Abstract
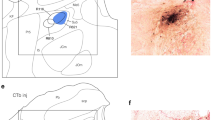
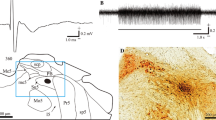
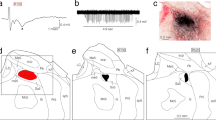
The cortical masticatory area (CMA) elicits rhythmic jaw movements in response to repetitive stimulation and is involved in the control of mastication. Based on jaw movement patterns, the CMA is divided into two parts. One is the part of the CMA in which a T-pattern similar to jaw movements during food transport in natural mastication is evoked by electrical stimulation. The other is more dorsomedially located, and during chewing a C-pattern similar to jaw movements can be induced. However, it is still not known which region of the putamen receives projections from the CMA and whether projections originate from both parts of the CMA. In this study, electrophysiological and histological experiments were undertaken in rabbits to investigate projections from the CMA to the putamen. Both experiments showed that the ventral region of the putamen received projections from the CMA. The density of the projections from the CMA area inducing the T-pattern seemed to be higher than that from the area inducing the C-pattern. Furthermore, the peak latency of the evoked potentials from stimulation of the CMA area inducing the T-pattern was shorter than that from stimulation of the area inducing the C-pattern. The data obtained from the present study indicate the functional role of the ventral region of the putamen in the regulation of mastication, and further suggest that the corticostriatal pathway is involved in the transition between behavioral jaw movement patterns.






Similar content being viewed by others
References
Alexander GE, DeLong MR (1985) Microstimulation of the primate striatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. J. Neurophysiol 53:1417–1430
Bremer F (1923) Physiologie nerveuse de la mastication chez le chat le lapin. Reflexes de mastication. Responses masticatrices corticales et centre cortical du gout. Arch Int Physiol 21:308–352
DiFiglia M, Pasik P, Pasik T (1976) A Golgi study of neuronal types in the neostriatum of monkeys. Brain Res 114:245–256
Ebrahimi A, Pochet R, Roger M (1992) Topographical organization of the projections from physiologically identified areas of the motor cortex to the striatum in the rat. Neurosci Res 14:39–60
Enomoto S, Schwartz G, Lund JP (1986) Behavior of cortical masticatory area neurons in the awake rabbit. Proc Intern U Physiol Sci, XVI:193
Enomoto S, Schwartz G, Lund JP (1987) The effects of cortical ablation on mastication in the rabbit. Neurosci Lett 82:162–166
Flaherty AW, Graybiel AM (1993) Output architecture of the primate putamen. J Neurosci 13:3222–3237
Hidaka O, Morimoto T, Kato T, Masuda Y, Inoue T, Takada K (1999) Behavior of jaw muscle spindle afferents during cortically induced rhythmic jaw movements in the anesthetized rabbit. J Neurophysiol 82:2633–2640
Huang CS, Sirisko MA, Hiraba H, Murray GM, Sessle BJ (1988) Organization of the primate face motor cortex as revealed by intracortical microstimulation and electrophysiological identification of afferent inputs and corticobulbar projections. J Neurophysiol 59:796–818.
Huang CS, Hiraba H, Murray G.M, Sessle BJ (1989) Topographical distribution and functional properties of cortically induced rhythmical jaw movements in the monkey (Macaca fascicularis). J Neurophysiol 61:635–650
Inoue T, Kato T, Masuda Y, Nakamura T, Kawamura Y, Morimoto T (1989) Modifications of masticatory behavior after trigeminal deafferentation in the rabbit. Exp Brain Res 74:579–591
Iwata K, Itoga H, Ikukawa A, Hanashima N, Sumino R (1985) Movements of the jaw and orofacial regions evoked by stimulation of two different cortical areas in cats. Brain Res 359:332–337
Jones EG, Coulter JD, Burton H, Porter R (1977) Cells of origin and terminal distribution of corticostriatal fibers arising in the sensory-motor cortex of monkeys. J Comp Neurol 173:53–80
Kemp JM, Powell TPS (1970) The cortico-striate projection in the monkey. Brain 93:525–546
Kolomiets BP, Deniau JM, Mailly P, Menetrey A, Glowinski J, Thierry AM (2001) Segregation and convergence of information flow through the cortico-subthalamic pathways. J Neurosci 21:5764–5772
Komuro A, Morimoto T, Iwata K, Inoue T, Masuda Y, Kato T, Hidaka O (2001) Putative feed-forward control of jaw-closing muscle activity during rhythmic jaw movements in the anesthetized rabbit. J Neurophysiol 86:2834–2844
Künzle H (1975) Bilateral projections from precentral motor cortex to the putamen and other parts of the basal ganglia. An autoradiographic study in Macaca fascicularis. Brain Res 88:195–209
Liles SL (1975) Cortico-striatal evoked potentials in the monkey (Macaca mulatta). Electroencephalogr Clin Neurophysiol 38:121–129
Liu ZJ, Masuda Y, Inoue T, Fuchihata H, Sumida A, Takada K, Morimoto T (1993) Coordination of cortically induced rhythmic jaw and tongue movements in the rabbit. J Neurophysiol 69:569–584
Lund JP (1991) Mastication and its control by the brain stem. Crit Rev Oral Biol Med 2:33–64
Lund JP, Sasamoto K, Murakami T, Olsson KA (1984) Analysis of rhythmical jaw movements produced by electrical stimulation of motor-sensory cortex of rabbits. J Neurophysiol 52:1014–1029
Marco LA, Copack P, Edelson AM, Gilman S (1973) Intrinsic connections of caudate neurons. I. Locally evoked field potentials and extracellular unitary activity. Brain Res 53:291–305
Masuda Y, Morimoto T, Hidaka O, et al. (1997) Modulation of jaw muscle spindle discharge during mastication in the rabbit. J Neurophysiol 77:2227–2231
Masuda Y, Kato T, Hidaka O, Matsuo R, Inoue T, Iwata K, Morimoto T (2001) Neuronal activity in the putamen and the globus pallidus of rabbit during mastication. Neurosci Res 39:11–19
Masuda Y, Tachibana Y, Inoue T, Iwata K, Morimoto T (2002) Influence of oro-facial sensory input on the output of the cortical masticatory area in the anesthetized rabbit. Exp Brain Res 146:501–510
Morimoto T, Kawamura Y (1973) Properties of tongue and jaw movements elicited by stimulation of the orbital gyrus in the cat. Arch Oral Biol 18:361–372
Morimoto T, Inoue T, Nakamura T, Kawamura Y (1985) Characteristics of rhythmic jaw movements of the rabbit. Arch Oral Biol 30:673–677
Morimoto T, Inoue T, Masuda Y, Nagashima T (1989) Sensory components facilitating jaw-closing muscle activities in the rabbit. Exp Brain Res 76:424–440
Narita N, Yamamura K, Yao DY, Martin R, Masuda Y, Sessle BJ (2002) Effects of reversible bilateral inactivation of primate lateral pericentral cortex on mastication. Arch Oral Biol 47:673–688
Pisa M (1988) Motor somatotopy in the striatum of rat: manipulation, biting, and gait. Behav Brain Res 27:21–35
Pisa M, Schranz JA (1988) Dissociable motor roles of the rat’s striatum conform to a somatotopic model. Behav Neurosci 102:429–440
Sawyer CH, Everette JW, Green JD (1954) The rabbit diencephalon in stereotaxic coordinates. J Comp Neurol 101:801–824
Schwartz G, Enomoto S, Valiquette C, Lund JP (1989) Mastication in the rabbit: a description of movement and muscle activity. J Neurophysiol 62:273–287
Takada M, Tokuno H, Nambu A, Inase M (1998) Corticostriatal projections from the somatic motor areas of the frontal cortex in the macaque monkey: segregation versus overlap of input zones from the primary cortex, the supplementary motor area, and the premotor cortex. Exp Brain Res 120:114–128
Acknowledgments
This study was supported by a grant-in-aid (no. 12671805) from the Japanese Ministry of Education, Science and Culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Masuda, Y., Kim, S.K., Kato, T. et al. Different corticostriatal projections from two parts of the cortical masticatory area in the rabbit. Exp Brain Res 161, 397–404 (2005). https://doi.org/10.1007/s00221-004-2073-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2073-0