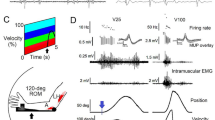
Abstract
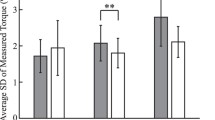
It is known that muscle activation patterns are changed when the force requirements of a task are increased (e.g., moving a heavier inertial load) or when the available muscle force is reduced (e.g., by inducing muscle fatigue). It is not known whether this is true when the torque-producing capability of a muscle is altered. Eight neurologically healthy subjects performed flexion and extension maximum voluntary isometric contractions (MVC) at five different joint positions (10°, 40°, 70°, 100°, and 130°, where 0° is full elbow extension). Flexion MVC increased by 138% and extension MVC increased by 74% as the elbow joint position changed from the most extended to the most flexed position tested. The same subjects then made rapid, 30° elbow flexion movements from each of four starting elbow positions (10°, 40°, 70°, and 100°). Muscle activation patterns for movements made from the more extended positions showed an increased first agonist burst duration and increased latency of the antagonist burst. There was no change in the initial rate of rise of the agonist burst across starting joint positions. Movements made from the most extended starting position were significantly slower and had longer acceleration and deceleration times than did movements made from the more flexed starting positions. The changes in muscle activation patterns were consistent with those seen when the force requirements of a task are increased or the available muscle force is reduced. We hypothesize that a fall in the ratio of available to required muscle forces causes the nervous system to change muscle activation patterns, to increase the ratio. Our results are consistent with this hypothesis.






Similar content being viewed by others
References
An KN, Hui FC, Morrey BF, Linscheid RL, Chao EY (1981) Muscles across the elbow joint: a biomechanical analysis. J Biomech 14:659–669
Basmajian JV, De Luca CJ (1985) Muscles alive, their functions revealed by electromyography, 5th edn. Williams and Wilkins, Baltimore, MD, pp 61–64
Brown SHC, Cooke JD (1981) Amplitude- and instruction-dependent modulation of movement-related electromyogram activity in humans. J Physiol (Lond) 316:97–107
Cheron G, Godaux E (1986) Self-terminated fast movement of the forearm in man: amplitude dependence of the triple burst pattern. J Biophys Biomec 10:109–117
Corcos DM, Jiang HY, Wilding J, Gottlieb GL (2002) Fatigue induced changes in phasic muscle activation patterns for fast elbow flexion movements. Exp Brain Res 142:1-12
Day BL, Marsden CD (1982) Accurate repositioning of the human thumb against unpredictable dynamic loads is dependent upon peripheral feedback. J Physiol (Lond) 327:393–407
Forget R, Lamarre Y (1987) Rapid elbow flexion in the absence of proprioceptive and cutaneous feedback. Hum Neurobiol 6:27–37
Gerilovsky L, Tsvetinov P, Trenkova G (1989) Peripheral effects on the amplitude of monopolar and bipolar H-reflex potentials from the soleus muscle. Exp Brain Res 76:173–181
Gottlieb GL (1996) On the voluntary movement of compliant (inertial-viscoelastic) loads by parcellated control mechanisms. J Neurophysiol 76:3207–3229
Gottlieb GL, Agarwal GC (1980) Response to sudden torques about the ankle in man. III. Suppression of stretch-evoked responses during phasic contraction. J Neurophysiol 44:233–246
Gottlieb GL, Corcos DM, Agarwal GC (1989a) Organizing principles for single-joint movements. I. A speed-insensitive strategy. J Neurophysiol 62:342–357
Gottlieb GL, Corcos DM, Agarwal GC (1989b) Strategies for the control of voluntary movements with one mechanical degree of freedom. Behav Brain Sci 12:189–210
Gottlieb GL, Corcos DM, Agarwal GC, Latash ML (1990) Organizing principles for single joint movements. III. Speed-insensitive strategy as a default. J Neurophysiol 63:625–636
Gottlieb GL, Latash ML, Corcos DM, Liubinskas TJ, Agarwal GC (1992) Organizing principles for single joint movement. V. Agonist-antagonist interactions. J Neurophysiol 67:1417–1427
Hallett M, Marsden CD (1979) Ballistic flexion movements of the human thumb. J Physiol (Lond) 294:33–50
Hallett M, Shahani BT, Young RR (1975) EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry 38:1154–1162
Hannaford B, Stark L (1985) Roles of the elements of the triphasic control signal. Exp Neurol 90:619–634
Hasan Z, Enoka RM (1985) Isometric torque–angle relationship and movement-related activity of human elbow flexors: implications for the equilibrium-point hypothesis. Exp Brain Res 59:441–450
Hoffman DS, Strick PL (1989) Force requirements and patterns of muscle activity. Behav Brain Sci 12:221–224
Hoffman DS, Strick PL (1993) Step-tracking movements of the wrist. III. Influence of changes in load on patterns of muscle activity. J Neurosci 13:5212–5227
Hutchins EL, Gonzalez RV, Barr RE (1993) Comparison of experimental and analytical torque–angle relationships of the human elbow joint complex. Biomed Sci Instrum. 29:17–24
Inman VT, Ralston HJ, Saunders JBCM, Feinstein B, Wright EW (1952) Relation of the human electromyogram to muscular tension. EEG Clin Neurophysiol 4:187–194
Jaric S, Radovanovic S, Milanovic S, Ljubisavljevic M, Anastasijevic R (1997) A comparison of the effects of agonist and antagonist muscle fatigue on performance of rapid movements. Eur J Appl Physiol 76:41–47
Jaskolski A, Kisiel K, Adach Z, Jaskolska A (2000) The influence of elbow joint angle on different phases of force development during maximal voluntary contraction. Can J Appl Physiol 25:453–465
Latash ML (1994) Control of fast elbow movement: a study of electromyographic patterns during movements against unexpectedly decreased inertial load. Exp Brain Res 98:145–152
Lee RG, Lucier GE, Mustard BE, White DG (1986) Modification of motor output to compensate for unanticipated load conditions during rapid voluntary movements. Can J Neurol Sci 13:97–102
Leedham JS, Dowling JJ (1995) Force-length, torque–angle and EMG-joint angle relationships of the human in vivo biceps brachii. Eur J Appl Physiol Occup Physiol 70:421–426
Marsden CD, Merton PA, Morton HB (1976) Servo action in the human thumb. J Physiol (Lond) 257:1-44
Mirkov DM, Milanovic S, Ilic DB, Jaric S (2002) Symmetry of discrete and oscillatory elbow movements: does it depend on torque that the agonist and antagonist muscle can exert? Mot Control 6:271–281
Murray WM, Buchanan TS, Delp SL (2000) The isometric functional capacity of muscles that cross the elbow. J Biomech 33:943–952
Pfann KD, Hoffman DS, Gottlieb GL, Strick PL, Corcos DM (1998) Common principles underlying the control of rapid, single degree-of-freedom movements at different joints. Exp Brain Res 118:35–51
Sanes JN (1986) Kinematics and end-point control of arm movements are modified by unexpected changes in viscous loading. J Neurosci 6:3120–3127
Shapiro MB, Gottlieb GL, Moore CG, Corcos DM (2002) Electromyographic responses to an unexpected load in fast voluntary movements: descending regulation of segmental reflexes. J Neurophysiol 88:1059–1063
Smeets JB, Erkelens CJ, Denier van der Gon JJ (1990) Adjustments of fast goal-directed movements in response to an unexpected inertial load. Exp Brain Res 81(2):303–312
Soechting JF, Dufresne JR, Lacquaniti F (1981) Time-varying properties of myotatic response in man during some simple motor tasks. J Neurophysiol 49(6):1226–1243
Sternad D, Corcos D (2001) Effect of task and instruction on patterns of muscle activation: Wachholder and beyond. Mot Control 5:307–336
Thomis MA, Van Leemputte M, Maes HH et al. (1997) Multivariate genetic analysis of maximal isometric muscle force at different elbow angles. J Appl Physiol 82:959–967
Wachholder K, Altenburger H (1926) Beiträge zur Physiologie der willkürlichen Bewegung. X. Mitteilung. Einzelbewegungen. Pflugers Arch 214:642–661
Winters JM, Woo SL (1990) Multiple muscle systems. Biomechanics and movement organization. Springer, New York
Acknowledgements
This study was supported in part by National Institutes of Health Grants AR33189, NS21827, and NS40902. We would also like to acknowledge the valuable comments of Dr. Ziaul Hasan and Dr. Mark Shapiro, and Rachel Musenge for her assistance in editing this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prodoehl, J., Gottlieb, G.L. & Corcos, D.M. The neural control of single degree-of-freedom elbow movements. Exp Brain Res 153, 7–15 (2003). https://doi.org/10.1007/s00221-003-1564-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-003-1564-8