patients are shown in Fig. 2b. The two patients on l-dopa committed a higher number of errors than other medicated and unmedicated patients throughout the three sessions (patients A and B in Fig. 2b). With the exception of two patients (D and F in Fig. 2b), eight of ten PD patients, including medicated subjects, showed an increased number of errors in the Session 3.
To be clear the possibility that general intelligence might contribute to the patients’ disability in Session 3, we examined the correlation of coefficients between the number of errors (Sessions 1 and 3) and other neuropsychological scores, or scales relevant to the severity of PD (Table 1). These results showed that visuospatial manipulation ability (copy score) and visual working memory (block tapping score) would be related to the performance of 2 × 8 task in Sessions 1 and 3. However, no neuropsychological factors specifically associated with the errors in Session 3. In Session 3 but not Session 1, a high and significant correlation between the Hoen and Yahr scale and the number of errors was found.
The scores for the declarative memory tasks revealed that it was difficult for both groups to recall the locations of eight sets. Mean scores of correctly recalled sets were shown in Fig 3. Two-way ANOVA with groups and sessions as factors revealed a significant interaction [F(2,40) = 3.68; P < 0.05], but no significant main effects [group: F(1,20) = 0.03; session: F(2,40) = 0.52]. Post hoc comparisons revealed that the mean score for the NC group was significantly lower than that for the PD group only in Session 3 (P < 0.05). While no significant difference was found (P = 0.055), NC subjects tended to show lower scores in the third session than in the first session, so they performed the 2 × 8 task more implicitly (automatically) in the third session than in the first session.
Discussion
Using a 2 × 8 task, we examined sequence-unselective learning ability in patients with PD. The PD patients made a significantly greater number of errors than NC participants only in the Session 3, in which they had to learn a new sequence during the 2 × 8 task. The results reflected the fact that the NC participants could reach the learning criterion with fewer errors in the Session 3 than in Session 1, which revealed normal sequence-unselective learning. However, PD patients could not exhibit such an improvement in Session 3. Our results suggest that PD patients failed to show signs of sequence-unselective learning. Fatigue could not explain the deficit because, in Session 3, PD patients demonstrated good scores in the declarative memory test which was executed after the 2 × 8 task. Habituation to the task apparatus also did not contribute to the deficit, since PD patients performed the task procedure well in Session 2. The results of the neuropsychological tests were normal, so other neuropsychological deficits could not explain this particular deficit. The results of correlational analyses showed that patients’ particular deficit in Session 3 might be connected to the severity of extrapyramidal symptoms caused by a dysfunction of the basal ganglia (evaluated by Hoen and Yahr scale) rather than other neuropsychological functions. These results suggest that basal ganglia degeneration may induce a deficit in sequence-unselective learning.
Role of the basal ganglia in 2 × 8 sequential learning
In Sessions 1 and 2, which involved using the same sequence (A), a significant difference was not detected in 2 × 8 task performance between PD patients and age-matched healthy participants. However, PD patients showed a significant number of errors in Session 3, which involved the use of a new sequence (B). Our results suggest that PD patients exhibit a difficulty with sequence-unselective learning rather than with sequence-selective learning. PD patients could not learn new sequences as flexibly and efficiently as healthy participants. These results do not conflict with those of previous neuropsychological studies (Contreras-Vidal and Schultz 1999; Exner et al. 2002; Krebs et al. 2001; Mochizuki-Kawai et al. 2004; Robertson and Flowers 1990). Exner et al. (2002) reported that the size of a lesion in the basal ganglia influenced general proficiency for sequential tasks, but this was not related to sequence-specific learning. The basal ganglia may contribute to a flexible strategy which is established through repeated experiences of learning with different stimuli, allowing us to efficiently learn multiple types of sequences, which is called sequence-unselective learning. This flexible strategy enables us to adapt to new and changing environments with minimal trial and error. In patients with PD, the lack of this flexible strategy may cause difficulties in their daily life when surrounding situations are frequently changing.
In the recall test in Session 3, the mean score of the NC participants was significantly lower than that of the PD patients. The NC participants exhibited poorer declarative knowledge in Session 3 than in Session 1. These results imply that their learning process with new sequences was precisely non-declarative. Sequence-unselective learning may shorten the declarative process in learning; that is, it may facilitate the transition from a declarative to an automatic process. However, their scores were very low (from 1 to 2 points) in comparison to the maximum score (8 points), so it is supposed that the significant difference in recall scores (declarative knowledge) had little effect on task performance. Further studies are required to discuss the contribution of declarative knowledge for sequence-unselective learning. There are two major types of memory: declarative and motor. Each is supported by a different neurological base (Milner 2005). PD patients were better than normal healthy people in declarative knowledge concerning sequence although they failed to learn the sequence by motor performance. This is the reverse pattern seen in the famous amnesic patient HM who could acquire motor skill but not declarative knowledge (Squire 1987). It has been suggested that PD patients have deficits in motor memory (non-declarative), while declarative memory is maintained (e.g., Bondi and Kaszniak 1991). The present results demonstrate the same pattern of memory deficit in a 2 × 8 button-press task.
There is an inconsistency between the present results and a previous animal study which reported that the lesions in the striatum were associated with an increased number of errors in sequence-selective learning during a 2 × 5 task (Miyachi et al. 1997). A possible explanation for the inferior performance observed in the previous study is that performance might have been influenced by a deficit in sequence-unselective learning; the monkeys had performed a different type of 2 × 5 task (Hikosaka et al. 1995; Miyashita et al. 1996) before the lesion study (Miyachi et al. 1997).
Cognitive inflexibility in Parkinson’s disease
The present results revealed an inflexibility in PD patients during the learning of new sequences. Their cognitive inflexibility has been reported and explained by cognitive deficits such as switching, probabilistic reversal learning, or set-shifting (Cools et al. 2001; Delazer et al. 2004; Monchi et al. 2004; Shohamy et al. 2006). The present sequence-unselective learning and previously reported cognitive mechanisms share the common point that subjects are required to change and reorganize their movements in accordance with new (or changed) stimuli. However, the previously reported mechanisms would not fully explain the present improvements in the learning of new sequences among healthy participants. It seems that the previous mechanisms enable us to manipulate new stimuli as well as old ones during a switching or set-shifting task. On the other hand, sequence-unselective learning enables us to perform better in new situations than we were able to in previous ones, so it refers to an active mechanism that minimizes trial and error in new situations. The present cognitive mechanism of sequence-selective learning may be similar to that of the learning-set (Harlow 1949; Yokoyama et al. 2005), rather than to that of switching and/or set-shifting. Harlow (1949) reported that subjects showed a progressive improvement in the rate of correct responses through the repeated experiencing of a discrimination task with different pairs of stimuli, and proposed that they acquired a learning-set, which is a strategy beyond a stationary association between a specific stimulus and response. Yokoyama et al. (2005) reported brain activations relevant to the learning-set in the striatum and frontal cortex in an animal study. Our results do not conflict with the results of the latter study. However, there are few studies investigating the neural basis of learning-sets with human subjects. The details of the neural mechanism are unclear. We need to reorganize several cognitive concepts, including sequence-unselective learning, set-switching and the learning-set, and determine these neural mechanisms to better understand flexible behavior.
l-Dopa medication and 2 × 8 button-press task
Several studies have reported different effects of dopaminergic medication in PD patients for previous cognitive mechanisms (Cools et al. 2001; Shohamy et al. 2006). l-Dopa medication ameliorates task performance in the switching paradigm by increasing the DA level in the dorsal caudate nucleus; however, this medication simultaneously causes a DA overdose in the ventral striatum, and induces impairment in other types of tasks involving probabilistic reversal learning, which measures the ability to alter behavior on the basis of the received feedback (Cools et al. 2001). In the present study, l-dopa-medicated patients failed to show normal sequence-unselective learning. The scores in Session 3 seemed to be independent of patients’ medications. Positive effects of l-dopa medication were not clear in the present study. Sequence-unselective learning and switching mechanisms may each contribute to flexible behavior, though in differing ways.
The present study has some limitations because of the small number of medicated or unmedicated PD patients. As a next step, a larger sample study is required to clarify the relationship in PD patients between sequence-unselective learning and medication with DA agonists. Additionally, multiple types of sequences should be used to avoid the effects of degree of difficulty or patient fatigue, in order to make clear the nature of learning inflexibility in greater detail.
References
Badgaiyan RD, Fischman AJ, Alpert NM (2007) Striatal dopamine release in sequential learning. Neuroimage 38:549–556
Bondi MW, Kaszniak AW (1991) Implicit and explicit memory in Alzheimer’s disease and Parkinson’s disease. J Clin Exp Neuropsychol 13:339–358
Contreras-Vidal JL, Schultz W (1999) A predictive reinforcement model of dopamine neurons for learning approach behavior. J Comput Neurosci 6:191–214
Cools R, Barker RA, Sahakian BJ, Robbins TW (2001) Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain 124:2503–2512
Delazer M, Domahs F, Lochy A, Karner E, Benke T, Poewe W (2004) Number processing and basal ganglia dysfunction: a single case study. Neuropsychologia 42:1050–1062
Dominey PF, Jeannerod M (1997) Contribution of frontostriatal function to sequence learning in Parkinson’s disease: evidence for dissociable systems. Neuroreport 8:3–9
Exner C, Koschack J, Irle E (2002) The differential role of premotor frontal cortex and basal ganglia in motor sequence learning: evidence from focal basal ganglia lesions. Learn Mem 9:376–386
Furtado JC, Mazurek MF (1996) Behavioral characterization of quinolinate-induced lesions of the medial striatum: relevance for Huntington’s disease. Exp Neurol 138:158–168
Harlow HF (1949) The formation of learning sets. Psychol Rev 56:51–65
Hikosaka O, Rand MK, Miyachi S, Miyashita K (1995) Learning of sequential movements in the monkey: process of learning and retention of memory. J Neurophysiol 74:1652–1661
Hikosaka O, Sakai K, Nakahara H, Miyachi S, Nakamura K, Rand MK (2000) Neural mechanisms for learning of sequential procedures. In: Gazzaniga MS (ed) The new cognitive neurosciences. MIT Press, Cambridge, pp 553–572
Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H (2001) Procedural motor learning in Parkinson’s disease. Exp Brain Res 141:425–437
Milner B (2005) The medial temporal-lobe amnesic syndrome. Psychiatr Clin N Am 28:599–611
Miyachi S, Hikosaka O, Miyashita K, Kárádi Z, Rand MK (1997) Differential roles of monkey striatum in learning of sequential hand movement. Exp Brain Res 115:1–5
Miyashita K, Rand MK, Miyachi S, Hikosaka O (1996) Anticipatory saccades in sequential procedural learning in monkeys. J Neurophysiol 76:1361–1366
Mochizuki-Kawai H, Kawamura M, Hasegawa Y, Mochizuki S, Oeda R, Yamanaka K, Tagaya H (2004) Deficits in long-term retention of learned motor skills in patients with cortical or subcortical degeneration. Neuropsychologia 42:1858–1863
Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A (2004) Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci 24:702–710
Robertson C, Flowers KA (1990) Motor set in Parkinson’s disease. J Neurol Neurosurg Psychiatry 53:583–592
Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA (2006) l-Dopa impairs learning, but spares generalization, in Parkinson’s disease. Neuropsychologia 44:774–784
Squire (1987) Memory and brain. Oxford University Press, New York
Yokoyama C, Tsukada H, Watanabe Y, Onoe H (2005) A dynamic shift of neural network activity before and after learning-set formation. Cereb Cortex 15:796–801
Acknowledgments
We are grateful to Dr Okihide Hikosaka of the National Institutes of Health for reading our manuscript. We would also like to thank Dr Takashi Tsukiura of National Institute of Advanced Industrial Science and Technology (AIST), and Yoshihiko Tanno of the University of Tokyo, for their helpful comments. This study was supported by a Grant-in-Aid for scientific research to H. Mochizuki-Kawai from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) (No. 19800061, No. 20688001). The study was also partly supported by a Showa University Grant-in-Aid for innovative collaborative research projects; a Special Research Grant-in-Aid for the Development of Characteristic Education from MEXT; and a Grant-in-Aid for Scientific Research on Priority Areas (C) from MEXT (No. 15590910). Mitsuru Kawamura was supported by CREST at the Japan Science and Technology Agency, and a Grant-in-Aid for Scientific Research on Priority Areas—System Study on Higher-order Brain Functions from MEXT (Nos. 17022035, 18020027 and 20020026). This study was also supported in part by a Showa University Grant-in-Aid for Innovative Collaborative Research Projects and a Special Research Grant-in-Aid for Development of Characteristic Education from MEXT.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Mochizuki-Kawai, H., Mochizuki, S. & Kawamura, M. A flexible sequential learning deficit in patients with Parkinson’s disease: a 2 × 8 button-press task. Exp Brain Res 202, 147–153 (2010). https://doi.org/10.1007/s00221-009-2119-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-2119-4