Abstract
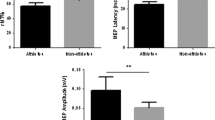
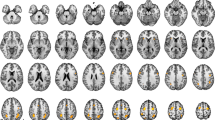
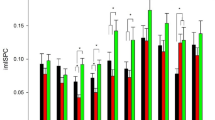
In this study, we measured primary motor cortex (MI) activity during a reaction time task to examine the appearance of MI activity that synchronized with the stimulus presentation (stimulus synchronous MI activity, SSMA). Because brain activity was expected to be enhanced by the repetitive/extensive activation, we hypothesized that the SSMA would be more clearly observable in athletes who were trained to perform reactive movements than in non-athletes. MI activity was measured in ten athletes and ten non-athletes by magnetoencephalography. The tasks were a simple reaction task and a Go/Nogo reaction task in which the subjects were asked to abduct their right index fingers in response to a visual stimulus. The Go/Nogo reaction time task was adopted to confirm the presence of the SSMA, because the MI activity in response to a Nogo stimulus did not overlap with the MI activity that was synchronous with the execution of the movement. The results show that the SSMA was clearly apparent in the athlete group (9/10). In the non-athlete group, however, only three subjects showed the SSMA (3/10). Moreover, the MI activity of the athletes tended to be larger than that of the non-athletes, even though the athletes did not specifically practice these index finger movements during their daily training. We concluded that long-term physical training promotes MI activity and the effects of reactive task repetition were more clearly apparent in the MI activity of the athletes.


Similar content being viewed by others
References
Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT (1990) Learning causes synaptogenesis, while motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA 87:5568–5572
Classen J, Liepert J, Wise SP, Hallett M, Cohen LG (1998) Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol 79:1117–1123
Coles MG (1989) Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology 26:251–269
De Jong R, Liang CC, Lauber E (1994) Conditional and unconditional automaticity: a dual-process model of effects of spatial stimulus-response correspondence. J Exp Psychol Hum Percept Perform 20:731–750
Eimer M (1995) Stimulus-response compatibility and automatic response activation: evidence from psychophysiological studies. J Exp Psychol Hum Percept Perform 21:837–854
Endo H, Kizuka T, Masuda T, Takeda T (1999) Automatic activation in the human primary motor cortex synchronized with movement preparation. Cogn Brain Res 8:229–239
Erdler M, Beisteiner R, Mayer D, Kaindl T, Edward V, Windischberger C, Lindinger G, Deecke L (2000) Supplementary motor area activation preceding voluntary movement is detectable with a whole-scalp magnetoencephalography system. Neuroimage 11:697–707
Geyer S, Matelli M, Luppino G, Zilles K (2000) Functional neuroanatomy of the primate isocortical motor system. Anat Embryol 202:443–474
Hallett M (2001) Plasticity of the human motor cortex and recovery from stroke. Brain Res Rev 36:169–174
Hämäläinen MS (1995) Functional localization based on measurements with a whole-head magnetometer system. Brain Topogr 7:283–289
Hayashi S, Hasegawa Y, Kasai T (2002) Transcranial magnetic stimulation study of plastic changes of human motor cortex after repetitive simple muscle contractions. Percept Mot Skills 95:699–705
Hoshiyama M, Kakigi R, Berg P, Koyama S, Kitamura Y, Shimojo M, Watanabe S, Nakamura A (1997) Identification of motor and sensory brain activities during unilateral finger movement: spatiotemporal source analysis of movement-associated magnetic fields. Exp Brain Res 115:6–14
Hung T, Spalding TW, Santa Maria DL, Hatfield BD (2004) Assessment of reactive motor performance with event-related brain potentials: attention processes in elite table tennis players. J Sport Exerc Psychol 26:317–337
Iwadate M, Mori A, Ashizuka T, Takayose M, Ozawa T (2005) Long-term physical exercise and somatosensory event-related potentials. Exp Brain Res 160:528–532
Kansaku K, Hanakawa T, Wu T, Hallett M (2004) A shared neural network for simple reaction time. Neuroimage 22:904–911
Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995) Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377:155–158
Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG (1998) The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA 95:861–868
Kato Y, Endo H, Kizuka T, Asami T (2006) Automatic and imperative motor activations in stimulus-response compatibility: magnetoencephalographic analysis of upper and lower limbs. Exp Brain Res 168:51–61
Kita Y, Mori A, Nara M (2001) Two types of movement-related cortical potentials preceding wrist extension in humans. Neuroreport 12:2221–2225
Kornblum S, Hasbroucq T, Osman A (1990) Dimensional overlap: cognitive basis for stimulus-response compatibility—a model and taxonomy. Psychol Rev 97:253–270
Kristeva R, Cheyne D, Deecke L (1991) Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalogr Clin Neurophysiol 81:284–298
Kurata K (1994) Information processing for motor control in primate premotor cortex. Behav Brain Res 61:135–142
Kwan HC, MacKay WA, Murphy JT, Wong YC (1985) Properties of visual cue responses in primate precentral cortex. Brain Res 343:24–35
Lamarre Y, Busby L, Spidalieri G (1983) Fast ballistic arm movements triggered by visual, auditory, and somesthetic stimuli in the monkey. I. Activity of precentral cortical neurons. J Neurophysiol 50:1343–1358
Lardon MT, Polich J (1996) EEG changes from long-term physical exercise. Biol Psychol 44:19–30
Leocani L, Cohen LG, Wassermann EM, Ikoma K, Hallett M (2000) Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain 123:1161–1173
McCloskey DP, Adamo DS, Anderson BJ (2001) Exercise increases metabolic capacity in the motor cortex and striatum, but in the hippocampus. Brain Res 891:168–175
Nagamine T, Toro C, Balish M, Deuschl G, Wang B, Sato S, Shibasaki H, Hallett M (1994) Cortical magnetic and electric fields associated with voluntary finger movements. Brain Topogr 6:175–183
Nagamine T, Kajola M, Salmelin R, Shibasaki H, Hari R (1996) Movement-related slow cortical magnetic fields and changes of spontaneous MEG- and EEG-brain rhythms. Electroencephalogr Clin Neurophysiol 99:274–286
Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M (1995) Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol 74:1037–1045
Polich J, Lardon MT (1997) P300 and long-term physical exercise. Electroencephalogr Clin Neurophysiol 103:493–498
Praamstra P, Schmitz F, Freund H-J, Schnitzler A (1999) Magneto-encephalographic correlates of the lateralized readiness potential. Cogn Brain Res 8:77–85
Riehle A (1991) Visually induced signal-locked neuronal activity changes in precentral motor areas of the monkey: hierarchical progression of signal processing. Brain Res 540:131–137
Riehle A, Kornblum S, Requin J (1997) Neuronal correlates of sensorimotor association in stimulus-response compatibility. J Exp Psychol Hum Percept Perform 23:1708–1726
Sanes JN, Donoghue JP (2000) Plasticity and primary motor cortex. Annu Rev Neurosci 23:393–415
Schlaug G, Knorr U, Seitz R (1994) Inter-subject variability of cerebral activations in acquiring a motor skill: a study with positron emission tomography. Exp Brain Res 98:523–534
Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT (2003) Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience 117:1037–1046
Tesche CD, Uusitalo MA, Ilmoniemi RJ, Huotilainen M, Kajola M, Salonen O (1995) Signal-space projections of MEG data characterize both distributed and well-localized neuronal sources. Electroencephalogr Clin Neurophysiol 95:189–200
Uusitalo MA, Ilmoniemi RJ (1997) Signal-space projection method for separating MEG or EEG into components. Med Biol Eng Comput 35:135–140
Valle-Inclán F (1996) The locus of interference in the Simon effect: an ERP study. Biol Psychol 43:147–162
Valle-Inclán F, Redondo M (1998) On the automaticity of ipsilateral response activation in the Simon effect. Psychophysiology 35:366–371
Wascher E, Schatz U, Kuder T, Verleger R (2001) Validity and boundary conditions of automatic response activation in the Simon task. J Exp Psychol Hum Percept Perform 27:731–751
Yoshino K, Mikami A, Kubota K (1998) Neuronal activities in the ventral premotor cortex during a visually guided jaw movement in monkeys. Neurosci Res 30:321–332
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The SSP method explains the measured magnetic fields by the linear combination of specific magnetic field patterns (the time-independent spatial distribution). The time course of activity amplitude of N sources was indicated by the time-varying amplitude \( {\mathbf{a}}(t) = \{ a_{1} (t),a_{2} (t), \ldots ,a_{N} (t)\} ^{{\text{T}}} \) corresponding to spatial pattern S = (s 1, s 2,...,s N ), where the vectors s i are unit vectors that characterize time-independent spatial distributions:
where b(t) is the magnetic field and n(t) is the noise. The estimate for the amplitude a(t) is
where S + is the pseudoinverse of S.
The procedure to estimate the time course of the activity is described below:
-
1.
The time-independent spatial distribution of the motor activity in MI was calculated from the SVM task. First, the dipole position of the motor activity was estimated in the SVM task using multi-dipole estimation software that was attached to the MEG system. Then, the spatial distribution of the magnetic field was calculated at this position. One motor dipole was assumed to be in each of the two hemispheres, and two orthogonal components were defined for each dipole so as not to fix the orientation of the motor dipole. Finally, four spatial patterns were defined for the motor activity: \( {\mathbf{S}}_{m} = ({\mathbf{s}}_{{m1}} ,{\mathbf{s}}_{{m2}} ,{\mathbf{s}}_{{m3}} ,{\mathbf{s}}_{{m4}} ) \).
-
2.
The visual activity was used not to precisely explain the brain activity in the visual cortex, but instead to achieve the explanation of the visually evoked magnetic fields. The spatial distribution of the visual activity was obtained from the VEF experiment. Since it was difficult to estimate a reliable visual dipole position from all of the subjects, the magnetic field pattern at one or two peak latencies up until 200 ms after the stimulus onset was used as the spatial pattern: S v = {s v1, (s v2)}. The value of the peak latency was determined from the measured waveforms of each subject.
-
3.
Then, two pairs of two motor activities and one or two visual activities were defined by S = (S m , S v ), and the time-varying amplitude vectors a(t) were calculated simultaneously using Eq. 2. The goodness of fit (GOF) and the root mean square (RMS) of the residual error were determined: cumulative GOF between 100 and 200 ms from the stimulus onset should be over 70%, or the error RMS between 100 and 200 ms should be less than twice the RMS of the measured data before the stimulus onset. Because the GOF is worse if the activity is weak, the RMS residual error was also determined. The equations for the GOF and the RMS are:
$$ {\text{cumulative}}\,{\text{GOF}} = {\left( {1 - \frac{{{\sum\nolimits_{t = ts}^{te} {{\sum\nolimits_{i = 1}^{64} {(m_{i} (t) - e_{i} (t))^{2} } }} }}} {{{\sum\nolimits_{t = ts}^{te} {{\sum\nolimits_{i = 1}^{64} {m_{i} (t)^{2} } }} }}}} \right)} \times 100\% , $$(3)$$ {\text{residual}}\,{\text{error}}\,{\text{RMS}}(t) = {\sqrt {\frac{{{\sum\nolimits_{i = 1}^{64} {(m_{i} (t) - e_{i} (t))^{2} } }}} {{64}}} }, $$(4)where m i (t) defines the magnetic field measured by the ith MEG sensor with a latency t in the time window between ts (100 ms) and te (200 ms), while e i (t) denotes the estimated magnetic field, which is calculated as follows:
$$ {\mathbf{e}}(t) = {\mathbf{Sa}}(t). $$(5) -
4.
Because the estimated amplitude a(t) is that for the unit vector of the spatial distribution, the dipole moment of the motor activity at its location was recalculated from the amplitude of the two orthogonal components. Hence, the dipole moment of the motor activity was always a positive value.
Rights and permissions
About this article
Cite this article
Endo, H., Kato, Y., Kizuka, T. et al. A comparison of stimulus synchronous activity in the primary motor cortices of athletes and non-athletes. Exp Brain Res 174, 426–434 (2006). https://doi.org/10.1007/s00221-006-0477-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0477-8