Abstract
The present work aimed to analyse the bioactive compounds and antioxidant activities of peel and seed extracts obtained from three tropical fruits: papaya, mango, and loquat, with different solvents (water, ethanol, and water:ethanol, 1:1) and evaluate their potential effects as antioxidants in a cooked chicken model under refrigerated storage. In the seed and peel extracts produced, bioactive compounds (total phenolic compounds, total flavonoids, and condensed tannins) were quantified. Additionally, antioxidant activities (ABTS, DPPH, and FRAP) were assayed spectrophotometrically. Seed extracts from the three fruits were selected for application in a cooked chicken model in which colour, lipids, and protein oxidation were evaluated during refrigerated storage. Moreover, compared with the other extracts, the mango seed extracts (MSEs), irrespective of the extraction solvent used, had the highest contents of bioactive compounds and antioxidant activities. MSEs significantly reduced the CIE L* and increased the CIE a* while effectively controlling lipid and protein oxidation in cooked chicken models during refrigerated storage. Due to their high antioxidant activity and high concentration of phenolic compounds, flavonoids, and condensed tannins, MSEs are interesting sources of natural antioxidants and bioactive compounds for use in the meat industry.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
In meat and meat products, lipid and protein oxidative processes result in changes in colour, flavour, odour, and texture, which adversely affect quality, shelf life, and nutritional value. These alterations serve as indicative signs of deterioration, impacting both quality and nutritional content and ultimately reducing shelf life. However, the addition of antioxidants can mitigate this deterioration by inhibiting oxidative reactions, thus preserving the original characteristics and extending the overall quality and shelf life of the products [1].
Synthetic antioxidants have been widely used as food additives to protect against oxidative degradation in meat and meat products. To improve the storage stability of food, synthetic antioxidants such as butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG), and tertiary butylhydroquinone (TBHQ) have been intensively used for industrial processing. Nevertheless, their use is under increasing scrutiny because of their potential genotoxic effects [2].
In recent years, a significant proportion of meat consumers have been willing to consume products with "natural origin" ingredients and without preservatives, which has led the industry to implement strategies based on the application of extracts derived from various plant materials for the control of oxidative processes [1, 3,4,5].
Vegetables, spices, aromatic plants, seeds, fruit, and byproduct (leaves, skin, seeds) extracts rich in antioxidant compounds are currently preferred for use in different meat products [6]. Most of the antioxidant capacity of these extracts is mainly due to the numerous phenolic compounds, flavonoids, anthocyanins, and other phytochemicals that act as free radical scavengers, reducing agents, chelators of prooxidant metals, or quenchers of singlet oxygen. These antioxidants delay the oxidative reactions in meat products derived from the processing and storage of meat products [2].
In addition, phytochemicals in the diet have a protective effect on human health, decreasing the abundance of biomarkers of oxidative damage by inhibiting or quenching free radicals and reactive oxygen species [7, 8]. A diet rich in phytochemicals with antioxidant activity has been shown to provide several subsequent health benefits, including a reduced risk of degenerative diseases, cancer, heart disease, hypertension, cataracts, and immune dysfunction [9].
Tropical fruits are characterized by high concentrations of biologically active compounds, mainly carotenoids and phenolic compounds [10]. In tropical fruits, the content of bioactive compounds is much greater than that found in traditional fruits [11]. The analysis of the different parts of the fruits revealed the different concentrations of bioactive compounds among them, and several papers have indicated that the amounts of such compounds are often considerably greater in the discarded fractions than in the edible parts, as reviewed by Villacís-Chiriboga [10]. These inedible parts, which are generally regarded as waste or byproducts with little value in the fruit industry, can be valorised as sources of bioactive compounds to improve economic benefits and ameliorate the adverse environmental problems generated [10].
For this purpose, natural extracts with antioxidant properties are produced from plant resources through the use of solid‒liquid extraction with a solvent or a mixture of solvents [12]. While the operating conditions (extraction time, number of extractions, temperature, and solvent/sample ratio) have important impacts on the extraction yield and antioxidant activity of the obtained extract, the solvent mixture plays a decisive role in its composition and antioxidant characteristics. The appropriate polarity of the solvent system will largely depend on the solubility of the bioactive compounds of interest to be extracted and will therefore determine the antioxidant properties of the extracts obtained [13].
Changing consumer preferences towards healthier lifestyles and exotic flavours have driven demand for tropical fruit juices. Production of juices from fruits like mango, papaya, and loquat has seen a noticeable surge in recent years. However, the generation of waste, including peels and seeds, presents environmental challenges. Utilizing these residues through techniques like extracting antioxidant compounds can help mitigate environmental impact and optimize natural resource utilization. Analysing the potential application of byproducts from tropical fruits as natural antioxidants is of particular interest to the food industry. It is of utmost importance to have adequate and accurate information on the compound profile and bioactive yield of these materials to assess their potential applicability accurately.
In this sense, the present work aimed to analyse the bioactive compounds and antioxidant activities of peel and seed extracts obtained from three tropical fruits, namely, papaya, mango, and loquat, with different solvents and evaluate their potential effects as natural antioxidants in a cooked chicken model under refrigerated storage.
Materials and methods
Chemicals and reagents
The Folin-Ciocalteu reagent, 2,2-diphenyl-2-picrylhydrazyl (DPPH), 2,2-azininobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), tripyridyl triazine (TPTZ), 6-hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid (Trolox), and 1,1,3,3-tetraethoxypropane (TEP) were purchased from Sigma‒Aldrich (St. Louis, MO).
Papaya, mango and loquat samples
Papaya (Carica papaya L.), mango (Mangifera indica L. var. osteen), and loquat (Eriobotrya japonica Thunb.) plants were purchased from a local fruit market (Cáceres, Spain). Seeds and peels were manually separated using a knife. Seeds and peels were cut into small pieces and subsequently were ground in a knife mill (Grindomix GM 200, Retsch GmbH, Haan, Germany) to a 1 mm particle size.
Preparation of the peel and seed extracts
The peel and seed extracts were prepared in a solvent ratio of 1:10 (w/v). The following three solvents were used: water, ethanol, and water:ethanol (1:1 v/v). The extraction was carried out in a shaking water bath at 25 °C for 120 min. Lapsed at this time, the samples were centrifuged at 4000 rpm for 5 min, filtered through a filter paper (Whatman® qualitative filter paper, Grade 1), and stored in amber bottles at – 20 °C. The extraction was repeated twice, and the supernatants were combined. Eight independent extractions were conducted for each type of fruit (mango, papaya, and loquat), fruit part (seed and peel) and extraction solvent (water, ethanol, and water:ethanol 1:1 v/v).
Determination of the antioxidant compound contents in peel and seed extracts
Total phenolic content (TPC)
The Folin–Ciocalteu method was modified for the determination of the total phenolic content of the extracts [14]. In a polypropylene test tube, 50 µL of extract, 450 µL of Milli-Q water, and 20 µL of Folin–Ciocalteu reagent were added, and the mixture was vortexed. To the tube, 50 µL of 20% sodium carbonate and 450 ml of water were added. After incubation at 25 °C for 60 min, the absorbance was measured at 765 nm in a Multiskan Go plate reader (Thermo Fisher Scientific, Inc., MA, USA). For quantification, a gallic acid calibration curve (10–500 µg/mL) was used. The results are expressed as mg of gallic acid eq./g sample.
Total flavonoid (FLAV)
The total flavonoid content in the extracts was analysed as previously described by Zhishen et al. [15]. In a 2 mL polypropylene test, 50 µL of sample was reacted with 400 µL of water and 15 µL of 10% sodium nitrite. After incubation (5 min), 15 µL of 20% aluminium chloride, 200 µL of 1 M sodium hydroxide and 320 µL of distilled water were added, and the absorbance was recorded at 510 nm. For quantification, a ( +) catechin calibration curve (1.0–0.1 mg/mL) was used. The results are expressed as mg catechin eq./g sample. Assays were performed in duplicate.
Condensed tannins (CTs)
The content of condensed tannins in the extracts was assessed by the method proposed by Watterman and Mole [16]. The assay was performed in a 96-well microplate. In each well, 25 µL of extract and 250 µL of vanillin (1% vanillin in methanol (MeOH): 10% HCl in MeOH) were added. After incubation (30 min), the absorbance was measured at 500 nm. For quantification, a ( +) catechin calibration curve (10.0–0.16 mg/mL) was used. The results are expressed as mg catechin eq./g sample. Assays were performed in duplicate.
In vitro antioxidant activity assays in peel and seed extracts
ABTS (2,2′-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid) antioxidant activity
The ABTS assay was performed according to Re et al. [17]. Equal volumes of 7 mM ABTS and 2.45 mM potassium persulfate were mixed and kept at room temperature for 16 h in the dark to generate the stable radical ABTS●+. After this time, the ABTS●+ radical was diluted with ethanol to achieve an absorbance of 0.7 ± 0.1 at 734 nm. In a 96-well plate, 5 µL of the extract was mixed with 245 µL of the ABTS●+ radical, and the absorbance at 734 nm was measured at 60-s intervals for 7 min. For quantification, a Trolox calibration curve (1.00–0.25 mg/mL) was generated. The results are expressed in mg Trolox equivalents activity (TEAC)/g sample. Assays were performed in duplicate.
DPPH (1,1-diphenyl-2-picrylhydrazyl) antioxidant activity
DPPH activity was determined according to the method previously proposed by Brand-Williams et al. [18]. The absorbance of the DPPH radical (0.1 mM DPPH●) was recorded at 515 nm. In a 96-well microplate, an aliquot (10 µL) of extract was mixed with 290 µL of DPPH● radical reagent. After incubation at 25 °C for 30 min, the absorbance was read at 515 nm.
The affinity of the fruit extracts for quenching DPPH radicals (% inhibition of DPPH at 515 nm) was calculated using the following equation (Eq. 1):
where A0 represents the absorbance of the DPPH solution measured at time zero and Af is the absorbance of each sample at 30 min after the addition of the DPPH solution. Assays were performed in duplicate.
FRAP (ferric reducing antioxidant power) antioxidant activity
The procedure was described by Benzie & Strain [19]. FRAP reagent was prepared by mixing 2.5 mL of 10 mM TPTZ in 40 mM HCl, 2.5 mL of 20 mM iron(III) chloride hexahydrate, and 25 mL of 0.3 mM acetate buffer, pH 3. Next, 200 µL of FRAP was added, and the absorbance at 593 nm was recorded. Subsequently, 7 µL of extract and 200 µL of distilled water were added. After incubation at 25 °C for 30 min, the absorbance was recorded at 593 nm. For quantification, a calibration curve of Trolox (4.0–0.25 mg/mL) was prepared and assayed under the same conditions as those used for the extracts. The results are expressed as TEAC eq./mL extract. Assays were performed in duplicate.
Cooked meat model: batter formulation, cooking, and storage
Commercial chicken breasts were obtained from a local meat market (Cáceres, Spain). The control chicken model systems were prepared with chicken breast (79 g/100 g), salt (2 g/100 g), and water (20 mL/100 g). In the extracts-treated models, water was partially replaced with selected extracts (mango, papaya, and loquat) at a concentration of 2 mL/100 g. Chicken breast was minced using a meat mincer with a 4-mm-diameter plate. (Mainca, Barcelona, Spain). Chicken, water, salt, and extracts were chopped and mixed in a Universal Machine UMC 5–12 (Stephan Machinery GmbH, Hameln, Germany) at 4 °C for 5 min.
Batters were produced, in duplicate, depending on the selected extract added to their formulations. Batters (∼50 g) were placed into 50 mL polypropylene tubes and cooked in a water bath to an internal temperature of 70 °C for 30 min. For each experimental group, six models (3 models/replica) were used to conduct the analytical determinations. The core temperature was measured and recorded using a K-type thermocouple temperature sensor coupled to a data logger (Data Harvest, Bedfordshire, UK). The cooked products were cooled in cold water for 10 min. To evaluate changes during refrigerated storage, the samples were kept in polypropylene plastic tubes under aerobic conditions and stored at + 4 °C for 0, 5 and 10 days in the dark. After measuring the instrumental colour, the samples were vacuum packed and frozen at –80 °C until lipid and protein oxidation analysis.
Instrumental colour of the cooked models
Color measurements were performed according to AMSA [20]. The instrumental colour coordinates (CIE L*a*b*) were measured using a Minolta CR-300 tristimulus reflectance colorimeter (Minolta Camera, Osaka, Japan) with an illuminant D65, a 10° standard observer, and a 0.8 cm port/viewing area. Previously, for colour measurements, the colorimeter was standardized to a white (CR-A43) or black tile (CM-A182). Three colour readings at three randomly selected locations were taken from each sample, and the average value was used. All the measurements were carried out on transverse cut sections of the cooked products after 0, 5, and 10 days of refrigeration. The total colour changes (ΔE) were evaluated by the following formula (Eq. 2):
where L*ref, a*ref, and b*ref represent the L*, a* and b* values in the control group, respectively.
Lipid oxidation of cooked models
Lipid oxidation was assessed by the 2-thiobarbituric acid (TBA) method [21]. Samples (2.5 g) and 7.5 mL of 3.86% perchloric acid and 250 µL of 4.2% BHT were homogenized at 11,500 rpm for 45 s with ice cooling. The homogenate was centrifuged (2000 rpm/5 min), and the resulting supernatant was filtered through glass wool. One mL of the filtered sample was mixed with 1 mL of TBA and incubated at 90 °C for 30 min. The tubes were centrifuged at 2000 rpm for 5 min, after which the absorbance of the supernatant was recorded at 532 nm. TBARS values were calculated from a standard curve of 1,1,3,3-tetraethoxypropane (TEP) (0.16–0.001 mM) and are expressed as mg malondialdehyde (MDA)/kg sample. Determinations were performed in duplicate.
Protein oxidation of cooked models
Protein oxidation was determined by estimating the protein carbonyl content [22]. Protein carbonyls were detected by reaction with 2,4-dinitrophenylhydrazine (DNPH) in 2 N hydrochloric acid to form protein hydrazones. The concentration of DNPH hydrazones was calculated by measuring the DNPH concentration based on the absorption at 22.0 mM−1 cm−1 at 370 nm. The protein concentration was determined spectrophotometrically at 280 nm in control samples using bovine serum albumin (BSA) as a standard. The results are expressed as nmol carbonyls/mg protein.
Statistical analysis
The data were analysed using one-way ANOVA with the statistical software Jamovi (https://www.jamovi.org) (version 2.0.0.0). When significant effects (extraction solvent, extract addition, time of storage) were detected (p < 0.05), the means were compared using Tukey’s HSD post hoc test. The results are presented as the mean ± standard deviation. Pearson correlations were determined, and the densities of the variables were plotted.
Results and discussion
Antioxidant compound contents of the extracts
Table 1 shows the contents of total phenolic compounds, total flavonoid compounds, and condensed tannins in water (H2O), ethanol (EtOH), and H2O:EtOH (1:1 v/v) extracts obtained from mango, loquat, and papaya peels and seeds. The ability of the different solvents to extract bioactive compounds varied according to their chemical nature (phenolics, flavonoids, or tannins), the fruit (mango, loquat, or papaya) and the material to be treated (seed or peel). In general, the seed extracts were richer in phenolic compounds than their peel counterparts were, and the binary mixture of H2O:EtOH (1:1 v/v) produced extracts with the highest contents of TPCs and FLAVs, but the aqueous extracts contained more CTs than their equivalents.
Several recent studies have reported that methanol, ethanol, and their water mixtures are the best solvents for obtaining the maximum yields of phenolic compounds from peels and seeds from tropical fruits [23,24,25]. The yield of phenolic compounds extraction depends on the polarity of the extraction solvent used. Therefore, the efficiency of solvents for extraction is also related to their ability to better solvate antioxidant compounds present in fruits, facilitated by interactions such as hydrogen bonds between the polar sites of the antioxidant molecules and ethanol or water. The different compositions of phenolic compounds with distinct polarities could explain their different concentrations depending on the extraction solvent used. The literature shows that there is no solvent generally acceptable for the extraction of phytochemicals; nevertheless, it is generally believed that solvents of higher polarity often perform best in terms of polyphenol extraction because of the high solubility of polyphenols in such solvents [26].
Despite the solvent used, mango seed extracts consistently exhibited high levels of antioxidant compounds. Specifically, mango seed extracts contained significantly higher levels of TPCs compared to papaya (ranging from 11- to 45-fold higher) and loquat extracts (ranging from 4.5- to 22.5-fold higher). Although mango seeds exhibited elevated levels of flavonoids and tannins in comparison to other fruits, the disparity was not as notable as observed in TPC levels. In contrast, extracts from papaya peel and seeds had the lowest contents of phenolic compounds. It is generally accepted that phenolic compounds are the most abundant secondary metabolites in plants, fruits, cereals, vegetables, and spices and are characterized by high antioxidant activity.
In line with observations on total phenolic content, mango seed extracts consistently exhibited the highest levels of flavonoids among all extracted samples, irrespective of the extraction solvent employed. However, the differences observed with other fruit extracts were less evident than those observed for total phenolic compounds. Flavonoid concentrations in mango seed extracts surpassed those in papaya and loquat extracts by a factor of 1.5 to 6. Moreover, mango and papaya peel extracts demonstrated comparatively lower flavonoid concentrations relative to their respective seed extracts.
In general, the seed extracts had higher content of CTs than peels. The effectiveness of the extraction solvent was dependent on the type of material extracted. Thus, the aqueous extracts from mango peel and seed contained the highest amounts of CTs. Moreover, the ethanolic extract from papaya seed was the richest among the CTs, and in the loquat seed extract, the binary mixture of water and ethanol extracted the highest amounts of these compounds.
These observations are consistent with previous studies on mango byproducts, indicating that ethanolic extracts from mango seeds and peels serve as significant sources of phenolic compounds [23]. Mango seeds have focused scientific interest due to their high phytochemical content, which has demonstrated antioxidant potential. Notable compounds among these include chlorogenic acid, gallic acid, caffeic acid, ellagic acid, rutin, and catechin [24, 25, 27]. The extracts from papaya and loquat seeds exhibited higher concentrations of phenolic compounds compared to those from their respective peels, regardless of the extraction solvent used. However, in both cases, the total phenolic compound levels were lower than those observed in mango seeds. These findings align with those reported by previous studies [10]. In a recent review, Fidelis et al. [28] examined the chemical composition and bioactivity of a variety of fruit seeds, along with discussing extraction techniques for water-soluble and lipophilic compounds, as well as their in vitro and in vivo functionalities, and explored the relationship between chemical composition and observed activity.
Antioxidant activities of the extracts
The use of multiple antioxidant assays for the determination of the antioxidant activity of a plant extract provides a comprehensive understanding of its efficacy in combating oxidative stress. The different in vitro assays target distinct mechanisms of antioxidant action, such as scavenging free radicals, chelating metal ions, or inhibiting lipid peroxidation, capturing the diverse antioxidant compounds present in the extract and assessing their potency across various pathways. By combining these distinct assays, it can gain a comprehensive understanding of the antioxidant properties of compounds. ABTS and DPPH evaluate the radical scavenging capacity toward a stable free radical (2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS)) and FRAP the reducing antioxidant potential of the ferric ion. Additionally, the applicability of the antioxidant test to both hydrophilic and lipophilic antioxidants. Thus, the ABTS tests can measure both hydrophilic, and lipophilic antioxidants, FRAP measures the hydrophilic antioxidants, and DPPH only applies to hydrophobic systems [29].
Table 2 shows the results of the antioxidant activities obtained by the ABTS, DPPH, and FRAP assays of the extracts obtained from the peels and seeds of mango, loquat, and papaya plants. Natural antioxidants are often multifunctional compounds with diverse mechanisms of antioxidant action, which is why a variety of methods must be used to measure their antioxidant activity [30]. In this work, the antioxidant properties of extracts recovered from mango, papaya, and loquat plants were evaluated by using two different free radical scavenging methods (ABTS, DPPH•) and one assay for the estimation of metal-reducing activity (FRAP). The antioxidant activities of the extracts varied depending on the fruit, the part of the fruit, and the extraction solvent used. The antioxidant activities were lower in the aqueous extracts than in the other two extracts. Specifically, the ABTS scavenging activity was high in the ethanolic extracts and low in the aqueous extracts. DPPH activity was equal in the aqueous: ethanolic and ethanolic extracts and higher in the aqueous extracts; moreover, FRAP activity was greater in the H2O:EtOH (1:1 v/v) extracts.
In general, the mango seed extracts had the highest antioxidant activity regardless of the solvent used, followed by the loquat plant extracts, while the lowest antioxidant activity was detected in the papaya plant extracts. The capacity to scavenge ABTS was found to be greatest for the mango seed extracts, irrespective of the solvent used, and lowest for the ethanol and water:ethanol papaya extracts. The mango extracts produced with ethanol or water:ethanol had higher ABTS activities than did the respective aqueous extracts. Similarly, mango extracts, especially the seeds, exhibited the highest DPPH radical scavenging potential. In contrast, the papaya extracts had low DPPH antioxidant activity, while intermediate activity was found for the loquat extracts. The FRAP activity was significantly greater for the mango extracts than for the loquat and papaya extracts. The FRAP activity of the mango seed extracts was significantly greater than that of the peel extracts, regardless of the solvent used. In line with what was previously described for ABTS and DPPH antioxidant activities, papaya extracts (peel and seed) showed limited antioxidant activity according to the FRAP assay. For the loquat extracts, no significant differences were found concerning the FRAP activity measured for the papaya extracts. The results obtained in the present paper agree with previous findings reported for mango [31], loquat [32, 33], and papaya extracts [34] obtained from different tissues of the fruit.
The differences in antioxidant activity are due to the distinct and specific compounds and their concentrations in the extracts, as described previously. In mango seeds and peels, the major polyphenols in terms of antioxidative capacity and/or quantity are mangiferin, catechins, quercetin, kaempferol, rhamnetin, anthocyanins, gallic and ellagic acids, propyl and methyl gallate, benzoic acid, and protocatechuic acid [31]. In different loquat tissues, phenolic compounds such as 3-p-coumaroylquininc acid, caffeoylquinic acid, 5-feruloylquinic acid, quercetin, and kaempferol derivatives (galactoside, glucoside, and rhamnoside) have been linked to the antioxidant activity of loquat [32, 33]. In papaya seeds and peels, ferulic, mandelic, syringic, vanillic acid, myricetin, and conifer-aldehyde, among other phenolic compounds, have been previously described as being responsible for the antioxidant activities of these plants [34].
The observed antioxidant activities reflected the differences in total phenolic, flavonoid, and condensed tannin contents. Thus, the higher the content of bioactive compounds is, the greater the antioxidant activity. Correlation analysis revealed strong correlations between the TPC and antioxidant activity and, to a lesser extent, with the FLAV content (Fig. 1). The highest correlations were found between the TPC and ABTS (r = + 0.94, p <.001), DPPH (r = + 0.92, p < .001) and FRAP (r = + 0.99, p < .001) assays. Similarly, the flavonoid content was positively correlated with the ABTS (r = + 0.53; p < .001), DPPH (r = + 0.45; p < .001) and FRAP (r = + 0.53; p < .001) assays. Phenolic compounds are the most common phytoconstituents of different fruits, vegetables, and medicinal and aromatic plants and are responsible for antioxidant activities [35, 36]. In contrast, the CTs showed no relationship with the antioxidant activity of the extracts, as reflected by the lack of a significant correlation between them. Our results contrast with many studies in vitro demonstrating the high antioxidant capacity of CTs [37, 38].
The water:ethanol extracts obtained from papaya and loquat seeds and the aqueous, ethanolic and water:ethanolic extracts produced from mango seeds were shown to possess high antioxidant activity and were therefore selected for evaluation of their activity in cooked chicken models. The ability of the selected extracts to inhibit discolouration and lipid and protein oxidation was evaluated in a cooked chicken model.
The effect of papaya, loquat, and mango extracts on the instrumental colour of cooked chicken models
The CIE L*a*b* values of the cooked chicken models during refrigerated storage are shown in Table 3. The models were characterized by high CIE L* values, moderate CIE b* values, and very low CIE a* values, resulting in a white or slightly yellowish product. The addition of extracts to cooked chicken models significantly affected the instrumental colour coordinates immediately after cooking and during refrigerated storage. The cooked chicken models did not experience marked changes in instrumental colour parameters during refrigerated storage, with no significant differences in CIE L*, a*, or b* values among days 0, 5, and 10.
The most noticeable changes occurred in the CIE L* and a* values, where the incorporation of mango seed extracts produced significant decreases in the CIE L* values and increases in the CIE a* values, resulting in darker and redder products than those of the control samples immediately after thermal treatment or after 5 and 10 days of refrigerated storage. Interactions between extract components, salt, and muscle proteins could be responsible for the darkening in samples treated with mango extracts [39]. Papaya and loquat seed extracts did not significantly change the lightness of the cooked samples, although they slightly increased the CIE a* value.
Small changes in b* were due to the use of the extracts. Only the aqueous mango seed extract produced a reduction in the CIE b* value in the cooked chicken models, compared with the control group and the other mango, papaya, and loquat seed extracts. In contrast, chicken models formulated with loquat and papaya seed extracts did not show differences in lightness (CIE L*) and redness (CIE a*) between them but did with those added with the mango seed extracts. Red‒orange carotenoids in mango extracts may be responsible for the increase in red colour in cooked chicken models before and after storage [40].
The total colour difference (ΔE) showed great variation due to the different extracts added. The addition of loquat and papaya seed extracts to cooked chicken models induced nonsignificant changes in colour (ΔE < 1.5) at any time of sampling. On the other hand, the addition of any of the mango seed extracts resulted in noticeable colour changes, as supported by the high values of ΔE (ΔE > 3.5). Colour changes in cooked models supplemented with the aqueous extract were more intense than those produced by the ethanolic or H2O:EtOH (1:1 v/v) extracts. A colour change greater than 3.5 reflects a clear difference in the colour perceptible to the consumer when products, control and treated, are compared [41].
The effect of papaya, loquat, and mango extracts on the formation of TBA-RS during the storage of cooked chicken models
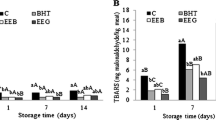
The addition of any of the selected papaya, loquat, or mango extracts to cooked chicken models significantly inhibited TBA-RS immediately after thermal treatment (0 days) and was highly effective at controlling the formation of malondialdehyde (MDA) during storage (5 days and 10 days) (Fig. 2). From day 0 to day 10 of refrigerated storage, the TBA-RS concentration increased in the control samples and in the papaya (H2O:EtOH, 1:1 v/v) and loquat seed (H2O:EtOH, 1:1 v/v) counterparts. In contrast, in cooked models in which mango seed extracts (H2O, EtOH, or H2O:EtOH, 1:1 v/v) were added, the TBA-RS values remained unchanged during refrigerated storage.
Effect of the incorporation of different extracts on lipid oxidation—measured as TBA-RS (mg MDA/kg sample)—in a cooked chicken model stored under refrigeration. N = 6 samples/batch/day of storage. ***p < .001. a, b, c: For the same day of storage, bars with different letters are significantly different (Tukey’s test, p < .05)
Papaya (H2O:EtOH, 1:1 v/v), loquat (H2O:EtOH, 1:1 v/v), and mango seed extracts (H2O, EtOH, and H2O:EtOH, 1:1 v/v) efficiently controlled lipid oxidation, although to varying degrees, by inhibiting the formation of malondialdehyde. Compared to those in the control samples, the papaya (H2O:EtOH, 1:1 v/v) and loquat (H2O:EtOH, 1:1 v/v) seed extracts in the cooked chicken models significantly reduced the TBA-RS concentration during storage. However, mango extracts showed a very high capacity to inhibit lipid peroxidation. The ability of mango seed extracts (H2O, EtOH, and H2O:EtOH, 1:1 v/v) to prevent lipid oxidation was greater than that of their papaya and loquat seed counterparts. Thus, the mango seed extract-treated models had significantly lower TBA-Rs than the models with added papaya and loquat seed extracts. No differences in TBA-RS were found among the groups treated with the different mango seed extracts.
In the treated samples, the lower TBA-Rs can be ascribed to the antioxidant activity of the phenolic compounds and the intensity of the inhibition of lipid oxidation linked to their contents in the extracts (Tables 1 and 2). The higher concentrations of phenolic compounds in the extracts resulted in greater antioxidant activity, resulting in greater inhibition of lipid peroxidation and thus lower TBA-RS values in the samples. According to Dias et al. [42], differences in phenolic profiles (type of phenolics present and their relative proportions) and synergistic or antagonistic effects among antioxidants cause the antioxidant activity of some extracts to be dependent not only on phenolic concentration but also on the structure and interactions between antioxidants.
The results agree with those reported in the scientific literature on the impact of the addition of phenolic‐rich extracts to meat and meat products on lipid oxidation; for example, samples treated with high phenolic-rich extracts maintained thiobarbituric acid‐reactive substances at basal levels and lower levels than those in control samples [3, 4, 6, 43]. The ability of phenolic compounds to protect against lipid oxidation in meat products can be attributed to both antioxidant mechanisms, free radical scavenging, and the ability to chelate metals, such as iron ions, resulting in the formation of stable complexes with heme and nonheme ions [1, 37].
The effect of papaya, loquat, and mango extracts on the formation of carbonyls during the storage of cooked chicken models
The effect of the incorporation of different extracts on carbonyls from protein oxidation in a cooked chicken model stored under refrigeration is shown in Fig. 3. The addition of mango, papaya, and loquat seed extracts affected the carbonyl content differently after cooking and during refrigerated storage. The formation of carbonyl groups is considered a general indicator of the level of protein oxidation in meat and meat products [44].
Effect of the incorporation of different extracts on protein oxidation—measured as carbonyls (nM carbonyls/mg protein)—in a cooked chicken model stored under refrigeration. n = 6 samples/batch/day of storage. ***p < .001. a, b, c: For the same day of storage, bars with different letters are significantly different (Tukey’s test, p < .05)
Immediately after heat treatment (day 0), the data did not show conclusive results on the antioxidant effect of proteins from tropical fruit extracts. An unexpected prooxidant effect on papaya- and loquat-treated models was found. In comparison with those in the control samples, the papaya and loquat extracts not only did not decrease the formation of carbonyl groups from protein oxidation but also increased but not to a significant extent the concentration of carbonyl groups in the samples on days 0, 5, and 10. In contrast, the ethanolic and aqueous: ethanolic mango seed extracts were observed to be responsible for greater control of the carbonylation of proteins in this study than was the control treatment. On days 5 and 10, the mango seed extracts inhibited the formation of carbonyl compounds by 30–54% for the ethanolic extract and 37–54% for the aqueous: ethanolic extract. The lower total carbonyl content in the mango extract group could be attributed to the intense antioxidant activity of the phenolic acids and flavonoids in these mango seed extracts, which are effective at inhibiting the formation of total protein carbonyl compounds. Several studies have reported the use of plant extracts rich in phenolic compounds to inhibit or decrease the oxidation rate of lipids and proteins [1, 3,4,5,6]. Additionally, a low concentration of reactive substances such as thiobarbituric acid could influence protein carbonylation. Protein oxidation can be induced by the presence of lipid oxidation-derived compounds, which easily oxidize protein constituents, including thiols and amino acids, leading to the generation of carbonyl compounds [44].
Conclusions
Extracts obtained from mango, papaya and loquat seeds can be used as natural antioxidants in meat products as substitutes for synthetic antioxidants. All the extracts obtained from mango seeds could be considered effective inhibitors of lipid oxidation and protein carbonylation; however, the ethanolic and aqueous extracts of mango seeds show very high antioxidant activity and high concentrations of phenolic compounds and flavonoids. Additional research is necessary to assess these extracts' effectiveness in controlling discoloration and oxidative deterioration. Moreover, their functionality in meats and meat products with higher fat and/or iron contents across various addition levels, processing methods, and storage conditions requires investigation. Additionally, studies exploring the impact of these extracts on the sensory properties of processed products should be considered.
Data availability
The data that support the findings of this study are openly available in Mendeley Data at.
References
Falowo AB, Fayemi PO, Muchenje V (2014) Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: a review. Food Res Int 64:171–181. https://doi.org/10.1016/j.foodres.2014.06.022
Jiang J, Xiong YL (2016) Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: a review. Meat Sci 120:107–117. https://doi.org/10.1016/j.meatsci.2016.04.005
Lavado G, Ladero L, Cava R (2021) Cork oak (Quercus suber L.) leaf extracts potential use as natural antioxidants in cooked meat. Ind Crops Prod 160:113086. https://doi.org/10.1016/j.indcrop.2020.113086
Munekata PES, Gullón B, Pateiro M et al (2020) Natural antioxidants from seeds and their application in meat products. Antioxidants 9:815. https://doi.org/10.3390/antiox9090815
Ribeiro JS, Santos MJMC, Silva LKR et al (2019) Natural antioxidants used in meat products: a brief review. Meat Sci 148:181–188. https://doi.org/10.1016/j.meatsci.2018.10.016
Ahmad SR, Gokulakrishnan P, Giriprasad R, Yatoo MA (2015) Fruit-based natural antioxidants in meat and meat products: a review. Crit Rev Food Sci Nutr 55:1503–1513. https://doi.org/10.1080/10408398.2012.701674
Aruoma OI (2003) Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat Res - Fundam Mol Mech Mutagen 523–524:9–20. https://doi.org/10.1016/S0027-5107(02)00317-2
Zhang YJ, Gan RY, Li S et al (2015) Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 20:21138–21156. https://doi.org/10.3390/molecules201219753
Jideani AIO, Silungwe H, Takalani T et al (2021) Antioxidant-rich natural fruit and vegetable products and human health. Int J Food Prop 24:41–67. https://doi.org/10.1080/10942912.2020.1866597
Villacís-Chiriboga J, Elst K, Van Camp J et al (2020) Valorization of byproducts from tropical fruits: extraction methodologies, applications, environmental, and economic assessment: a review (Part 1: general overview of the byproducts, traditional biorefinery practices, and possible applications). Compr Rev Food Sci Food Saf 19:405–447. https://doi.org/10.1111/1541-4337.12542
Gorinstein S, Poovarodom S, Leontowicz H et al (2011) Antioxidant properties and bioactive constituents of some rare exotic Thai fruits and comparison with conventional fruits. In vitro and in vivo studies. Food Res Int 44:2222–2232. https://doi.org/10.1016/j.foodres.2010.10.009
Fan R, Yuan F, Wang N et al (2015) Extraction and analysis of antioxidant compounds from the residues of Asparagus officinalis L. J Food Sci Technol 52:2690–2700. https://doi.org/10.1007/s13197-014-1360-4
Muhamad II, Hassan ND, Mamat SNH, et al (2017) Extraction technologies and solvents of phytocompounds from plant materials : physicochemical characterization and identification of ingredients and bioactive compounds from plant extract using various instrumentations. Elsevier Inc.
Medina MB (2011) Determination of the total phenolics in juices and superfruits by a novel chemical method. J Funct Foods 3:79–87. https://doi.org/10.1016/j.jff.2011.02.007
Zhishen J, Mengcheng T, Jianming W (1999) The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem 64:555–559. https://doi.org/10.1016/S0308-8146(98)00102-2
Waterman PG, Mole S (1994) Methods in ecology: analysis of phenolic plant metabolites. 74–93
Re R, Pellegrini N, Proteggente A et al (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci Technol 28:25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Benzie I, Strain J (1996) The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant power”: the FRAP assay analytical biochemistry. Anal Biochem 239:70–76
AMSA (2012) Meat Color Measurement Guidelines. Champaign, Illinois, USA
Salih AM, Smith DM, Price JF, Dawson LE (1987) Modified extraction 2-thiobarbituric acid method for measuring lipid oxidation in poultry. Poult Sci 66:1483–1488. https://doi.org/10.3382/ps.0661483
Oliver CN, Ahn BW, Moerman EJ et al (1987) Age-related changes in oxidized proteins. J Biol Chem 262:5488–5491. https://doi.org/10.1016/S0021-9258(18)45598-6
Agatonovic-Kustrin S, Kustrin E, Morton DW (2018) Phenolic acids contribution to antioxidant activities and comparative assessment of phenolic content in mango pulp and peel. South African J Bot 116:158–163. https://doi.org/10.1016/j.sajb.2018.03.013
da Silva Sauthier MC, da Silva EGP, da Silva Santos BR et al (2019) Screening of Mangifera indica L. functional content using PCA and neural networks (ANN). Food Chem 273:115–123. https://doi.org/10.1016/j.foodchem.2018.01.129
Ballesteros-Vivas D, Álvarez-Rivera G, Morantes SJ et al (2019) An integrated approach for the valorization of mango seed kernel: efficient extraction solvent selection, phytochemical profiling and antiproliferative activity assessment. Food Res Int 126:108616. https://doi.org/10.1016/j.foodres.2019.108616
Alara OR, Abdurahman NH, Ukaegbu CI (2021) Extraction of phenolic compounds: a review. Curr Res Food Sci 4:200–214. https://doi.org/10.1016/j.crfs.2021.03.011
Torres-León C, Rojas R, Contreras-Esquivel JC et al (2016) Mango seed: functional and nutritional properties. Trends Food Sci Technol 55:109–117. https://doi.org/10.1016/j.tifs.2016.06.009
Fidelis M, De Moura C, Kabbas T et al (2019) Fruit seeds as sources of bioactive compounds: sustainable production of high value-added ingredients from by-products within circular economy. Molecules 24:1–54. https://doi.org/10.3390/molecules24213854
Munteanu IG, Apetrei C (2021) Analytical methods used in determining antioxidant activity: a review. Int J Mol Sci. https://doi.org/10.3390/ijms22073380
Yang DJ, Chang YZ, Chen YC et al (2012) Antioxidant effect and active components of litchi (Litchi chinensis Sonn.) flower. Food Chem Toxicol 50:3056–3061. https://doi.org/10.1016/j.fct.2012.06.011
Masibo M, Qian H (2008) Major mango polyphenols and their potential significance to human health. Compr Rev Food Sci Food Saf 7:309–319. https://doi.org/10.1111/j.1541-4337.2008.00047.x
Liu Y, Zhang W, Xu C, Li X (2016) Biological activities of extracts from Loquat (Eriobotrya japonica Lindl).: a review. Int J Mol Sci. https://doi.org/10.3390/ijms17121983
Zhang W, Zhao X, Sun C et al (2015) Phenolic composition from different loquat (Eriobotrya japonica Lindl.) cultivars grown in China and their antioxidant properties. Molecules 20:542–555. https://doi.org/10.3390/molecules20010542
Gonçalves Rodrigues LG, Mazzutti S, Vitali L et al (2019) Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal Agric Biotechnol 22:101367. https://doi.org/10.1016/j.bcab.2019.101367
Galanakis CM, Tsatalas P, Galanakis IM (2018) Implementation of phenols recovered from olive mill wastewater as UV booster in cosmetics. Ind Crops Prod 111:30–37. https://doi.org/10.1016/j.indcrop.2017.09.058
Ge L, Li S-P, Lisak G (2020) Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J Pharm Biomed Anal 179:112913. https://doi.org/10.1016/j.jpba.2019.112913
Rice-Evans CA, Miller NJ, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Barbehenn RV, Jones CP, Karonen M, Salminen J-P (2006) Tannin composition affects the oxidative activities of tree leaves. J Chem Ecol 32:2235–2251. https://doi.org/10.1007/s10886-006-9142-8
Devatkal SK, Kumboj R, Paul D (2014) Comparative antioxidant effect of BHT and water extracts of banana and sapodilla peels in raw poultry meat. J Food Sci Technol 51:387–391. https://doi.org/10.1007/s13197-011-0508-8
Baria B, Upadhyay N, Singh AK, Malhotra RK (2019) Optimization of ‘green’ extraction of carotenoids from mango pulp using split plot design and its characterization. Lwt 104:186–194. https://doi.org/10.1016/j.lwt.2019.01.044
Mokrzycki WS, Tatol M (2011) Color difference delta E - a survey. Mach Graph Vis 20:383–411
Dias JL, Mazzutti S, de Souza JAL et al (2019) Extraction of umbu (Spondias tuberosa) seed oil using CO2, ultrasound and conventional methods: evaluations of composition profiles and antioxidant activities. J Supercrit Fluids 145:10–18. https://doi.org/10.1016/j.supflu.2018.11.011
Dorta E, Lobo MG, Gonzalez M (2012) Reutilization of mango byproducts: study of the effect of extraction solvent and temperature on their antioxidant properties. J Food Sci 77:80–89. https://doi.org/10.1111/j.1750-3841.2011.02477.x
Guyon C, Meynier A, de Lamballerie M (2016) Protein and lipid oxidation in meat: a review with emphasis on high-pressure treatments. Trends Food Sci Technol 50:131–143. https://doi.org/10.1016/j.tifs.2016.01.026
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.C.; methodology, R.C.; formal analysis, L.L.; investigation, L.L.; resources, R.C. and L.L.; data curation, R.C.; writing—original draft preparation, R.C.; writing—review and editing, R.C. and L.L.; supervision, R.C.; project administration, R.C.; funding acquisition, R.C. All the authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
The study does not involve research on human participants and/or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cava, R., Ladero, L. Using polyphenol-rich extracts from tropical fruit byproducts to control lipid and protein oxidation in cooked chicken models. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04577-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04577-x