Abstract
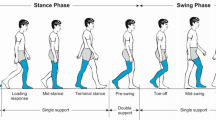
The bilateral coordination of locomotion has been described in detail in animal studies and to some degree in man; however, the mechanisms that contribute to the bilateral coordination of gait in humans are not fully understood. The objective of the present study was to develop a measure for quantifying the bilateral coordination of gait and to evaluate the effects of aging and Parkinson’s disease (PD) on this new metric. To this end, we compared the gait of healthy older adults to that of healthy young adults and patients with PD. Specifically, we defined the stride duration of one foot as a gait cycle or 360°, determined the relative timing of contra-lateral heel-strikes, and defined this as the phase, ϕ (ideally, ϕ = 180° for every step). The sum of the coefficient of variation of ϕ and the mean absolute difference between ϕ and 180° was defined as the phase coordination index (PCI), representing variability and inaccuracy, respectively, in phase generation. PCI values were higher (poorer bilateral coordination) in patients with PD in comparison to the healthy older adults (P < 0.006). Although gait speed and stride time variability were similar in the healthy young and older adults, PCI values were significantly higher among the healthy elderly subjects compared to the young adults (P < 0.001). Regression analysis suggests that only about 40% of the variance in the values of PCI can be explained by the combination of gait asymmetry (as defined by the differences in each leg’s swing times), gait speed and stride time variability, pointing to the independent nature of this new metric. This study demonstrates that bilateral coordination of gait deteriorates with aging, further deteriorates in PD, and is not strongly associated with other spatio-temporal features of gait.



Similar content being viewed by others
References
Abe K, Asai Y, Matsuo Y, Nomura T, Sato S, Inoue S, Mizukura I, Sakoda S (2003) Classifying lower limb dynamics in Parkinson’s disease. Brain Res Bull 61:219–226
Ashtom-Miller JA (2005) In: Hausdorff JM, Alexander NB (eds) Gait disorders evaluation and management. Tayor & Francis Group, Boca Raton, pp 63–100
Baltadjieva R, Giladi N, Gruendlinger L, Peretz C, Hausdorff JM (2006) Marked alterations in the gait timing and rhythmicity of patients with de novo Parkinson’s disease. Eur J Neurosci 24:1815–1820
Berger W, Dietz V, Quintern J (1984) Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. J Physiol 357:109–125
Bohannon RW (1997) Comfortable and maximum walking speed of adults aged 20–79 years: reference values and determinants. Age Ageing 26:15–19
Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP (2004) Cerebellar and premotor function in bimanual coordination: parametric neural responses to spatiotemporal complexity and cycling frequency. Neuroimage 21:1416–1427
Dietz V (2002) Do human bipeds use quadrupedal coordination?. Trends Neurosci 25:462–467
Dietz V (2003) Spinal cord pattern generators for locomotion. Clin Neurophysiol 114:1379–1389
Dietz V, Zijlstra W, Prokop T, Berger W (1995) Leg muscle activation during gait in Parkinson’s disease: adaptation and interlimb coordination. Electroencephalogr Clin Neurophysiol 97:408–415
Fahn S, Elton R, Members of the UPDRS Developmental Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein MS (eds) Recent developments in Parkinson’s disease. Florham Park, pp 153–163
Folstein MF, Folstein SE, McHugh PR (1975) “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson disease. Arch Neurol 56:33–39
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L (2004) Movement disorder society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19:1020–1028
Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL (1997) Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol 82:262–269
Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL (1998) Gait variability and basal ganglia disorders: stride-to-stride variations of gait cycle timing in Parkinson’s disease and Huntington’s disease. Mov Disord 13:428–437
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression, and mortality. Neurology 57:S11–S26
Hughes AJ, Ben Shlomo Y, Daniel SE, Lees AJ (2001) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. 1992. Neurology 57:S34–S38
Kjaerulff O, Kiehn O (1996) Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16:5777–5794
Lee TD, Blandin Y, Proteau L (1996) Effects of task instructions and oscillation frequency on bimanual coordination. Psychol Res 59:100–106
Morley JE (2003) Mobility performance: a high-tech test for geriatricians. J Gerontol A Biol Sci Med Sci 58:712–714
Morris ME, Iansek R, Matyas TA, Summers JJ (1996) Stride length regulation in Parkinson’s disease. Normalization strategies and underlying mechanisms. Brain 119:551–568
Obhi SS, Haggard P, Taylor J, Pascual-Leone A (2002) rTMS to the supplementary motor area disrupts bimanual coordination. Motor Control 6:319–332
Pang MY, Yang JF (2001) Interlimb co-ordination in human infant stepping. J Physiol 533:617–625
Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM (2005) Is freezing of gait in Parkinson’s disease related to asymmetric motor function?. Ann Neurol 57:656–663
Prokop T, Berger W, Zijlstra W, Dietz V (1995) Adaptational and learning processes during human split-belt locomotion: interaction between central mechanisms and afferent input. Exp Brain Res 106:449–456
Sadeghi H, Allard P, Prince F, Labelle H (2000) Symmetry and limb dominance in able-bodied gait: a review. Gait Posture 12:34–45
Sofuwa O, Niuwboer A, Desloovere K, Willems AM, Chavert F, Jonkers I (2005) Quantitative gait analysis in Parkinson’s disease: comparison with healthy control group. Arch Phys Med Rehabil 86:1007–1013
Ullen F, Forssberg H, Ehrsson HH (2003) Neural networks for the coordination of the hands in time. J Neurophysiol 89:1126–1135
Winter DA, Patla AE, Frank JS, Walt SE (1990) Biomechanical walking pattern changes in the fit and healthy elderly. Phys Ther 70:340–347
Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM (2005) Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding?. Eur J Neurosci 22:1248–1256
Yogev G, Plotnik M, Peretz C, Giladi N, Hausdorff JM (2006) Gait asymmetry in patients with Parkinson’s disease and elderly fallers: when does the bilateral coordination of gait require attention? Exp Brain Res (e-pub a head of print)
Zehr EP, Duysens J (2004) Regulation of arm and leg movement during human locomotion. Neuroscientist 10:347–361
Zmitrewicz RJ, Neptune RR, Walden JG, Rogers WE, Bosker GW (2006) The effect of foot and ankle prosthetic components on braking and propulsive impulses during transtibial amputee gait. Arch Phys Med Rehabil 87:1334–1339
Acknowledgments
We thank the subjects for their participation, time and effort. We thank Ms. Galit Yogev, Ms. Michal Leshem and Mr. Ronny Bartsch for their invaluable assistance and Dr. Eli Plotnik for mathematical advice. This work was supported in part by the Inheritance Fund of the Israeli Ministry of Health, NIH grants AG-14100, RR-13622, HD-39838 and AG-08812, by the US-Israel Bi-National Science Foundation, by the Parkinson’s Disease Foundation (PDF), New York and the National Parkinson Foundation (NPF), Miami USA, and by the European Union Sixth Framework Program, FET contract no. 018474-2, Dynamic Analysis of Physiological Networks (DAPHNet).
Author information
Authors and Affiliations
Corresponding author
Appendix: Analytical and numerical analyses of the relationship between the parameters ϕ_CV and ϕ_ABS
Appendix: Analytical and numerical analyses of the relationship between the parameters ϕ_CV and ϕ_ABS
In this appendix, we present analytical and numerical analyses that describe the relationship between the two components of the PCI defined to incorporate the estimates by which a subject is constantly and accurately generating left–right stepping phases while walking.
Analytical analysis
Denote by ϕ = ϕ1,ϕ2, …, ϕN, a set of N measurements of the phase ϕ.
Let \( \ifmmode\expandafter\bar\else\expandafter\=\fi{\varphi } \) be the mean value of ϕ, i.e.
and δ the standard deviation of ϕ given by
The coefficient of variation of ϕ is defined by
Define:
From (4) we obtain:
and (5) yields:
But the left term in the square parentacese of Eq. 7 can be written as:
where [Δϕ] is defined as follows: \( [\Delta \varphi ] = \overline{\varphi } - 180^{^\circ } \)
Note that the middle term in Eq. 8 can be written as:
Returning to Eq. 7 and using the relation described in Eq. 6, ABS2 (ϕ) can be now written as:
or alternatively:
Equation 11 suggests that quadratic–quadratic relation describes in part the analytic dependency between ϕ_CV and ϕ_ABS. At the same time this dependency may vary substantially, given a series of ϕ i as expressed by the middle and the left terms of the right part of the equation. In the following paragraphs, a numerical analysis describes how the relation between ϕ_CV and ϕ_ABS is altered with accordance to different series of ϕ i .
Numerical analysis
To explore a priori relations between ϕ_CV and ϕ_ABS, three separate artificial data sets were established. For each data set 31 left–right stepping phase series of 267 strides each were established. Each of the 31 series represents data obtained from a different walking human subject. Across all series in each data set, the mean values of ϕ ranged from 165° to 195° by increments of 1° (total of 31 mean values). The distribution of ϕ values around the mean value was identical for all 31 series, i.e. the standard deviation of the mean (SD) was identical for all 31 series. SD values were assigned to be 1.1, 4.4, 17.6 in the first (A), second (B) and thirds (C), data sets, respectively. Thus for each data set, 31 pairs of ϕ_CV and ϕ_ABS could be generated. Figure 4, depicts the relationships between ϕ_ABS and ϕ_CV for each of the data sets. On the abscissa the increase in the values of ϕ_CV corresponds to the decrease in the mean value of ϕ from 195° to 165° (a trivial outcome from the fact that SD was maintained constant). It can be seen that the corresponding ϕ_ABS values decrease as the mean value of ϕ approaches 180° and increase again as the mean value of ϕ continues to decrease towards 165°. It may be concluded that in a given value of SD there would be a monotonous change in the values of ϕ_ABS as a function of ϕ_CV, in half of the range of ϕ_CV, and opposite monotonous change in the second range of ϕ_CV. As can be seen by the different range of variation which is covered by ordinate axes in all three panels, we may conclude that actual experimental data collected may in part be more influenced by the direct dependency between ϕ_CV and ϕ_ABS, while for other terms this direct dependency is less obvious. Therefore, a priori for critical statistical tests based on this analysis it was determined to treat ϕ_CV and ϕ_ABS as inter-correlated parameters.
Numerical analyses depicting the relation between ϕ_ABS and ϕ_CV. The trace in each panel shows calculated values of ϕ_ABS and ϕ_CV when the standard deviation of the mean value of ϕ is kept constant while the mean value of ϕ varies between 165° and 195° (left most and right most pairs of values, respectively, in all panels)
Rights and permissions
About this article
Cite this article
Plotnik, M., Giladi, N. & Hausdorff, J.M. A new measure for quantifying the bilateral coordination of human gait: effects of aging and Parkinson’s disease. Exp Brain Res 181, 561–570 (2007). https://doi.org/10.1007/s00221-007-0955-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0955-7