Abstract
Polymethoxyflavones (PMFs) occur naturally in citrus peels and citrus-derived foods as well as in other plants. Many in vitro and some in vivo studies have shown potentially relevant biological effects of PMFs, including anticancer, anti-inflammatory, anti-atherosclerosis, and neuroprotective activities. These promising biological effects still require further research to establish their impact on human health. This review updates the current clinical trials data. It highlights the limited information available on the bioavailability and metabolism of PMFs (pharmacokinetics, human phase I and II metabolites in biological fluids and tissues, and gut microbiota metabolism).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Citrus species rank among the globally predominant cultivated plants, with an annual production of approximately 140 million tons. Cultivated in over 100 countries, primarily in tropical and subtropical regions, these fruits are consumed fresh, transformed into juices, and utilized as ingredients in various culinary creations and beverages. Additionally, a myriad of supplements and functional foods have been developed using citrus or citrus-derived products [1]. Citrus products are rich in phenolic compounds, mainly flavanones and polymethoxyflavones (PMFs), but also contain C-glycosyl flavones, O-glycosyl flavones, hydroxycinnamic acid derivatives, coumarins, and psoralens. Terpenoids, such as essential oils, limonoids and carotenoids, and nitrogen-containing compounds such as synephrine are also characteristic of citrus fruits [2].
Flavonoids play a key role in conferring a diverse range of health benefits [3]. Among these, polymethoxylated flavones (PMFs), a specific group of flavonoids found in citrus fruits, have been highlighted for their potential positive impacts on health. These effects include antioxidative, anticancer, anti-inflammatory, and disease-preventive activities, particularly in relation to neurodegenerative diseases [4]. These effects have been mainly evidenced through in vitro assays and in some cases using preclinical studies with animal models. However, the understanding of PMF metabolism in humans and their direct effects on human health remains limited. The main sources of information on these health effects in humans are derived from two recent clinical trials [5, 6], an observational study [7], and a pilot study [8]. However, no data on bioavailability and metabolism (pharmacokinetics, human phase I and II metabolism in biological fluids and tissues, gut microbiota metabolism) were reported. This review discusses the available research results related to the occurrence of these flavonoids in the diet, and their potential for human health considering their bioavailability and metabolic fate in humans.
Chemical structure
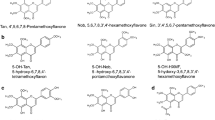
Citrus PMFs are fully methoxylated flavone aglycones with different oxygenation patterns going from two to seven methoxylations (Fig. 1, Table 1). Thirty-five PMFs have been identified from citrus species so far [9], not counting hydroxylated PMFs. Among them, nobiletin (5,6,7,8,3’,4’-hexamethoxyflavone, 30, Table 1) and tangeretin (5,6,7,8,4’-pentamethoxyflavone, 22, Table 1) are the most abundant. Other less distributed PMFs are 5,6,7,4’-tetramethoxyflavone, sinensetin (5,6,7,3’,4’-pentamethoxyflavone, 23, Table 1), isosinensetin (5,7,8,3’,4’-pentamethoxyflavone 24, Table 1), 3,5,6,7,3’,4’-hexamethoxyflavone (28, Table 1), and 3,5,6,7,8,3’,4’-heptamethoxyflavone (35, Table 1) [9]. Moreover, demethylated PMFs in which one or several methyl residues from the methoxy groups are removed, leading to the corresponding free hydroxyl groups, have also been described in some citrus fruits or in citrus-derived medicinal products. The nomenclature of these demethylated derivatives of PMFs is often difficult and can lead to confusion. The most accepted names are those based on the common name in which the demethylation is shown. Thus, 5-desmethyl nobiletin and 5-hydroxy-6,7,8,3’,4’-pentamethoxyflavone are both acceptable names that do not lead to confusion. The major citrus desmethyl PMFs identified to date are produced from PMFs in aged and long-term stored citrus fruits and peels [9].
Occurrence in citrus fruit. Differences among citrus species and different cultivars
PMFs are distributed mainly in the peels, where they are components of the essential oil fraction (oil glands), while little is present in the pulp. The two most abundant PMFs in citrus peels are nobiletin and tangeretin, commonly detected in the Citrus genus, specifically, mandarins (Citrus reticulata), and sweet oranges (Citrus sinensis) [10, 11]. Depending on the Citrus species and their degree of maturity, a significant difference can be found in the content and type of PMFs in the pericarp. Moreover, tetramethyl-isoscutellarein (5,7,8,4’-tetramethoxyflavone, 15, Table 1) is also a newly identified citrus PMF that occurs in the pericarp of immature Shiranui fruit (Citrus unshiu x sinensis) [11, 12]. This fact revealed that the ripeness of citrus fruit might primarily change the PMF composition of the peel [12, 13]. Some specific types of citrus fruits growing abundantly in Asia (China, Japan, and Korea) are responsible for the diversity of citrus PMFs. For example, sudachitin (5,7,4’-trihydroxy,6,8,3’-trimethoxyflavone) is mainly found in Citrus sudachi, native to Tokushima Prefecture in Japan [12,13,14,15].
Citrus essential oils were also studied for their content in PMFs, particularly those obtained by cold press methodology [16]. Sweet orange (Citrus sinensis (L.) Osbek) essential oil contains six PMFs: sinensetin, hexamethoxyflavone, nobiletin, tetra-O-methyl-scutellarein, heptamethoxyflavone, and tangeretin. Similarly, mandarin essential oil is characterized by several PMFs, being tangeretin the most abundant one. Still, sinensetin, hexamethoxyflavone, and tetra-O-methyl-scutellarein are present in trace quantities [15]. Bitter orange essential oil contains nobiletin, tetra-O-methyl-scutellarein, heptamethoxyflavone, and tangeretin. Clementine essential oil showed a qualitative profile in PMFs like that of sweet orange essential oil and is characterized by the presence of heptamethoxyflavone [15].
Identification and quantification of PMFs in citrus fruits and processed juices
PMFs are mainly present in processed citrus juices [16, 17]. However, hand-squeezed juice samples also have them, although in low concentrations [16, 17]. Thus, the PMFs of three juice samples (conventional thermal processing, high pressure pasteurization, and hand-squeezed) were evaluated by UPLC-Single Quadrupole and HPLC–DAD-IonTrap-MSn in the positive mode. The HPLC–MS analyses showed a similar PMF profile in the three juices although with different total contents. Two tetramethoxyflavones [5,6,7,4′-tetramethoxyflavone (14) and 5,7,8,4′-tetramethoxyflavone (15)], three pentamethoxyflavones [5,6,7,8,4′-pentamethoxyflavone (tangeretin) (22), 5,6,7,3′,4′-pentamethoxyflavone (sinensetin) (23), and 5,7,8,3′,4′-pentamethoxyflavone (isosinensetin) (24)] and two hexamethoxyflavones [3,5,6,7,3′,4′-hexamethoxyflavone (28) and 5,6,7,8,3′,4′-hexamethoxyflavone (nobiletin) (31)] were detected, as reported in a previous study [16,17,18]. The content of PMFs in the juices was assessed by DAD (diode array detection) and quantification against authentic tangeretin and nobiletin standards. The content was significant (1 − 9 μg/mL) although lower than the juices’ flavanone content (200 − 450 μg/mL). These values are similar to those previously reported in orange juice samples [16,17,18]. When demethylated PMFs (hydroxy-trimethoxyflavone, hydroxy-tetramethoxyflavone, and hydroxy-pentamethoxyflavone) were searched in the juices using UPLC QTOF (extracted ion chromatograms), these metabolites were not detected. Table 2 summarizes the differences in PMFs in the juice among different citrus species and cultivars.
Biosynthesis and transformation
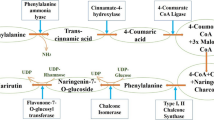
As far as we know, no study has thoroughly verified PMFs biosynthesis so far. The flavonoid biosynthesis location is in the cytoplasm of the flavedo cells and then they are transferred to the vacuoles. Citrus fruits are a rich source of O-methylated flavonoids and PMFs. A previous study showed that five O-methyl transferase (OMT) genes (Citrus depressa from OMT 1, 3, 4, 5, and 6) isolated from Citrus depressa promoted the accumulation of nobiletin in the flavedo [12]. Therefore, PMFs might be synthesized from flavone aglycones by OMTs. Another potential biosynthesis pathway is the methoxylation of flavonoids with the catalysis of methyl transferase [12].
The demethylation is identified as the common metabolic biotransformation pathway of PMFs in vivo. Many studies have shown that some metabolites of PMFs, mainly demethylated PMFs, have better biological activities [12, 13]. Among demethylated PMFs, 5-desmethylnobiletin and 5-desmethyltangeretin are the two most common and abundant hydroxylated PMFs in citrus fruits [18].
The major animal and human metabolites of nobiletin are 3’-desmethylnobiletin, 4’-desmethylnobiletin, and 3’,4’-bidesmethylnobiletin while 4’-desmethyltangeretin, 6–4’-dihydroxy-5,7,8-trimethoxyflavone and 3’-4’-dihydroxy-5,6,7,8-tetramethoxyflavone are the major metabolites for tangeretin, which indicate that 3’- and 4’-positions of the B ring of PMFs are the major biotransformation sites [17].
Similarly, Blautia sp. showed both demethylation and deglycosylation activities against PMF and flavanones, respectively, and ultimately yielded their parent aglycones or corresponding demethylated flavones. Nobiletin could also be demethylated by Aspergillus niger to give 4’-hydroxy-5,6,7,8,3’-pentamethoxyflavone that exhibited a more robust anti-mutagenic activity [12]. Sinensetin and tangeretin are major PMFs found in tangerine and other citrus peels and were biotransformed by A. niger to yield 4’-hydroxy-5,6,7,3’-tetramethoxyflavone and 4’-desmethyl tangeretin with high yield [12]. It was evident that the microbial demethylation of PMFs abundant in citrus occurs for most structures at the C-3’ and C-4’ methoxy groups of the B ring. In general, all the O-demethylated biotransformed products exhibited higher biological activity and yield compared to their fully methylated flavonoids [12].
Extraction and isolation of PMFs from other sources
To maximize the yield of PMFs extracted from citrus peel, several extraction methods have been reported in the literature [3]. Recommended methods include (1) chemical methods, such as hot water extraction, solvent extraction, and alkaline extraction, and (2) advanced methods, such as ultrasound-assisted extraction, supercritical fluid extraction, microwave-assisted extraction, and enzyme-assisted extraction [3].
The following hydroxylated PMFs have been isolated from sweet oranges (Citrus sinensis): salvigenin (5-hydroxy-6,7,4′-trimethoxyflavone), gardenin B or 5-desmethyl tangeretin (5-hydroxy-6,7,8,4′-tetramethoxyflavone), 3’-hydroxy-5,6,7,4′-tetramethoxyflavone, 3-hydroxytangeretin (3-hydroxy-5,6,7,8,4′-pentamethoxyflavone), 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone, retusin or 3,3′,4′,7-tetramethyl quercetin (5-hydroxy- 3,7,3′,4′-tetramethoxyflavone), 5-hydroxy-3,7,8,3′,4′-pentamethoxyflavone, and 5-hydroxy-nobiletin [19].
The majority of hydroxylated PMFs have been isolated from the citrus plants, but some of them are isolated from other sources mainly from Lamiaceae and Asteraceae. The demethylated derivative of heptamethoxyflavone, 5,3’-dihydroxy-3,6,7,8,4’-pentamethoxyflavone, has been found in Zieridium pseudo-obtusifolium, Acronychia porter, Polanisia dodecandra, and the leaves of the Thai plant Gardenia obtusifolia. Also, 5-desmethyltangeretin has been found in Calamintha ashei and herbal medicines based on bitter orange, Fructus aurantii and Fructus aurantii immaturus [19]. The hydroxylated derivative of sinensetin, 5-hydroxy-6,7,3’,4’-tetramethoxyflavone, is present in Thymus satureioides, Orthosiphon stamineus, and the flowers of Citrus aurantium L. var. amara Engl and Artemisia amygdalina Decne. Another derivative of sinensetin, 3’-hydroxy-5,6,7,4’-tetramethoxyflavone, was isolated from the leaves of the traditional herb Orthosiphon stamineus [19]. The PMFs 5,7,3′,4′,5′-pentamethoxyflavone, 5,7,8,3′,4′,5′-hexamethoxyflavone, 3,5,7,3′,4′,5′-hexamethoxyflavone, and 3,5,7,8,3′,4′,5′-heptamethoxyflavone were isolated from the leaves of Murraya paniculata (Rutaceae). 5,7,4′-Trimethoxyflavone and 5,7,3′,4′-tetramethoxyflavone were isolated from Kaempferia parviflora (Zingiberaceae), and 5,7-dimethoxyflavone, 4′,5,7-trimethoxyflavone, and 3′,4′,5,7-tetramethoxyflavone were isolated from the aerial parts of Piper porphyrophyllum [19].
Bioavailability and metabolism in humans (pharmacokinetics, human metabolism, gut microbiota metabolism)
To the best of our knowledge, only one human clinical trial has been conducted to investigate the health benefits and safety of a functional food including a nobiletin-rich extract from C. depressa peel in healthy elderly subjects [5]. The scores of “general memory” or “visual memory” in the indices of WMS-R (Wechsler Memory Scale-Revised) were significantly higher in the nobiletin-containing test food group than in the placebo group, indicating that the tested food was beneficial for improving memory dysfunction in healthy elderly subjects. However, no direct correlation between nobiletin and the observed effects was demonstrated.
Furthermore, another human clinical trial [6], the NIRVANA study, evaluated the effect of a nutraceutical preparation on lipid profile, endothelial function, and oxidative stress. Each capsule consisted of red yeast rice containing monacolin K, PMFs from a tangerine extract (mainly nobiletin and tangeretin), hydroxytyrosol from an olive fruit extract, phenolic acids, and flavonoids from an Ipomoea batatas extract, vitamin E and coenzyme Q10. However, this clinical study might not be considered as an effect of PMFs on human health as they are constituents of a mixture of nutrients in which they are at a very low concentration among other compounds and therefore the beneficial effect might be due to other bioactives or the synergy among all of them.
An observational study [7] was performed to evaluate the effectiveness of “Yokukansankachimpihange”, a formulation combined with nobiletin-rich Citrus reticulata and donepezil on improving the behavioral and psychological symptoms of dementia (BPSD). A total of 46 patients with dementia were selected for the study and grouped in the “Yokukansankachimpihange” group (23 patients) and donepezil group (23 patients). The Frequency-Weighted Behavioral Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD-FW) was used to evaluate the BPSD, while the Mini-Mental State Examination and the Digit Symbol test of WAIS-R (Wechsler Adults Intelligence Scale-Revised) were used to evaluate impairment of global cognitive and executive function. The study reported a positive clinical effect on improving behavioral abnormalities of the combined donepezil “Yokukansankachimpihange” group and no effect was observed on cognitive functions [7].
Finally, a pilot clinical study was performed to examine the safety and feasibility of a nobiletin-rich Citrus reticulata (NChinpi) co-administration with donepezil in donepezil-pre-administered patients [8]. A total of six patients with mild to moderate AD were selected for the NChinpi treatment group and five patients were selected for the donepezil treatment group. The treatment was continued for 1 year and after 1 year, the baseline cognitive assessment was assessed with Mini-Mental State Examination and Japanese Version of AD assessment Cognitive Subscale. After 1 year of treatment, there were no significant changes in the NChinpi-treated group as compared to the baseline, however, the donepezil-administered group showed cognition impairment. NChinpi was also found to be safe, with no adverse effects and digestive symptoms [8].
However, none of the above studies have described any information on the bioavailability and metabolism of PMFs in humans. As far as we know, there are still no human clinical trials describing the main metabolites present in circulation, distribution in tissues or biological fluids, and their respective concentrations. There is a need for detailed clinical studies, to understand factors related to PMF pharmacokinetics, to bring such results to human clinical practice.
A recent study has been the first to describe the bioavailability and metabolism of citrus juice PMFs in humans [16]. Thus, three isomers of hydroxy-tetramethoxyflavone sulfate and two isomers of hydroxy-pentamethoxyflavone sulfate were found, as well as only one isomer of hydroxy-trimethoxyflavone sulfate and two isomers of hydroxy-pentamethoxyflavone glucuronide after the intake of citrus juices manufactured by different processing technologies. Remarkably, in demethylated PMFs, sulfates were the main phase II metabolites, while glucuronides were only minor ones. The PMF conjugates present in urine reached around 1 μM concentration. Also, an interindividual variability in the excretion of demethylated PMFs was described [16].
Health-promoting effects of PMFs (In vitro human cell cultures and in vivo animal models). Evidence of the health effects
In vitro human cell cultures
Typically, demethylated metabolites show different, even more potent bioactivities than the parent PMFs [21,22,23,24,25]. Various studies have demonstrated that nobiletin and its demethylated metabolites exhibit anti-inflammatory effects in the order 3’-desmethylnobiletin > 3’,4’ -desmethylnobiletin > 4’-desmethylnobiletin > nobiletin [20,21,22].
Among their health-promoting properties, the anticancer activities of PMFs have been extensively studied [20, 21, 23,24,25,26,27,28]. Most commonly, they can induce cell cycle arrest at different phases (G0/G1, G1, G1/S, and G2/M) and apoptosis [23, 29,30,31]. Further investigations have demonstrated that they are associated with the regulation of some key signal kinases, including the up-regulation of p53/p21 and down-regulation of cyclin D1, cyclin-dependent kinase 2 (CDK2), cyclin-dependent kinase 4 (CDK4), and mitogen-activated protein kinase (MAPK) (Table 3).
The health-promoting functions (antioxidant, anti-inflammatory, anti-atherosclerotic, and anti-diabetic effects) of PMFs as well as the corresponding mechanisms have been studied extensively in recent years (Table 3). PMFs have been shown to inhibit reactive oxygen species (ROS) production and improve the activity of antioxidant enzymes, including superoxide dismutase, catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) [33,34,35,36]. Some hydroxylated PMFs, such as 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone and 5-demethylnobelitin, exhibit 2,2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity at levels more potent than those of nobiletin [39]. This activity might be attributable to the hydrogen-donating ability of the demethylated derivatives (hydroxylated PMFs). The anti-inflammatory mechanisms of PMFs include inhibition of the production of inflammatory mediators (e.g., cyclooxygenase-2 COX-2, inducible nitric oxide synthase iNOS, interleukin 6 IL-6, interleukin IL-1α, interleukin IL-1β, and tumor necrosis factor alpha TNF-α) and prostaglandin E2 (PGE2) [22, 38,39,40] suppression of the enzymes involved in mitogen-activated protein kinase (MAPK) pathways (e.g., matrix metalloproteinase proMMP-1, proMMP-3, and MMPs) [44], and regulation of the nuclear factor-κB (NF-ƙB) level (Table 3) [38].
Many other mechanisms, such as the reduction of IL-1, IL-6, iNOS, and COX-2 levels [41], activation or up-regulation of caspase-3, miR-410 p21, p27, p53, and Bax [28, 42], inhibition or down-regulation of MMP-1, MMP-7, MMP-9, and NF-ƙB [44], have also been identified. The anti-atherosclerotic effects of citrus PMFs have also been reported widely. The mechanisms are associated with the inhibition of platelet-derived growth factor, angiotensin II, and platelet aggregation, suppression of the expression of di-acyl-glycerol acyltransferase, lectin-like oxidized low-density lipoprotein receptor-1 LOX-1, platelet membrane CD36 and scavenger receptor class A (SR-A), reduction of very-low-density lipoprotein (VLDL)-triglyceride secretion, and inhibition of SR-A mRNA expression and ox-LDL uptake (Table 3) [44, 45]. In addition, PMFs have excellent anti-diabetic effects through the improvement of hyperglycemia and insulin resistance, stimulation of glucose uptake by the regulation of activated protein kinase (AMPK) signaling pathways [46], regulation of glucose transporter protein type 1 (Glut1), Glut4, and adipokines [47], reduction of enzymes involved in carbohydrate metabolism to normal levels [48], impairment of lipid homeostasis by activation of MAPK, extracellular signal-regulated kinase (ERK) signaling [39], and reduction of di-acyl-glycerol acyltransferase DGAT1/2 mRNA expression (Table 3) [49].
Although the in vitro results have been described as promising, most of the reported concentrations of nobiletin evaluated (> 20 µM) were not achievable in physiological conditions as demonstrated by in vivo pharmacokinetic studies of nobiletin [50]. Comparing the high experimental levels used against the relatively low peak plasma concentration (4.4 µM) after one hour of oral administration of 50 mg/kg nobiletin and the rapid elimination from the body points out a limitation to suggesting nobiletin in its unaltered natural form as a human health-promoting compound [50].
Preclinical studies (animal models)
This review in addition to reporting data on in vitro models, reports health-promoting functions of in vivo preclinical studies using different animal models.
Anti-cancer
PMFs in citrus peels may produce much stronger active anticancer compounds through biotransformation [50]. PMFs and hydroxylated PMFs show synergistic effects with an anticancer drug. Although some studies show hydroxylated PMFs, acetylated-PMF, and PMF metabolites have better anticancer activity in specific cancer type; the comparison of anticancer ability among them needs more research [51]. 5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone is an effective antitumor agent and its inhibitory effect is through the down-regulation of inflammatory iNOS and COX-2 gene expression in mouse skin [39]. A study reported that 3,5,6,7,8,3′,4′-heptamethoxyflavone (35, Table 1) not only significantly suppressed the tumor necrosis factor (TNF-α) response in LPS-treated mice, but also rendered adverse effects on macrophage inflammatory protein 1α (MIP-1α) and IL-10 expression, although no impact was found toward cytokines IL-6, IL-8 and IL-1β [44, 45]. Three metabolites, 5,3’-didesmethylnobiletin, 5,4’-didesmethylnobiletin, and 5,3’,4’-tridesmethylnobiletin, were identified in mouse urine after oral administration of 5-desmethylnobiletin. All of them displayed stronger cytotoxic effects than 5-desmethylnobiletin on human colon cancer cells, indicating the biotransformation of 5-desmethylnobiletin and its beneficial effects in vivo [52, 53].
An orange peel extract (OPE) on intestinal tumor growth in ApcMin/ + mice was studied. The OPE contained 30% PMFs, a mixture that included tangeretin (19.0%), heptamethoxyflavone (15.24%), tetramethoxyflavone (13.6%), nobiletin (12.49%), hexamethoxyflavone (11.06%), and sinensetin (9.16%). After 9 weeks of feeding a new Western-style diet (NWD) to the ApcMin/ + mice, tumors increased, mainly in the colon. After feeding 0.5% OPE in NWD, the development of tumors markedly decreased, by 49% in the small intestine and 38% in the colon [21].
Anti-inflammation
Various studies, including in vivo and in vitro models, have demonstrated nobiletin and its demethylated metabolites exhibit anti-inflammatory potency in the order of 3’- desmethylnobiletin > 3’,4’-desmethylnobiletin > 4’-desmethylnobiletin > nobiletin [23]. The anti-inflammatory effects of nobiletin metabolites were compared in murine macrophages. The major nobiletin metabolite of mouse urine is identified as 4′-desmethylnobiletin, whereas 3′-desmethylnobiletin is a minor metabolite [19].
In a lipopolysaccharide (LPS)-induced inflammatory response on a mouse macrophage model, nobiletin and its metabolites 3’-desmethylnobiletin, 4’-desmethylnobiletin, and 3’,4’-didesmethylnobiletin moderately attenuated iNOS and COX-2 gene expression [19] and suppressed the activation of activator protein 1 (AP-1), NF-κB, and cyclic amp-response element binding protein (CREB) [44]. Moreover, 3′,4′-didesmethylnobiletin was examined for its anti-inflammatory effects and significantly inhibited 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced mouse skin inflammation by decreasing inflammatory parameters [54]. 5-Desmethylnobiletin also showed anti-inflammatory activity in TPA-induced ear edema and acute mouse paw edema induced by carrageenan and phospholipase A2 (PLA2). 5-Hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone significantly inhibited TPA-induced mouse skin inflammation by decreasing inflammatory parameters. Its inhibitory effect is through the down-regulation of inflammatory iNOS and COX-2 gene expression in mouse skin, suggesting that it is a novel functional agent capable of preventing inflammation-associated tumorigenesis [39].
The effects of 3,5,6,7,8,3′,4′-heptamethoxyflavone (35, Table 1) on inflammation in the brain in vivo using mice injected intra-hippocampally with lipopolysaccharide were investigated. This study demonstrated that subcutaneously injected 3,5,6,7,8,3′,4′-heptamethoxyflavone (35, Table 1) suppressed: LPS-induced losses in body weight, LPS-induced microglial activation in the hippocampus, and LPS-induced interleukin-1β mRNA expression in the hippocampus, suggesting that 3,5,6,7,8,3′,4′-heptamethoxyflavone can reduce neuro-inflammation in the brain [55].
Anti-atherosclerosis
The anti-atherosclerotic effects of citrus PMFs have also been reported widely [56]. The mechanisms are associated with the inhibition of platelet-derived growth factor, angiotensin II, and platelet aggregation, suppression of the expression of di-acyl-glycerol acyltransferase, LOX-1, CD36, SR-A, and scavenger receptor, reduction of very-low-density lipoprotein (VLDL)-triglyceride secretion, and inhibition of SR-A mRNA expression and ox-LDL uptake [52, 53]. PMF food supplementation may ameliorate hypertriacylglyceridemia and its anti-diabetic effects in hamsters through adipocytokine regulation and peroxisome proliferators activated receptor-a (PPARa) and PPARc activation [56].
Metabolic syndrome
PMFs showed positive effects in controlling metabolic syndrome, including dyslipidemia, insulin resistance, obesity, and cardiovascular diseases.
Regulation of lipid metabolism and dyslipidemia
PMFs extract from citrus peels decreased the total cholesterol (TC), triglycerides, low-density lipoprotein (LDL), and VLDL in hamsters with hypercholesterolemia or insulin resistance [57]. Also, an effective modulation of lipid profile in vivo was observed for nobiletin [49], tangeretin [58], heptamethoxyflavone [59], and sudachitin [60].
Regulation of glucose metabolism and insulin resistance (IR)
PMFs have been shown to improve glucose homeostasis in different animal models. A treatment of PMFs (62.5- and 125 mg/kg) for 4 weeks can decrease the serum insulin level in high-fructose diet (HFD) induced IR hamsters [65]. Nobiletin is reported to attenuate hyperglycemia and insulin resistance in HFD-induced obese mice, obese diabetic ob/ob mice, and HFD-fed LDLR-deficient mice [49, 61].
Anti-obesity
Several in vivo studies show that hydroxylated PMFs reduce adipose mass in diet-induced obese mice [62]. The PMFs extract/mixture with a higher concentration of hydroxyl PMFs has a better anti-obesity effect [63, 64]. Hydroxylated PMFs decrease adipogenesis by down-regulating several transcription factors peroxisome proliferator-activated receptor and sterol-regulatory-element-binding protein 1c (PPAR and SREBP-1c) and activating adenosine monophosphate-activated protein kinase (AMPK) signaling in 3T3-L1 adipocytes. Intake of hydroxylated PMFs by mice reduces high-fat diet-induced weight gain and adiposity, suggesting the potential of hydroxylated PMFs for the prevention and treatment of obesity [63].
Regulation of cardiovascular diseases
The treatment of nobiletin (0.3% in diets) for 12 weeks effectively reduced the aortic cholesterol accumulation and plaque macrophage content [65]. Tangeretin decreased the blood pressure in the hypertensive rats [66]. PMFs showed a protective effect against trimethylamine N-oxide (TMAO) in animals treated with choline chloride and L-carnitine in the diets. Nobiletin is found to reduce cardiovascular inflammation by lowering the systemic oxidative stress and pro-inflammatory cytokines in choline chloride-treated rats.
Conclusions and future perspectives
Bioactivities of PMFs in vitro and in vivo for the benefits of human health include to regulate differentiation, proliferation, angiogenesis, and metabolism through acting on modulation of signaling cascades, gene transcription, and protein function and enzyme activity. However, there are limitations to be solved. Great differences in dosage and administration approach of citrus PMFs. Thus, intraperitoneal injection with nobiletin could significantly restore ischemic stroke, while it exerts no efficacy when administrated orally. Also, the dosage of PMFs, which ranged from 50 mg/kg to 200 mg/kg, are not realistic and too high for clinical therapy. Also, the systematic comparison of the activities of various individual PMF in the same biological experimental system will clarify the influence of the position and numbers of hydroxyl groups and methoxyl groups on the bioactivity. The synergic effects among different PMFs should also be explored since the pharmacological actions of citrus extracts are not the simple sum of various components. Furthermore, most of the bioactivities mentioned above are only conducted on animal models. Additional studies in healthy volunteers are needed to determine efficacy of these potential PMFs. A huge gap is found in google scholar when the keywords “polymethoxyflavones and human clinical trial” or “bioavailability and metabolism of PMF in humans” are used. Yes, indeed, there is no information about these keywords. It is therefore a great opportunity to set up a clinical study in human with dietary supplements based on PMFs since any result will be relevant for being the first. There remains a need for detailed factors related to pharmacokinetics and the main metabolites present in circulation, tissues or biological fluids and their respective concentrations to bring such results to human health-promoting. Therefore, the future perspectives are very challenging.
Data availability
The authors do not have permission to share data.
References
Ballistreri, G., Fabroni, S., Romeo, F.V., Timpanaro, N., Amenta, M., Rapisarda, P., 2019. Chapter 13 - Anthocyanins and Other Polyphenols in Citrus Genus: Biosynthesis, Chemical Profile, and Biological Activity, Editor(s): Ronald Ross Watson, Polyphenols in Plants (Second Edition), Academic Press, 191–215. https://doi.org/10.1016/B978-0-12-813768-0.00014-1
Asmaa I. Owis, Chapter 15 - Citrus Polymethoxyflavones: Biofunctional Molecules of Therapeutic Interest, Studies in Natural Products Chemistry, Volume 59, 2018, Pages 509–530, ISSN 1572–5995, ISBN 9780444641793, https://doi.org/10.1016/B978-0-444-64179-3.00015-3.
Koolaji N, Shammugasamy B, Schindeler A, Dong Q, Dehghani F, Valtchev P (2020) Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr Devel Nutr. https://doi.org/10.1093/cdn/nzaa025
Li S, Wang H, Guo L, Zhao H, Ho CT (2014) Chemistry and bioactivity of nobiletin and its metabolites. J Funct Foods 6:2–10. https://doi.org/10.1016/j.jff.2013.12.011
Yamada S, Shirai M, Ono K, Teruya T, Yamano A, Tae Woo J (2021) Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects. A multicenter, randomized, double-blind, placebo-controlled study. Food Sci Nutr 9:6844–6853. https://doi.org/10.1002/fsn3.2640
Cimaglia P, Vieceli Dalla Sega F, Vitali F, Lodolini V, Bernucci D, Passarini G, Fortini F, Marracino L, Aquila G, Rizzo P, Ferrari R, Campo G (2019) Effectiveness of a novel nutraceutical compound containing red yeast rice, polymethoxyflavones and antioxidants in the modulation of cholesterol levels in subjects with hypercholesterolemia and low-moderate cardiovascular risk: the NIRVANA study. Front Physiol. https://doi.org/10.3389/fphys.2019.00217
Meguro K, Yamaguchi S (2018) Decreased behavioral abnormalities after treatment with combined donepezil and yokukansankachimpihange in Alzheimer disease: An observational tudy. The Osaki-Tajiri Project. Neurol Ther 7:333–340. https://doi.org/10.1007/s40120-018-0109-9
Seki T, Kamiya T, Furukawa K, Azumi M, Ishizuka S, Takayama S, Nagase S, Arai H, Yamakuni T, Yaegashi N (2013) Nobiletin-rich Citrus reticulata peels, a Kampo medicine for Alzheimer’s Disease: A case series. Geriatr Gerontol Int 13:236–238. https://doi.org/10.1111/j.1447-0594.2012.00892.x
Fontana G, Bruno M, Sottile F, Badalamenti N (2023) The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus. Antioxidants 12:23. https://doi.org/10.3390/antiox12010023
Lai C, Wu J, Ho CT, Pan M (2015) Disease chemopreventive effects and molecular mechanisms of hydroxylated polymethoxyflavones. BioFactors 41:301–313. https://doi.org/10.1002/biof.1236
Barreca D, Mandalari G, Calderaro A, Smeriglio A, Trombetta D, Felice MR, Gattuso G (2020) Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 9:288. https://doi.org/10.3390/plants9030288
Seoka M, Ma G, Zhang L, Yahata M, Yamanaki K, Kan, t., Kato, M. (2020) Expression and functional analysis of nobiletin biosynthesis-related gene CitOMT in citrus fruit. Sci Rep 10:15288. https://doi.org/10.1038/s41598-020-72277-z
Wang X, Li S, Wei C, Huang J, Pan M, Shahidi F, Ho C (2018) Anti-inflammatory effects of polymethoxyflavones from citrus peels: a review. J Food Bioactiv 3:76–86
Karn A, Zhao C, Yang F, Cui J, Gao Z, Wang M, Wang F, Xiao H, Zheng J (2021) In-vivo biotransformation of citrus functional components and their effects on health. Critical Rev Food Sci Nutr 61:756–776. https://doi.org/10.1080/10408398.2020.1746234
Russo M, Rigano F, Arigò A, Dugo P, Mondello L (2021) Coumarins, psoralens and polymethoxyflavones in cold-pressed citrus essential oils: a review. J Essential Oil Res 33:221–239. https://doi.org/10.1080/10412905.2020.1857855
Tomás-Navarro M, Navarro JL, Vallejo F, Tomás-Barberán FA (2021) Novel urinary biomarkers of orange juice consumption, interindividual variability, and differences with processing methods. J Agric Food Chem 69:4006–4017. https://doi.org/10.1021/acs.jafc.0c08144
Li, S., Pan, M.H.C., Wang, Z., Lambros, T., Ho, C.T. 2008. Biological activity, metabolism and separation of polymethoxyflavonoids from citrus peels. Tree For. Sci. Biotech. 2, 36–51. http://www.globalsciencebooks.info/Online/GSBOnline/images/0812/TFSB_2(SI1)/TFSB_2(SI1)36-51o.pdf
Li S, Lo CY, Ho CT (2006) Hydroxylated polymethoxyflavones and methylated flavonoids in sweet orange (citrus sinensis) peel. J Agric Food Chem 54:4176–4185. https://doi.org/10.1021/jf060234n
Han S, Kim HM, Lee JM, Mok SY, Lee S (2010) Isolation and identification of polymethoxyflavones from the hybrid Citrus hallabong. J Agric Food Chem 58:9488–9491. https://doi.org/10.1021/jf102730b
Song M, Charoensinphon N, Wu X, Zheng J, Gao Z, Xu F, Wang M, Xiao H (2016) Inhibitory effects of metabolites of 5- demethylnobiletin on human nonsmall cell lung cancer cells. J Agric Food Chem 64:4943–4949. https://doi.org/10.1021/acs.jafc.6b01367
Zheng JK, Song MY, Dong P, Qiu PJ, Guo SS, Zhong ZM, Li SM, Ho CT, Xiao H (2013) Identification of novel bioactive metabolites of 5-demethylnobiletin in mice. Mol Nutr Food Res 57:1999–2007. https://doi.org/10.1002/mnfr.201300211
Wu X, Song MY, Gao ZL, Sun Y, Wang MF, Li F, Zheng JK, Xiao H (2017) Nobiletin and its colonic metabolites suppress colitis-associated colon carcinogenesis by down-regulating iNOS, inducing antioxidative enzymes and arresting cell cycle progression. J Nutr Biochem 42:17–25. https://doi.org/10.1016/j.jnutbio.2016.12.020
Wu X, Song M, Qiu P, Li F, Wang M, Zheng J, Wang Q, Xu F, Xiao H (2018) A metabolite of nobiletin, 4’-demethylnobiletin and atorvastatin synergistically inhibits human colon cancer cell growth by inducing G0/G1 cell cycle arrest and apoptosis. Food Func 9:87–95. https://doi.org/10.1039/c7fo01155e
Qiu P, Cui Y, Xiao H, Han Z, Ma H, Tang Y, Xu H, Zhang L (2017) 5-Hydroxy polymethoxyflavones inhibit glycosaminoglycan biosynthesis in lung and colon cancer cells. J Func Foods 30:39–47. https://doi.org/10.1016/j.jff.2017.01.008
Song M, Lopez-Peña CL, McClements DJ, Decker EA, Xiao H (2017) Safety evaluation and lipid-lowering effects of food-grade biopolymer complexes (e-polylysine-pectin) in mice fed a high-fat diet. Food Func 8:1822–1829. https://doi.org/10.1039/C7FO00222J
Wu X, Song M, Wang M, Zheng J, Gao Z, Xu F, Zhang G, Xiao H (2015) Chemopreventive effects of nobiletin and its colonic metabolites on Colon carcinogenesis. Mol Nutr Food Res 59:2383–2394. https://doi.org/10.1002/mnfr.201500378
Charoensinphon N, Qiu P, Dong P, Zheng J, Ngauv P, Cao Y, Li S, Ho CT, Xiao H (2013) 5-demethyltangeretin inhibits human nonsmall cell lung cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. Mol Nutr Food Res 57:2103–2111. https://doi.org/10.1002/mnfr.201300136
Qiu P, Guan H, Dong P, Guo S, Zheng J, Li S, Chen Y, Ho CT, Pan MH, McClements DJ, Xiao H (2011) The inhibitory effects of 5-hydroxy-3,6,7,8,3′,4′-hexamethoxyflavone on human colon cancer cells. Mol Nutr Food Res 55:1523–1532. https://doi.org/10.1002/mnfr.201100070
Lien LM, Wang MJ, Chen RJ, Chiu HC, Wu JL, Shen MY, Chou DS, Sheu JR, Lin KH, Lu WJ (2016) Nobiletin, a polymethoxylated flavone, inhibits glioma cell growth and migration via arresting cell cycle and suppressing MAPK and Akt pathways. Phytother Res 30:214–221. https://doi.org/10.1002/ptr.5517
Arivazhagan L, Sorimuthu PS (2014) Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7,12-dimethylbenz(α)anthracene-induced rat mammary carcinoma. J Nutr Biochem 25:1140–1153. https://doi.org/10.1016/j.jnutbio.2014.06.007
Ma X, Jin S, Zhang Y, Wan L, Zhao Y (2014) Inhibitory effects of nobiletin on hepatocellular carcinoma in vitro and in vivo. Pharmacol Res 28:560–567. https://doi.org/10.1002/ptr.5024
Yoon JH, Lim TG, Lee KM, Jeon AJ, Kim SY, Lee KW (2011) Tangeretin reduces ultraviolet B (UVB)-induced cyclooxygenase- 2 expression in mouse epidermal cells by blocking mitogen-activated protein kinase (MAPK) activation and reactive oxygen species (ROS) generation. J Agric Food Chem 59:222–228. https://doi.org/10.1021/jf103204x
Zhang L, Xu X, Jiang T, Wu K, Ding C, Liu Z, Zhang X, Yu T, Song C (2018) Citrus aurantium naringenin prevents osteosarcoma progression and recurrence in the patients who underwent osteosarcoma surgery by improving antioxidant capability. Oxid Med Cell Longev 8713263:1–16. https://doi.org/10.1155/2018/8713263
Ramalingam S, Shanthi P, Panchanadham S (2015) Tangeretin, a polymethoxylated flavone, modulates lipid homeostasis and decreases oxidative stress by inhibiting NF-κB activation and proinflammatory cytokines in cardiac tissue of streptozotocin-induced diabetic rats. J Func Foods 16:315–333. https://doi.org/10.1016/j.jff.2015.03.024
Gao Z, Gao W, Zeng SL, Li P, Liu EH (2018) Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J Funct Foods 40:498–509. https://doi.org/10.1016/j.jff.2017.11.036
Hamdan D, El-Readi MZ, Tahrani A, Herrmann F, Kaufmann D, Farrag N, El-Shazly A, Wink M (2011) Chemical composition and biological activity of Citrus jambhiri Lush. Food Chem 127:394–403. https://doi.org/10.1016/j.foodchem.2010.12.129
Yi L, Ma S, Ren D (2017) Phytochemistry and bioactivity of Citrus flavonoids: a focus on antioxidant, anti-inflammatory, anticancer and cardiovascular protection activities. Phytochem Rev 16:479–511. https://doi.org/10.1007/s11101-017-9497-1
Arab H, Mohamed WR, Barakat BM, Arafa ES (2016) Tangeretin attenuates cisplatin-induced renal injury in rats: Impact on the inflammatory cascade and oxidative perturbations. Chem Biol Interact 258:205–213. https://doi.org/10.1016/j.cbi.2016.09.008
Chen XM, Tait AR, Kitts DD (2017) Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem 218:15–21. https://doi.org/10.1016/j.foodchem.2016.09.016
Shin HS, Kang SI, Ko HC, Kim HM, Hong YS, Yoon SA, Kim SJ (2011) Antiinflammatory effect of the immature peel extract of Jinkyool (Citrus sunki Hort. ex Tanaka). Food Sci Biotechnol 20:1235–1241. https://doi.org/10.1007/s10068-011-0170-y
Song M, Wu X, Zheng J, Xiao H (2014) 5-Demethylnobiletin inhibits Colon carcinogenesis in azoxymethane/dextran sulfate sodium-treated mice. FASEB J 2:3–4. https://doi.org/10.1096/fasebj.28.1_supplement.123.3
Dong Y, Cao A, Shi J, Yin P, Wang L, Ji G, Xie J, Wu D (2014) Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol Reports 31:1788–1794. https://doi.org/10.3892/or.2014.3034
Chien SY, Hsieh MJ, Chen CJ, Yang SF, Chen MK (2015) Nobiletin inhibits invasion and migration of human nasopharyngeal carcinoma cell lines by involving ERK1/2 and transcriptional inhibition of MMP-2. Exp Opin Ther Targets 19:1–14. https://doi.org/10.1517/14728222.2014.992875
Eguchi A, Murakami A, Li S, Ho CT, Ohigashi H (2007) Suppressive effects of demethylated metabolites of nobiletin on phorbol ester-induced expression of scavenger receptor genes in THP-1 human monocytic cells. BioFactors 31:107–116. https://doi.org/10.1002/biof.5520310201
Kou MC, Fu SH, Yen JHC, Weng Y, Li SM, Ho CT, Wu MJ (2013) Effects of citrus flavonoids, 5-hydroxy-3,6,7,8,3’,4’-hexamethoxyflavone and 3,5,6,7,8,3’,4’-heptamethoxyflavone, on the activities of macrophage scavenger receptors and the hepatic LDL receptor. Food & Funct 4:602–609. https://doi.org/10.1039/C3FO30301B
Kim MS, Hur HJ, Kwon DY, Hwang JT (2012) Tangeretin stimulates glucose uptake via regulation of AMPK signaling pathways in c2c12 myotubes and improves glucose tolerance in high-fat diet induced obese mice. Mol Cel Endocrinol 358:127–134. https://doi.org/10.1016/j.mce.2012.03.013
Lee YS, Cha BY, Saito K, Yamakawa H, Choi SS, Yamaguchi K, Yonezawa T, Teruya T, Nagai K, Woo JT (2010) Nobiletin improves hyperglycemia and insulin resistance in obese diabetic ob/ob mice. Biochem Pharm 79:1674–1683. https://doi.org/10.1016/j.bcp.2010.01.034
Sundaram R, Shanthi P, Sachdanandam P (2015) Tangeretin, a polymethoxylated flavone, modulates lipid homeostasis and decreases oxidative stress by inhibiting NF-jB activation and proinflammatory cytokines in cardiac tissue of streptozotocin-induced diabetic rats. J Funct Foods 16:315–333. https://doi.org/10.1016/j.jff.2015.03.024
Mulvihill EE, Assini JM, Lee JK, Allister EM, Sutherland BG, Koppes JB, Sawyez CG, Edwards JY, Telford DE, Charbonneau A, St-Pierre P, Marette A, Huff MW (2011) Nobiletin attenuates VLDL overproduction, dyslipidemia, and atherosclerosis in mice with diet-induced insulin resistance. Diabetes 60:1446–1457. https://doi.org/10.2337/db10-0589
Wang L, Wang J, Fang L, Zheng Z, Zhi D, Wang S, Li S, Ho CT, Zhao H (2014) Anticancer activities of citrus peel polymethoxyflavones related to angiogenesis and others. BioMed Res Inter 453972:1–10. https://doi.org/10.1155/2014/453972
Singh SP, Tewari D, Patel K, Jain GK (2011) Permeability determination and pharmacokinetic study of nobiletin in rat plasma and brain by validated high-performance liquid chromatography method. Fitoterapia 82:1206–1214. https://doi.org/10.1016/j.fitote.2011.08.010
Tung YC, Chou YC, Hung WL, Cheng AC, Yu RC, Ho CT, Pan MH (2019) Polymethoxyflavones: Chemistry and Molecular Mechanisms for Cancer Prevention and Treatment. Curr Pharmacol 5:98–113. https://doi.org/10.1007/s40495-019-00170-z
Zheng J, Bi J, Johnson D, Sun Y, Song M, Qiu P, Dong P, Decker E, Xiao H (2015) Analysis of 10 metabolites of polymethoxyflavones with high sensitivity by electrochemical detection in high performance liquid chromatography. J Agric Food Chem 63:509–516. https://doi.org/10.1021/jf505545x
Fan KN, Kurihara N, Abe S, Ho CT, Ghai G, Yan K (2007) Chemopreventive effects of orange peel extract (OPE) I. OPE inhibits intestinal tumor growth in ApcMin/+ mice. J Med Food 10:11–17. https://doi.org/10.1089/jmf.2006.0214
Lai CS, Li S, Chai CY, Lo CY, Dushenkov S, Ho CT, Pan MH, Wang YJ (2008) Antiinflammatory and antitumor promotional effects of a novel urinary metabolite, 3’,4’-didemethylnobiletin, derived from nobiletin. Carcinogenesis 29:2415–2424. https://doi.org/10.1093/carcin/bgn222
Okuyama S, Miyoshi K, Tsumura Y, Amakura Y, Yoshimura M, Yoshida T, Nakajima M, Furukawa Y (2015) 3,5,6,7,8,3′,4′-heptamethoxyflavone, a citrus polymethoxylated flavone, attenuates inflammation in the mouse hippocampus. Brain Sci 5:118–129. https://doi.org/10.3390/brainsci5020118
Zhang M, Zhu S, Ho CT, Huang Q (2021) Citrus polymethoxyflavones as regulators of metabolic homoeostasis: Recent advances for possible mechanisms Trends Food Sci. Technol 110:743–753. https://doi.org/10.1016/j.tifs.2021.02.046
Kim YJ, Choi MS, Woo JT, Jeong MJ, Kim SR, Jung UJ (2017) Longterm dietary supplementation with low-dose nobiletin ameliorates hepatic steatosis, insulin resistance, and inflammation without altering fat mass in diet-induced obesity. Mol Nutr Food Res 61:1600889. https://doi.org/10.1002/mnfr.201600889
Feng K, Lan Y, Zhu X, Li J, Chen T, Huang Q, Ho CT, Chen Y, Cao Y (2020) Hepatic lipidomics analysis reveals the anti-obesity and cholesterol-lowering effects of tangeretin in high-fat diet-fed rats. J Agric Food Chem 68:6142–6153. https://doi.org/10.1021/acs.jafc.0c01778
Feng K, Zhu X, Chen T, Peng B, Lu M, Zheng H, Huang Q, Ho CT, Chen Y, Cao Y (2019) Prevention of obesity and hyperlipidemia by heptamethoxyflavone in high-fat diet-induced rats. J Agric Food Chem 67:2476–2489. https://doi.org/10.1021/acs.jafc.8b05632
Tsutsumi R, Yoshida T, Nii Y, Okahisa N, Iwata S, Tsukayama M, Hashimoto R, Taniguchi Y, Sakaue H, Hosaka T, Shuto E, Sakai T (2014) Sudachitin, a polymethoxylated flavone, improves glucose and lipid metabolism by increasing mitochondrial biogenesis in skeletal muscle. Nutr Metab 11:32. https://doi.org/10.1186/1743-7075-11-32
Lee YS, Cha BY, Choi SS, Choi BK, Yonezawa T, Teruya T, Nagai K, Woo JT (2013) Nobiletin improves obesity and insulin resistance in high-fat diet-induced obese mice. J Nutr Biochem 24:156–162. https://doi.org/10.1016/j.jnutbio.2012.03.014
Pan MH, Yang G, Li S, Li MY, Tsai ML, Wu JC, Badmaev V, Ho CT, Lai CS (2017) Combination of citrus polymethoxyflavones, green tea polyphenols, and Lychee extracts suppresses obesity and hepatic steatosis in high-fat diet induced obese mice. Mol Nutr Food Res 61:1601104. https://doi.org/10.1002/mnfr.201601104
Lai CS, Ho MH, Tsai ML, Li S, Badmaev V, Ho CT, Pan MH (2013) Suppression of adipogenesis and obesity in high-fat induced mouse model by hydroxylated polymethoxyflavones. J Agric Food Chem 61:10320–10328. https://doi.org/10.1021/jf402257t
Tung YC, Chang WT, Li S, Wu JC, Badmeav V, Ho CT, Pan MH (2018) Citrus peel extracts attenuated obesity and modulated gut microbiota in mice with high-fat diet-induced obesity. Food & Func 9:3363–3373. https://doi.org/10.1039/C7FO02066J
Chen PY, Li S, Koh YC, Wu JC, Yang MJ, Ho CT, Pan MH (2019) Oolong tea extract and citrus peel polymethoxyflavones reduce transformation of L-Carnitine to trimethylamine-N-oxide and decrease vascular inflammation in L-Carnitine feeding mice. J Agric Food Chem 67:7869–7879. https://doi.org/10.1021/acs.jafc.9b03092
Acknowledgements
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature.
Author information
Authors and Affiliations
Contributions
R. Toledo: investigation, data curation, formal analysis. M. Tomás-Navarro: investigation, data curation, writing—original draft. J.E. Yuste: investigation, data curation, formal analysis. P. Crupi: review and editing, F. Vallejo: writing original draft—review and editing, supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Compliance with ethics requirements
There is no research using human or animal subjects in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Toledo, R., Tomás-Navarro, M., Yuste, J.E. et al. An update on citrus polymethoxyflavones: chemistry, metabolic fate, and relevant bioactivities. Eur Food Res Technol 250, 2179–2192 (2024). https://doi.org/10.1007/s00217-024-04529-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04529-5