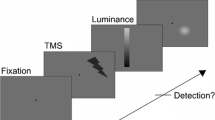
Abstract
Phosphenes represent a perceptual effect of transcranial magnetic stimulation (TMS) or electric stimulation of visual cortical areas. One likely neural basis for the generation of static phosphenes is the primary visual cortex (V1) although evidence is controversial. A peculiar feature of V1 is that it has sparse callosal connections with the exception of a central portion of visual field representation. In contrast, visually responsive cortical areas in the parietal lobe have widespread callosal connections. Thus, interhemispheric transfer (IT) time of off-centre phosphenes should be slower when generated by V1 than by visual parietal areas. To verify this possibility, in Exp. 1 we measured IT of phosphenes generated by TMS applied to V1 and in Exp. 2 we measured IT of phosphenes obtained by TMS applied to posterior parietal cortex. In both experiments, we obtained static bright circular phosphenes appearing in the contralateral hemifield. We measured IT time behaviorally by comparing unimanual simple reaction time to the onset of a phosphene under crossed or uncrossed hemifield-hand condition (Poffenberger paradigm). In keeping with our prediction, we found a substantially longer IT time for V1 than for parietal phosphenes. Additionally, an IT similar to that obtained with V1 stimulation was found when participants were asked to imagine the phosphenes previously experienced during TMS. In conclusion, the present results suggest that IT of phosphenes either generated by V1 TMS or imagined is subserved by slower callosal channels than those of real visual stimuli or parietal phosphenes.




Similar content being viewed by others
References
Aboitiz F, Scheibel AB, Fisher RS, Zaidel E (1992) Fiber composition of the human corpus callosum. Brain Res 598:143–153
Amassian VE, Cracco RQ, Maccabee PJ, Cracco JB, Rudell AP, Eberle L (1998) Transcranial magnetic stimulation in study of the visual pathway. J Clin Neurophysiol 15:288–304
Amassian VE, Maccabee PJ, Cracco RQ, Cracco JB, Somasundaram M, Rothwell JC, Eberle L, Henry K, Rudell AP (1994) The polarity of the induced electric field influences magnetic coil inhibition of human visual cortex: implications for the site of excitation. Electroencephalogr Clin Neurophysiol 93:21–26
Anand S, Hotson J (2002) Transcranial magnetic stimulation: neurophysiological applications and safety. Brain Cogn 50:366–386
Antal A, Nitsche MA, Kincses TZ, Lampe C, Paulus W (2004) No correlation between moving phosphene and motor thresholds: a transcranial magnetic stimulation study. Neuroreport 15:297–302
Anzola GP, Bertoloni G, Buchtel HA, Rizzolatti G (1977) Spatial compatibility and anatomical factors in simple and choice reaction time. Neuropsychologia 15:295–302
Berlucchi G (1972) Anatomical and physiological aspects of visual functions of corpus callosum. Brain Res 37:371–392
Berlucchi G, Crea F, Di Stefano M, Tassinari G (1977) Influence of spatial stimulus-response compatibility on reaction time of ipsilateral and contralateral hand to lateralized light stimuli. J Exp Psychol Hum Percept Perform 3:505–517
Berlucchi G, Heron W, Hyman R, Rizzolatti G, Umiltà C (1971) Simple reaction times of ipsilateral and contralateral hand to lateralized visual stimuli. Brain 94:419–430
Boroojerdi B, Prager A, Muellbacher W, Cohen LG (2000) Reduction of human visual cortex excitability using 1-Hz transcranial magnetic stimulation. Neurology 54:1529–1531
Brown WS, Jeeves MA, Dietrich R, Burnison DS (1999) Bilateral field advantage and evoked potential interhemispheric transmission in commissurotomy and callosal agenesis. Neuropsychologia 37:1165–1180
Chiang TC, Lavidor M (2005) Magnetic stimulation and the crossed-uncrossed difference (CUD) paradigm: selective effects in the ipsilateral and contralateral hemispheres. Exp Brain Res 160:404–418
Chiang TC, Walsh V, Lavidor M (2004) The cortical representation of foveal stimuli: evidence from quadrantanopia and TMS-induced suppression. Brain Res Cogn Brain Res 21:309–316
Clarke S, Miklossy J (1990) Occipital cortex in man: organization of callosal connections, related myelo- and cytoarchitecture, and putative boundaries of functional visual areas. J Comp Neurol 298:188–214
Corballis MC (2002) Hemispheric interactions in simple reaction time. Neuropsychologia 40:423–443
Cowey A, Walsh V (2000) Magnetically induced phosphenes in sighted, blind and blindsighted observers. Neuroreport 11:3269–3273
Cowey A, Walsh V (2001) Tickling the brain: studying visual sensation, perception and cognition by transcranial magnetic stimulation. Prog Brain Res 134:411–425
Deblieck C, Thompson B, Iacoboni M, Wu AD (2008) Correlation between motor and phosphene thresholds: a transcranial magnetic stimulation study. Hum Brain Mapp 29:662–670
Fendrich R, Hutsler JJ, Gazzaniga MS (2004) Visual and tactile interhemispheric transfer compared with the method of Poffenberger. Exp Brain Res 158:67–74
Fernandez E, Alfaro A, Tormos JM, Climent R, Martínez M, Vilanova H, Walsh V, Pascual-Leone A (2002) Mapping of the human visual cortex using image-guided transcranial magnetic stimulation. Brain Res Brain Res Protoc 10:115–124
Ffytche DH, Howseman A, Edwards R, Sandeman DR, Zeki S (2000) Human area V5 and motion in the ipsilateral visual field. Eur J Neurosci 12:3015–3025
Grefkes C, Fink GR (2005) The functional organization of the intraparietal sulcus in humans and monkeys. J Anat 207:3–17
Gross CG, Bender DB, Mishkin M (1977) Contributions of the corpus callosum and the anterior commissure to visual activation of inferior temporal neurons. Brain Res 131:227–239
Grubbs F (1969) Procedures for detecting outlying observations in samples. Technometrics 11:1–21
Herwig U, Satrapi P, Schönfeldt-Lecuona C (2003) Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr 16:95–99
Hoptman MJ, Davidson RJ, Gudmundsson A, Schreiber RT, Ershler WB (1996) Age differences in visual evoked potential estimates of interhemispheric transfer. Neuropsychology 10:263–271
Iacoboni M (2006) Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia 44:2691–2699
Iacoboni M, Zaidel E (2004) Interhemispheric visuo-motor integration in humans: the role of the superior parietal cortex. Neuropsychologia 42:419–425
Ilmoniemi RJ, Virtanen J, Ruohonen J, Karhu J, Aronen HJ, Näätänen R, Katila T (1997) Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. NeuroReport 8:3537–3540
Ipata A, Girelli M, Miniussi C, Marzi CA (1997) Interhemispheric transfer of visual information in humans: the role of different callosal channels. Arch Ital Biol 135:169–182
Kammer T (1999) Phosphenes and transient scotomas induced by magnetic stimulation of the occipital lobe: their topographic relationship. Neuropsychologia 37:191–198
Kammer T (2007) Masking visual stimuli by transcranial magnetic stimulation. Psychol Res 71:659–666
Kammer T, Beck S, Erb M, Grodd W (2001) The influence of current direction on phosphene thresholds evoked by transcranial magnetic stimulation. Clin Neurophysiol 112:2015–2021
Kammer T, Puls K, Erb M, Grodd W (2005) Transcranial magnetic stimulation in the visual system. II. Characterization of induced phosphenes and scotomas. Exp Brain Res 160:129–140
Kastner S, Paul I, Ziemann U (1998) Transient visual field defects induced by transcranial magnetic stimulation over the occipital lobe. Exp Brain Res 118:19–26
Kosslyn SM, Thompson WL (2003) When is early visual cortex activated during visual mental imagery? Psychol Bull 129:723–746
Kosslyn SM, Ganis G, Thompson WL (2001) Neural foundations of imagery. Nat Rev Neurosci 2:635–642
Lavidor M, Walsh V (2004) The nature of foveal representation. Nat Rev Neurosci 5:729–735
Libet B (2006) Reflections on the interaction of the mind and brain. Prog Neurobiol 78:322–326
Lines CR, Rugg MD, Milner AD (1984) The effect of stimulus intensity on visual evoked potential estimates of interhemispheric transmission time. Exp Brain Res 57:89–98
Marg E, Rudiak D (1994) Phosphenes induced by magnetic stimulation over the occipital brain: description and probable site of stimulation. Optom Vis Sci 71:301–311
Marzi CA (1999) The Poffenberger paradigm: a first, simple, behavioural tool to study interhemispheric transmission in humans. Brain Res Bull 50:421–422
Marzi CA, Antonini A, Di Stefano M, Legg CR (1982) The contribution of the corpus callosum to receptive fields in the lateral suprasylvian visual areas of the cat. Behav Brain Res 4:155–176
Marzi CA, Bisiacchi P, Nicoletti R (1991) Is interhemispheric transfer of visuomotor information asymmetric? Evidence from a meta-analysis. Neuropsychologia 29:1163–1177
Marzi CA, Mancini F, Metitieri T, Savazzi S (2006) Retinal eccentricity effects on reaction time to imagined stimuli. Neuropsychologia 44:1489–1495
Marzi CA, Miniussi C, Maravita A, Bertolasi L, Zanette G, Rothwell JC, Sanes JN (1998) Transcranial magnetic stimulation selectively impairs interhemispheric transfer of visuo-motor information in humans. Exp Brain Res 118:435–438
Meyer BU, Diehl R, Steinmetz H, Britton TC, Benecke R (1991) Magnetic stimuli applied over motor and visual cortex: influence of coil position and field polarity on motor responses, phosphenes and eye movements. EEG Clin Neurophysiol Suppl 43:121–134
Milner AD, Lines CR (1982) Interhemispheric pathways in simple reaction time to lateralized light flash. Neuropsychologia 20:171–179
Omura K, Tsukamoto T, Kotani Y, Ohgami Y, Minami M, Inoue Y (2004) Different mechanisms involved in interhemispheric transfer of visuomotor information. Neuroreport 15:2707–2711
Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, Wardak C, Durand JB, Vanduffel W (2006) Mapping the parietal cortex of human and non-human primates. Neuropsychologia 44:2647–2667
Pandya DN, Seltzer B (1986) The topography of commissural fibers. In: Lepore F, Ptito M, Jaspers HH (eds) Two hemispheres—one brain: functions of the corpus callosum. Alan R. Liss, New York, pp 47–73
Pascual-Leone A, Walsh V (2001) Fast backprojections from the motion to the primary visual area necessary for visual awareness. Science 292:510–512
Pascual-Leone A, Walsh V, Rothwell J (2000) Transcranial magnetic stimulation in cognitive neuroscience-virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol 10:232–237
Poffenberger AT (1912) Reaction time to retinal stimulation with special reference to the time lost in conduction through nervous centers. Arch Psychol 23:1–73
Pollen DA (2004) Brain stimulation and conscious experience. Conscious Cogn 13:626–645
Pollen DA (2006) Brain stimulation and conscious experience: electrical stimulation of the cortical surface at a threshold current evokes sustained neuronal activity only after a prolonged latency. Conscious Cogn 15:560–565
Ray PG, Meador KJ, Epstein CM, Loring DW, Day LJ (1998) Magnetic stimulation of visual cortex: factors influencing the perception of phosphenes. J Clin Neurophysiol 15:351–357
Rugg MD, Lines CR, Milner AD (1984) Visual evoked potentials to lateralized visual stimuli and the measurement of interhemispheric transmission time. Neuropsychologia 22:215–225
Saron CD, Foxe JJ, Simpson GV, Vaughan HG (2003) Interhemispheric visuomotor activation: spatiotemporal electrophysyiology related to reaction time. In: Zaidel E, Iacoboni M (eds) The parallel brain: the cognitive neuroscience of the corpus callosum. MIT Press, Cambridge, MA, pp 171–219
Savazzi S, Mancini F, Marzi CA (2008) Interhemispheric transfer and integration of imagined visual stimuli. Neuropsychologia 46:803–880
Savazzi S, Fabri M, Rubboli G, Paggi A, Tassinari CA, Marzi CA (2007) Interhemispheric transfer following callosotomy in humans: role of the superior colliculus. Neuropsychologia 45:2417–2427
Schluppeck D, Glimcher P, Heeger DJ (2005) Topographic organization for delayed saccades in human posterior parietal cortex. J Neurophysiol 94:1372–1384
Schönfeldt-Lecuona C, Thielscher A, Freudenmann RW, Kron M, Spitzer M, Herwig U (2005) Accuracy of stereotaxic positioning of transcranial magnetic stimulation. Brain Topogr 17:253–259
Sereno MI, Pitzalis S, Martinez A (2001) Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science 294:1350–1354
Silvanto J, Cowey A, Lavie N, Walsh V (2005) Striate cortex (V1) activity gates awareness of motion. Nat Neurosci 8:143–144
Silvanto J, Cowey A, Lavie N, Walsh V (2007) Making the blindsighted see. Neuropsychologia 45:3346–3350
Silver MA, Ress D, Heeger DJ (2005) Topographic maps of visual spatial attention in human parietal cortex. J Neurophysiol 94:1358–1371
Sparing R, Buelte D, Meister IG, Paus T, Fink GR (2008) Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp 29:82–96
Sparing R, Dambeck N, Stock K, Meister IG, Huetter D, Boroojerdi B (2005) Investigation of the primary visual cortex using short-interval paired-pulse transcranial magnetic stimulation (TMS). Neurosci Lett 382:312–316
Sparing R, Mottaghy FM, Ganis G, Thompson WL, Topper R, Kosslyn SM, Pascual-Leone A (2002) Visual cortex excitability increases during visual mental imagery—a TMS study in healthy human subjects. Brain Res 938:92–97
Stoerig P (2001) The neuroanatomy of phenomenal vision: a psychological perspective. Ann N Y Acad Sci 929:176–194
Swisher JD, Halko MA, Merabet LB, McMains SA, Somers DC (2007) Visual topography of human intraparietal sulcus. J Neurosci 27:5326–5337
Tettamanti M, Paulesu E, Scifo P, Maravita A, Fazio F, Perani D, Marzi CA (2002) Interhemispheric transmission of visuomotor information in humans: fMRI evidence. J Neurophysiol 88:1051–1058
Tlauka M, McKenna FP (1998) Mental imagery yields stimulus–response compatibility. Acta Psychologica 98:67–79
Wassermann EM (1998) Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section 108:1–16
Weber B, Treyer V, Oberholzer N, Jaermann T, Boesiger P, Brugger P, Regard M, Buck A, Savazzi S, Marzi CA (2005) Attention and interhemispheric transfer: a behavioral and fMRI study. J Cogn Neurosci 17:113–123
Zaidel E, Iacoboni M (2003) Introduction: Poffenberger’s simple reaction time paradigm for measuring interhemispheric transfer time. In: Zaidel E, Iacoboni M (eds) The parallel brain: the cognitive neuroscience of the corpus callosum. MIT Press, Cambridge, MA, pp 1–7
Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM (2006) Functional anatomy of interhemispheric cortical connections in the human brain. J Anat 209:311–320
Acknowledgments
The study has been financed by the Italian MIUR. We thank Paola Cesari for the use of the TMS facility at the Faculty of Motor Science of the University of Verona.
Author information
Authors and Affiliations
Corresponding author
Addendum
Addendum
This article is dedicated to Giovanni Berlucchi who has taught the senior author how to do science honestly, creatively and enthusiastically. Giovanni, and his colleagues and friends Carlo Umiltà and Giacomo Rizzolatti have the historical merit of having revitalized the Poffenberger paradigm with their seminal 1971 paper, which is an example of great behavioral science, neat and clever. Since then the Poffenberger paradigm has been employed many times in both normal and brain damaged people always providing important information for understanding how the cerebral hemispheres interact. In fact, the present study has been carried out using stimuli that do not really exist in the external world but are in the mind of the beholder. We trust Giovanni will appreciate this bizarre use of the Poffenberger paradigm.
Rights and permissions
About this article
Cite this article
Marzi, C.A., Mancini, F. & Savazzi, S. Interhemispheric transfer of phosphenes generated by occipital versus parietal transcranial magnetic stimulation. Exp Brain Res 192, 431–441 (2009). https://doi.org/10.1007/s00221-008-1496-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1496-4