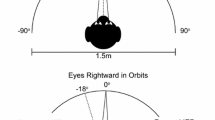
Abstract
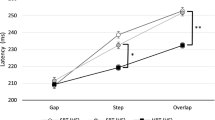
The ability to dissociate eye movements from head movements is essential to animals with foveas and fovea-like retinal specializations, as these species shift the eyes constantly, and moving the head with each gaze shift would be impractical and energetically wasteful. The processes by which the dissociation is effected remain unclear. We hypothesized that the dissociation is accomplished by means of a neural gate, which prevents a common gaze-shift command from reaching the neck circuitry when eye-only saccades are desired. We further hypothesized that such a gate would require a finite period to reset following opening to allow a combined eye–head saccade, and thus the probability of generating a head movement during a saccade would be augmented when a new visual target (the ‘test’ target) appeared during, or soon after, a combined eye–head saccade made to an earlier, ‘conditioning’ target. We tested human subjects using three different combinations of targets—a horizontal conditioning target followed by a horizontal test target (H/H condition), horizontal conditioning followed by vertical test (H/V), and vertical conditioning followed by horizontal test (V/H). We varied the delay between the onset of the conditioning head movement and the presentation of the test target, and determined the probability of generating a head movement to the test target as a function of target delay. As predicted, head movement probability was elevated significantly at the shortest target delays and declined thereafter. The half-life of the increase in probability averaged 740, 490, and 320 ms for the H/H, H/V, and V/H conditions, respectively. For the H/H condition, the augmentation appeared to outlast the duration of the conditioning head movement. Because the augmentation could outlast the conditioning head movement and did not depend on the head movements to the conditioning and test targets lying in the same directions, we could largely exclude the possibility that the augmentation arises from mechanical effects. These results support the existence of the hypothetical eye–head gate, and suggest ways that its constituent neurons might be identified using neurophysiological methods.







Similar content being viewed by others
References
van Alphen AM, Stahl JS, Koekkoek SKE, De Zeeuw CI (2001) The dynamic characteristics of the mouse vestibulo-ocular and optokinetic response. Brain Res 890:296–305
Alstermark B, Pinter MJ, Sasaki S (1992) Tectal and tegmental excitation in dorsal neck motoneurones of the cat. J Physiol (Lond) 454:517–532
Andre-Deshays C, Revel M, Berthoz A (1991) Eye–head coupling in humans. II. Phasic components. Exp Brain Res 84:359–366
Collewijn H (1977) Eye- and head movements in freely moving rabbits. J Physiol (Lond) 266:471–498
Corneil BD, Munoz DP (1999) Human eye–head gaze shifts in a distractor task. II. Reduced threshold for initiation of early head movements. J Neurophysiol 82:1406–1421
Corneil BD, Olivier E, Munoz DP (2002) Neck muscle responses to stimulation of monkey superior colliculus. I. Topography and manipulation of stimulation parameters. J Neurophysiol 88:1980–2002
Cowie RJ, Robinson DL (1994) Subcortical contributions to head movements in macaques. I. Contrasting effects of electrical stimulation of a medial pontomedullary region and the superior colliculus. J Neurophysiol 72:2648–2664
Evinger C, Kaneko CR, Fuchs AF (1982) Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J Neurophysiol 47:827–844
Freedman EG (2001) Interactions between eye and head control signals can account for movement kinematics. Biol Cybern 84:453–462
Freedman EG, Sparks DL (1997) Activity of cells in the deeper layers of the superior colliculus of the rhesus monkey: Evidence for a gaze displacement command. J Neurophysiol 78:1669–1690
Freedman EG, Stanford TR, Sparks DL (1996) Combined eye–head gaze shifts produced by electrical stimulation of the superior colliculus in rhesus monkeys. J Neurophysiol 76:927–952
Fuller JH (1980) Linkage of eye and head movements in the alert rabbit. Brain Res 194:219–222
Fuller JH (1981) Eye and head movements during vestibular stimulation in the alert rabbit. Brain Res 205:363–381
Fuller JH (1992) Comparison of head movement strategies among mammals. In: Berthoz A, Graf W, Vidal PP (eds) The Head-Neck Sensory Motor System. Oxford University Press, New York, pp 101–112
Gandhi NJ, Sparks DL (2000) Microstimulation of the pontine reticular formation in monkey: Effects on coordinated eye–head movements. Soc Neurosci Abstr 26:109–108
Gilchrist ID, Brown V, Findlay JM (1997) Saccades without eye movements. Nature 390:130–131
Gresty MA (1975) Eye, head and body movements of the guinea pig in response to optokinetic stimulation and sinusoidal oscillation in yaw. Pflugers Arch 353:201–214
Guitton D, Munoz DP, Galiana HL (1990) Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. J Neurophysiol 64:509–531
Hughes A (1977) The topography of vision in mammals of contrasting life style: Comparative optics and retinal organisation. In: Crescitelli F (ed) The Visual System in Vertebrates. Springer-Verlag, New York, pp 615–756
Isa T, Naito K (1995) Activity of neurons in the medial pontomedullary reticular formation during orienting movements in alert head-free cats. J Neurophysiol 74:73–95
Iwamoto Y, Sasaki S, Suzuki I (1990) Input-output organization of reticulospinal neurones, with special reference to connexions with dorsal neck motoneurones in the cat. Exp Brain Res 80:260–276
Martinez-Trujillo JC, Klier EM, Wang H, Crawford JD (2003a) Contribution of head movement to gaze command coding in monkey frontal cortex and superior colliculus. J Neurophysiol 90:2770–2776
Martinez-Trujillo JC, Wang H, Crawford DJ (2003b) Electrical stimulation of the supplementary eye fields in the head-free macaque evokes kinematically normal gaze shifts. J Neurophysiol 89:2961–2974
Oommen BS, Smith RM, Stahl JS (2003) The influence of future gaze orientation upon eye–head coupling during saccades. Exp Brain Res 155:9–18
Oommen BS, Smith RM, Stahl JS (2004) Probability of head movement during saccades increases when saccades initiate during ongoing head movements. Soc Neurosci Abstr 30:990.994
Quessy S, Freedman EG (2004) Electrical stimulation of rhesus monkey nucleus reticularis gigantocellularis. I. Characteristics of evoked head movements. Exp Brain Res 156:342–356
Robinson FR, Phillips JO, Fuchs AF (1994) Coordination of gaze shifts in primates: brainstem inputs to neck and extraocular motoneuron pools. J Comp Neurol 346:43–62
Sasaki S (1999) Direct connection of the nucleus reticularis gigantocellularis neurons with neck motoneurons in cats. Exp Brain Res 128:527–530
Silverman BW (1986) Density Estimation for Statistics and Data Analysis. Chapman and Hall, New York
Stahl JS (1999) Amplitude of human head movements associated with horizontal saccades. Exp Brain Res 126:41–54
Stahl JS (2001) Adaptive plasticity of head movement propensity. Exp Brain Res 139:201–208
Tu TA, Keating GE (2000) Electrical stimulation of the frontal eye field in a monkey produces combined eye and head movements. J Neurophysiol 84:1103–1106
Walls GL (1942) The Vertebrate Eye and Its Adaptive Radiation. Cranbrook Press, Bloomfield Hills
Zangemeister WH, Stark L (1982) Gaze latency: Variable interactions of head and eye latency. Exp Neurol 75:389–406
Zee DS, FitzGibbon EJ, Optican LM (1992) Saccade-vergence interactions in humans. J Neurophysiol 68:1624–1641
Acknowledgements
This work was supported by a grant from the Department of Veterans Affairs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oommen, B., Stahl, J. Overlapping gaze shifts reveal timing of an eye–head gate. Exp Brain Res 167, 276–286 (2005). https://doi.org/10.1007/s00221-005-0036-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0036-8