Abstract
Petroleum-based plastics have been widely used as packaging materials because of their low-cost availability and good mechanical properties. However, the use of plastics has become restricted as they are highly resistant to biodegradation, causing environmental problems. This work aimed to produce and characterize emulsified pullulan films incorporating geraniol for application as food packaging materials with potential to substitute the conventional plastics. When geraniol was incorporated in the films, they showed antimicrobial activity against Enterococcus faecalis ATCC 29212 (inhibition zone diameter = 15.19 ± 0.66 mm) and Pseudomonas aeruginosa ATCC 27853 (inhibition zone diameter = 10.99 ± 1.82 mm). Furthermore, scanning electron microscopy showed the inhibition of Enterococcus faecalis ATCC 29212 biofilms when they were directly formed on the emulsified pullulan films incorporating geraniol. The produced films also demonstrated high transparency (> 90%) and hydrophilic surfaces (water contact angle < 90°). This work demonstrated the viability of using geraniol to produce pullulan active films as new food packaging materials.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plastics are widely employed in a variety of industrial fields because they are lightweight, manageable, resistant, and affordable. Most plastic packaging materials are made of synthetic polymers (70–99%), which are always mixed with different additives like plasticizers, antioxidants, dyes, antistatic agents, fillers, and many other substances [1]. As petrochemical corporations make substantial investments in new production facilities and infrastructures, there is a predicted rise in global demand for conventional plastics [2]. However, plastics are creating several environmental problems and, therefore, it is urgent to develop alternatives to their use, namely biopolymer films.
Nowadays, food packaging plays more than a passive role in protecting and promoting an edible product [3]. An active package material is considered to be one that contains components that can interact with the internal environment and the food to extend its shelf life and increase its margin of safety [4]. Among the compounds of natural origin most used in active packaging are essential oils, plant extracts, and their isolated compounds. However, when incorporating essential oils in aqueous filmogenic solutions, they tend to not mix completely, usually remaining on the surface, resulting in a non-uniform distribution in the films.
Biopolymers are polymers of natural origin made up of monomeric units that are covalently bound to one another, producing molecules that resemble chains [5]. Among the biopolymers most used for the development of films, polysaccharides stand out. By combining two or more constituent materials with different properties, a film forming dispersion is obtained. After casting and drying processes, a heterogeneous structural material mix called biopolymer composites is obtained [6]. Either a stable emulsion or a bilayer can be used to produce a composite film [7].
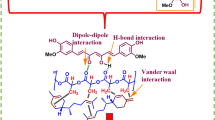
Emulsified films require the dispersion of the lipophilic component (like essential oils or their isolated compounds) into the aqueous filmogenic solution to make a stable emulsion. Polysaccharides stabilize emulsions through stearic effects, which are primarily stabilized by electrostatic forces. To generate a polymeric layer or network with a substantial thickness, polysaccharides must be tightly bonded to the surface of the lipophilic compound and significantly protrude into the continuous phase [8]. Nevertheless, polysaccharides frequently have a limited amphiphilic character, therefore, adding emulsifiers, like polysorbate, is necessary to increase the emulsion stability [7]. Because of their hydrophilic nature coming from the available hydroxyl groups, polysaccharides typically produce films with strong mechanical properties but poor moisture barriers [9]. The incorporation of lipophilic compounds to films causes an increase in oxygen permeability and a decrease in moisture barrier [10].
The extracellular polysaccharide pullulan, which is produced by the polymorphic fungus Aureobasidium pullulans, is a linear α-D-glucan that has a regularly repeating trisaccharide residue [11]. Pullulan has good fiber and film forming capacities, is water soluble, biodegradable and exhibits great adhesion. Additionally, pullulan is ‘generally recognized as safe’ (GRAS) [12]. Glycerol that acts as a plasticizer, reduces the intermolecular forces between polymer chains and increases their mobility, reducing the H-bonding interactions. These effects are the main reasons for the interest of using glycerol [13]. Tween 40, or Polysorbate 40, is a surfactant used as an emulsifier. Emulsifiers could make primary emulsion formation easier and boost their stability after formation by reducing interfacial tension through adsorption at the boundary between the oil and aqueous phases [14]. Geraniol is an acyclic monoterpene alcohol that can be obtained from Cymbopogon martinii essential oil. Geraniol appears as a transparent to pale yellow oil that is soluble in most organic solvents but insoluble in water [15]. A review of geraniol's antimicrobial properties reveals that its antimicrobial activity may be primarily due to its non-polar nature, which may allow it to interact with the components of the microorganisms’ cell membrane and disrupt its lipid structure. Geraniol has also the ability to enter the cells and bind to intracellular sites crucial for the survival of the bacteria, hence limiting its growth [16].
As the replacement and/or reduction of plastics is one of the biggest challenges facing the food industry towards the implementation of a circular economy, the objective of this work was to develop emulsified pullulan films with incorporation of geraniol for future application as food packaging materials The barrier, mechanical and active properties of the produced emulsified films were further evaluated.
Materials and methods
Reagents
Pullulan (CAS No.: 9057-02-7), molar mass 574.57 g/mol was acquired from TCI Europe NV. Glycerol (anhydrous, extra pure) (CAS No.: 56-81-5) was purchased at Merck. Geraniol (CAS No.: 106-24-1), molar mass 154.24 g/mol was bought in TCI Europe NV. Tween 40 (CAS No.: 9005-66-7), molar mass 1283.63 g/mol was acquired from Alfa Aesar.
Preparation of films
The initial preparation of the pullulan aqueous solution (3%, w/v) involved magnetic stirring (300 rpm) at room temperature for 5 min of 3 g of pullulan with 100 mL of distilled water. Then, 0.45 g of glycerol were added as plasticizer (15%, w/w relative to pullulan), and the solution was stirred for 30 min at 50 °C. Subsequently, 150 mg of geraniol (5%, w/w relative to pullulan) and 0.5 mL of Tween 40 were added to the solution and agitated for 10 min under the same conditions. Before the distribution of the solution to the polystyrene Petri dishes (16 mL/plate), it was subjected to homogenization at 21,500 rpm for 5 min using an IKA T25 Digital Ultra-Turrax rotor–stator homogenizer. This process leads to formation of foam in the solution so, a vacuum cycle with a Rotavapor RE 111 regulated with a Vacuum Controller V-850 up to 130 mbar was required to remove the foam bubbles. After that, the Petri dishes were placed for 3 h in a ventilated oven at 60 °C. For the preparation of the control films, the method used was similar, except the addition of Tween 40 (in the PuGer films), the addition of Ger (in the Pu40 films) and the addition of Tween 40 and Ger (in the Pu films). After being dried, the films were removed from the plates and stored at a temperature of 23 ± 2 °C and a relative humidity (RH) of 50 ± 5% [23].
Characterization of films
Grammage, thickness and mechanical properties
According to ISO 536:1995, the grammage of the films was determined using the mass-to-area ratio (g/m2) [40]. The thickness was measured using an Adamel Lhomargy type MI 20 µm in accordance with ISO 534:2011. Each film was subjected to five measurements at random positions, and the mean values were used to determine the mechanical properties [41]. Elongation (%), tensile strength (MPa), tensile index (N.m/g), and elastic modulus (MPa) of the films were measured in accordance with ISO 1924/2 using a tensile tester (Thwing-Albert Instrument Co., West Berlin, NJ, USA), with the initial grip set at 50 mm and the crosshead speed set at 10 mm/min [27].
Optical properties
A Technidyne Color Touch 2 spectrophotometer was used to evaluate the optical properties of the films (color coordinates and transparency). Several random measurements were carried out on each sample using the D65 illuminant (daytime light with the ultraviolet component) and the observation angle of 10°. Color coordinates L* (lightness), a* (redness; ± red-green) and b* (yellowness, ± yellow-blue) were obtained. The ISO 22891 Eq. (1) was used to calculate the transparency (T) of the samples [42]:
where, RW is the reflectance of the sample in % when positioned against a white background, R0 is the reflectance of the sample against a black background and R(w) = 90.41 is the reflectance of the standard white background used in the test.
Fourier transform infrared spectroscopy (FTIR)
Using a Nicolet iS10 smart iTRBasic (Thermo Fisher Scientific, Waltham, MA, USA) model with 64 scans and a 4 cm−1 resolution, FTIR spectra of the films were acquired between 4000 and 600 cm−1 [43].
Differential scanning calorimetry (DSC)
With a calorimeter Netzsch DSC 204 operating in an inert atmosphere and heating the films at a rate of 10 °C/min from 25 to 400 °C, DSC thermograms of the films were acquired. Samples of the films remained at 105 °C for 24 h prior the thermal analysis to totally evaporate the water [44].
Contact angle and surface free energies
The sessile drop contact angle method was used to determine the contact angles of the films, and the model OCAH 200 (DataPhysics Instruments, Filderstadt, Germany) that allowed image acquisition and data analysis was used. Deionized water, ethyleneglycol, and diiodomethane were used to measure the contact angles to allow the determination of the surface free energy (total, dispersive, and polar components) of the films. The software of the equipment provided the surface tension components of the reference liquids [40]. For each liquid and each sample, contact angles were acquired from at least six measurements, and the surface free energies of the samples were determined using the Owens, Wendt, Rabel and Kaelble (OWRK) method [41].
Barrier properties
Oil permeability
First, test tubes were filled with 5 mL of edible vegetal oil from sunflower seeds, which were then covered with each film. The tubes were placed upside-down on a filter paper surface. The weight difference of the filter paper (before and after oil exposition), the thickness of the films, the effective contact area, and the storage time (24 h) were used to calculate the oil permeability (OP) (g.mm/m2.day) as follows:
where ∆W is the weight difference of the filter paper (g), e corresponds to the thickness of the film (mm), A is the contact area (m2), and t is the storage period (days) [32].
Water vapor permeability
According to the standard procedure ASTM E96-00, water vapor permeability (WVP) (g/Pa.day.m) and water vapor transmission rate (WVTR) (g/m2.day) were measured. The films were adhered to the top of adjusted cups that contained a desiccant (13 g of anhydrous CaCl2, dried at 105 °C before use). After that, the test cups were kept in a cabinet at 23 ± 2 °C and 50 ± 5% RH. Over the course of 48 h, the weight variations were tracked every 2 h. The slope of a linear regression of the weight gain vs time was used to calculate the gradient. Equations (3) and (4) were used to determine WVTR and WVP, respectively [31]:
where ∆m is the weight changes of test cups (g), A is the test area (m2), and t is the test time (day).
where p is the vapor pressure of water at 23 °C (Pa), RH1 is the RH of the cabinet (50%), RH2 is the RH inside the cups (0%), and e is the thickness (m) of the films.
Evaluation of the antibacterial activity of the films
Three Gram-positive bacterial strains (Enterococcus faecalis ATCC 29212, Listeria monocytogenes LMG 16779, and Staphylococcus aureus ATCC 25923) and three Gram-negative bacterial strains (Salmonella Typhimurium ATCC 13311, Pseudomonas aeruginosa ATCC 27853 and Escherichia coli ATCC 25922) were employed for the antibacterial experiments. The American Type Culture Collection (ATCC, Manassas, VT, USA) and the BCCM/LMG Bacteria Collection (Belgian Co-Ordinated Collections of Microorganisms, Gent, Belgium) provided the reference strains. The bacterial strains' stock cultures were stored at – 80 °C in 20% (v/v) glycerol (Himedia, Mumbai, India). 24 h prior to the assays, all the strains for the antibacterial activity were sub-cultured in brain–heart infusion agar (BHI).
Solid diffusion assay
Bacteria were suspended in sterile saline solution (NaCl, 0.85% (w/v)) to a cell suspension of 0.5 McFarland (1–2 × 108 colony-forming units/mL (CFU/mL)) to create the inoculums for this test. 6 mm diameter films’ discs were prepared. Then, the previously prepared discs were placed on the top of the BHI or Müeller–Hinton Agar (MHA) plates that were previously inoculated. The plates were then incubated for 18 h at 37 °C. Following incubation, the diameters of the inhibition zones were measured with a digital pachymeter on each plate [45]. This assay was performed three independent times.
Anti-biofilm activity
Scanning electron microscopy (SEM) was used to study the antibiofilm activity of the films for one Gram-positive bacterial specie (E. faecalis ATCC 29212). Bacterial biofilms were formed for that purpose directly on the discs of the films (about 1 cm2) that were set up on 12-well plates. The suspensions' turbidity was adjusted to have an OD610 nm of 0.7. The BHI medium was then added (700 µL) to the bacterial suspensions (300 µL). The plates were incubated for 24 h at 37 °C. The biofilms were then fixed with 2.5% (v/v) glutaraldehyde (Sigma–Aldrich, USA) diluted with phosphate-buffered saline solution (PBS) and incubated at 4 °C for 4 h after being washed twice with sterile saline solution. After that, samples were given a single PBS wash before being dehydrated in ethanol for 20 min at each of the following concentrations: 30, 50, 70, 80, and 90% (v/v) and absolute. The samples were then left to dry in a desiccator overnight. Then they were mounted on the stubs and coated with gold using a metal evaporator (Quorum Q150R ES, East Sussex, UK). VP SEM Hitachi S-3400N was used to observe the biofilms, with a voltage of 20.0 kV and a 120.0 A emission [31].
Statistical analysis
Generally, the results were presented as mean ± standard deviation (SD). Microsoft Excel® was used to evaluate the data. Student’s t test was used to examine significant variations in mean (assuming the normal distribution of the continuous variables). p values lower than 0.05 were considered as statistically significant.
Results and discussion
Characterization of films
Four types of films were produced. PuGer40 (pullulan, glycerol, geraniol, and Tween 40), PuGer (pullulan, glycerol, and geraniol), Pu40 (pullulan, glycerol, and Tween 40) and Pu (pullulan and glycerol).
Grammage, thickness and mechanical properties
Elastic modulus, elongation and other mechanical properties are influenced by the films’ formation process as well as their constituents [17]. Because packaging films are designed to endure external forces while preserving their integrity, these properties are crucial. By measuring the mechanical properties, it is possible to forecast how the material would respond under the food processing conditions and to compare it with commercial polymers [18].
The results obtained for grammage, thickness and mechanical properties of the emulsified films produced in this work are presented on the Table 1. The grammage is higher in Pu40 and PuGer40 films due to the presence of Tween 40. However, it was found that these films have the lowest apparent density due to the increase in their thickness caused by the presence of foam produced during the homogenization process. Although the vacuum cycle, some films keep bubbles in their composition.
Polysaccharides films are stiff in terms of their mechanical properties; hence, plasticizers are required to increase their flexibility [19]. The tensile strength of the films with Tween 40 decreased compared to Pu and PuGer films, which means that the presence of foam probably influences this property. Similar results were observed for elongation and elastic modulus. These results could be compared with a previous work with plasticized pullulan films where, the tensile strength, elongation and elastic modulus also decreased with nanocrystals addition [20]. The mechanical properties of the emulsified films now developed are weaker when compared with plastic films used in the food industry, such as low-density polyethylene (LDPE) [20].
Optical properties
The results of the color coordinates and transparency are summarized in Table 2. It was possible to verify that the addition of Tween 40 did not affect the color coordinates in comparison to the Pu film, an interesting conclusion since Tween 40 is a surfactant with greasy appearance.
The incorporation of geraniol in the Pu films has not affected the color coordinates, with similar results being found previously [21]. However, when adding geraniol together with Tween 40, the values turn into more light than in Pu or PuGer films. This result can be explained because Tween 40 is an emulsifier and can make the PuGer40 films more homogenous than PuGer, increasing their lightness.
The incorporation of geraniol did not affect the transparency of the films because geraniol, without Tween 40, may have evaporated during the casting process. It was also found that with the incorporation of Tween 40, the transparency of the films (with or without geraniol) decreased. In a previous work, the transparency of the films also decreased with glycerin incorporation [22]. In general, the transparency of the produced films was above 85%, so it can be concluded that the emulsified films have great optical properties as transparent food packaging materials.
Fourier transform infrared spectroscopy (FTIR)
With the aim of identifying the main functional groups present in all compounds used in the films' formulation, as well as for emulsified films produced in this work, FTIR was carried out. The FTIR spectra obtained are presented in Fig. 1, where it is possible to observe some of the main functional groups of all the compounds.
In Fig. 1a, the spectrum correspondent to Tween 40 showed that it possesses a methyl group, an ester group, an alkene group, and an ether group. A previous report showed that Tween 80 presented a band near 1735 cm−1 associated to the stretching vibration of the carbonyl group [23]. In Fig. 1b, the spectrum correspondent to glycerol showed that it possesses an alcohol group, a methylene group, and an alkyne group, which is corroborated by previous research [24]. In Fig. 1c, the spectrum correspondent to geraniol showed that it possesses a methylene group, an alkyne group, and an ether group. Similar results were obtained previously [25]. In Fig. 1d, the spectrum correspondent to pullulan showed that it owns a bending oscillation of hydroxyl groups (observed frequently in the structure of polysaccharides), a methyl group, an alkene group, and an ether group, as it was mentioned earlier [26].
Comparing the FTIR spectra of the films’ components with the spectra of the produced films it was possible to conclude that pullulan spectra and Pu spectra are very similar, Pu40 spectra just has a different peak comparing to Pu spectra (marked with an arrow in Fig. 1e) that can be associated with the presence of Tween 40. Same result was observed in Fig. 1g with the same peak associated to Tween 40.
Differential scanning calorimetry (DSC)
It is possible to detect changes in the physical and chemical properties of materials as a function of temperature using the DSC: below the glass transition temperature (Tg), a material is rigid and fragile, whereas above, it becomes more flexible and brittle. The Tg is the temperature at which a material undergoes a structural transition from a solid, amorphous state to one that is more viscous and elastic [27].
The thermal profiles of pullulan films with (PuGer40) or without (Pu) geraniol incorporated were evaluated by DSC (Fig. 2).
The pullulan Tg is 154.5 °C. The DSC thermogram showed that Tg of PuGer40 films is 71 °C (marked with a cross), which transition ends in a baseline. The peaks that appear bellow this baseline represent exothermic reactions and the peaks above the baseline represent endothermic reactions. The decrease of the Tg means that geraniol presented a plasticizing effect on the produced films. In accordance with the results now obtained, the plasticizing effect of one essential oil was also observed previously because its incorporation caused a decrease in Tg in the produced pullulan films [27].
Contact angle and surface free energies
Through the measurement of the contact angle, the interaction between a surface and a certain liquid can be examined. The contact angle is defined as the angle be-tween a plan parallel to a liquid drop and a plan containing the surface where the liquid is deposited [28]. The results of the contact angles obtained for the emulsified pullulan films are shown in Table 3.
The experiments conducted with a water droplet showed that the produced films have a hydrophilic behavior since their water contact angles are smaller than 90°. The incorporation of geraniol into Pu films increased the water contact angle, which can be explained by the hydrophobic nature of geraniol that keeps the droplets less dispersed. However, when geraniol was emulsified with Tween 40, the water contact angle decreased because geraniol is in the emulsion, reducing cohesion forces allowing greater adhesion and consequent spreading.
The sum of the polar and dispersive components of the surface free energy can be used to represent the total surface free energy of the solid and liquid phases [29]. In this work, the OWRK method was applied, which calculates a solid surface's total energy as the sum of all interactions at the solid/liquid interface, divided into two contributions: polar and dispersive [30]. This study indicated that the dispersive component was higher than the polar component (Table 4).
Comparing Pu and PuGer films, it was observed that geraniol decreased the total surface free energy due to the decrease of both dispersive and polar components, being the ratio dispersive/polar quite similar. The addition of Tween 40 in Pu40 films, increased their total surface free energy due to the increase of dispersive component, when compared with Pu films, which caused an increase of the ratio dispersive/polar. In the emulsion, Tween 40 will decrease the interfacial tension between the molecules of water and geraniol (two liquid phases). After the films’ preparation, the hydrophilic phase evaporated, suggesting that Tween 40 molecules were preferentially oriented towards the surface of the films since their total surface free energy increased mainly due to the increase of the dispersive component. PuGer40 films presented similar values of the total surface free energy of the Pu films, but with higher values of dispersive component. This result suggests that the total sur-face free energy of PuGer40 films was influenced by the presence of Tween 40. The ratio dispersive/polar obtained for these films indicates the highest adhesion when compared with Pu40 films, being lower than the other types of films.
In a previous work with pullulan films containing rockrose essential oil, the total surface free energy of the films and the corresponding polar component were significantly reduced, but the dispersive component increased. These results are also connected to the rockrose essential oil hydrophobicity [31]. Contrariwise, in the present study, the incorporation of geraniol in the PuGer40 film increased the dispersive component and the total surface free energy, when compared with Pu film; however, the polar component decreased.
Barrier properties
To preserve the integrity of the packaged food during the entire handling process until it reaches the ultimate consumer, one of the major properties of the food materials is their ability to create a protective atmosphere [27]. Good barrier properties are a reliable predictor of packaging materials over time [32]. The oil and water vapor permeability results, as the barrier properties studied, are presented in Table 5.
Analyzing the results, the oil permeability (OP) of Pu and PuGer films are similar, which may be explained by the absence of Tween 40. Without the emulsifier, geraniol may evaporate during casting, presenting these films very similar characteristics with Pu films. The addition of Tween 40 increased the OP of the films, this compound may have enlarged the free volume between the pullulan molecules, which made oil to permeate easily. Also, the films with Tween 40 presented higher thickness and lower apparent density, which also corroborates the different matrix of the films obtained with Tween 40 incorporation, affecting the OP.
When assessing the practical uses of functional films, water vapor permeability (WVP) is a crucial factor to calculate or estimate the shelf life of the packaged food. Plastics typically have a strong barrier to tiny molecules like gases, water vapor, organic vapors, or liquids. These substances might penetrate the polymer package wall from the interior or exterior environment, causing a permanent change in the quality of the product and shortening its shelf life [33].
Due to the hydrophobic nature of geraniol, it was expected that the films incorporating it presented lower water permeability (Table 5). However, the results showed that the geraniol incorporation increased the WVP, but not significantly (p value > 0.05). Since PuGer film is thinner than Pu film, it could explain the rise of water vapor transmission rate (WVTR). These findings demonstrated that the water vapor barrier of the films was weakened by foam, which also affected their thickness. Furthermore, pullulan is a linear polymer that has the possibility of establishing in-termolecular bonds in the polymer chains, which can give rise to a more closed matrix, thus making it difficult for water vapor molecules to pass through, which was also corroborated by the mechanical resistance results showed previously. In a previous study with pullulan films incorporating rice wax (hydrophobic), the presence of wax decreased the WVP in contrast with what was observed in the present study [34].
Evaluation of the antibacterial and anti-biofilm activities of the films
The antibacterial agents can stop or slow down the microbial growth that cause rapid food spoilage and disease transmission [35]. Given that geraniol has antimicrobial activity, the antibacterial and anti-biofilm activities of the emulsified films produced were also evaluated. The results of the solid diffusion assay are presented in Table 6.
From the results presented, it was possible to verify that only Pu40 and PuGer40 films presented activity against Enterococcus faecalis ATCC 29212. All the emulsified films produced were able to inhibit the growth of Pseudomonas aeruginosa ATCC 27853. The Pu40 film showed contact inhibition against Staphylococcus aureus ATCC 25923.
The potential for applications in antimicrobial packaging systems that could lower the risk of foodborne illnesses related to microbial contamination in food products is demonstrated by packaging materials using antibacterial agents [36]. To the best of our knowledge, no studies regarding the incorporation of geraniol in active films were performed yet.
Since the emulsified films produced showed interesting results of antibacterial activity against Enterococcus faecalis ATCC 29212 and considering the ability of this bacterium to form biofilms, the anti-biofilm activity of the films was then evaluated.
Biofilms were described as organized bacterial cell populations that adhered to inert or alive surfaces inside of a self-produced polymeric matrix [37]. Evidence suggests that anti-biofilm agents change biotic and abiotic surfaces as well as the physical characteristics of bacterial surfaces involved in processes of cell-to-cell adhesion and aggregation [38]. In fact, the images of SEM (Fig. 3) it was observed that the incorporation of geraniol, as active agent, and Tween 40, as emulsifier, increased the anti-biofilm activity of pullulan films. While in the Pu film a biofilm is formed with a regular 3D structure and several layers of bacteria, in the Pu40 and PuGer40 films no biofilm was formed and a substantial reduction in the number of bacterial cells was even observed. These findings may suggest that the addition of geraniol alters the sur-face charges of the polymer, as verified in a previous work that studied the an-ti-biofilm activity of chitosan [39]. In this work, it was proven that charged interactions alter the permeability of the cell membrane and cause cytoplasmic material to flow, which ultimately resulted in cell death [39].
Conclusions
In this work, pullulan-Tween 40 emulsified films containing geraniol were produced and characterized as active food packaging materials. The mechanical properties of the films were influenced by the geraniol incorporation. That is, the grammage and thickness increased, while the tensile strength, elongation and the elastic modulus decreased. Concerning the optical properties, the films presented great transparency. The films have promising antibacterial and anti-biofilm activities, particularly against Enterococcus faecalis, a known foodborne pathogen.
Further studies applying the developed films in a food model will be necessary to evaluate the release kinetics of geraniol over time.
Data availability
Data will be available by request to the corresponding author.
References
Piergiovanni L, Limbo S (2016) Plastic packaging materials. Food packaging materials. Springer, Cham, pp 33–49
Nielsen TD, Hasselbalch J, Holmberg K, Stripple J (2020) Politics and the plastic crisis: a review throughout the plastic life cycle. WIREs Energy Environ 9:e360
Petkoska AT, Daniloski D, D’Cunha NM, Naumovski N, Broach AT (2021) Edible packaging: sustainable solutions and novel trends in food packaging. Food Res Int 140:109981
Rangappa SM, Parameswaranpillai J, Thiagamani SMK, Krishnasamy S, Siengchin S (2021) Food packaging: advanced materials, technologies, and innovations. CRC Press
Othman SH (2014) Bio-nanocomposite materials for food packaging applications: types of biopolymer and nano-sized filler. Agr Agr Sci Proc 2:296–303
Christian SJ (2020) Natural fibre-reinforced noncementitious composites (biocomposites). Nonconventional and vernacular construction materials. Elsevier, pp 169–187
Pérez-Gago MB, Krochta JM (2005) Emulsion and bi-layer edible films. Innovations in food packaging. Elsevier, pp 384–402
Callegarin F, Gallo JQ, Debeaufort F, Voilley A (1997) Lipids and biopackaging. J Am Oil Chem S 74:1183–1192
Hambleton A, Perpiñan-Saiz N, Fabra MJ, Volley A, Debeaufort F (2012) The Schroeder paradox or how the state of water affects the moisture transfer through edible films. Food Chem 132:1671–1678
Galus S, Kadzińska J (2015) Food applications of emulsion-based edible films and coatings. Trends Food Sci Tech 45:273–283
Catley BJ, Ramsay A, Servis C (1986) Observations on the structure of the fungal extracellular polysaccharide, pullulan. Carbohydr Res 153:79–86
Niu B, Shao P, Chen H, Sun P (2019) Structural and physiochemical characterization of novel hydrophobic packaging films based on pullulan derivatives for fruits preservation. Carbohydr Polym 208:276–284
Lavorgna M, Piscitelli F, Mangiacapra P, Buonocore GG (2010) Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr Polym 82:291–298
Marhamati M, Ranjbar G, Rezaie M (2021) Effects of emulsifiers on the physicochemical stability of oil-in-water nanoemulsions: a critical review. J Mol Liq 340:117218
Chen W, Viljoen AM (2010) Geraniol: a review of a commercially important fragrance material. South Afr J Bot 76:643–651
Maczka W, Winska K, Grabarczyk M (2020) One hundred faces of geraniol. Molecules 25:3303
Vieira MGA, Silva MA, Santos LO, Beppu MM (2011) Natural-based plasticizers and biopolymer films: a review. Eur Polym J 47:254–263
Cazón P, Velazquez G, Ramírez JA, Vásquez M (2017) Polysaccharide-based films and coatings for food packaging: a review. Food Hydrocoll 68:136–148
Sun H, Shao X, Jiang R, Shen Z, Ma Z (2018) Mechanical and barrier properties of corn distarch phosphate-zein bilayer films by thermocompression. Int J Biol Macromol 118:2076–2081
Kristo E, Biliaderis C (2007) Physical properties of starch nanocrystal-reinforced pullulan films. Carbohydr Pol 68:146–158
Agarwal S, Hogue M, Bandara N, Pal K, Sarkar P (2020) Synthesis and characterization of tamarind kernel powder-based antimicrobial edible films loaded with geraniol. Food Pack Shelf Life 26:100562
Trinetta V, Cutter CN, Floros JD (2011) Effects of ingredient composition on optical and mechanical properties of pullulan film for food-packaging applications. LWT - Food Sci Technol 44:2296–2301
Chu Y, Xu T, Gao C, Liu X, Zhang N, Feng X, Liu X, Shen X, Tang X (2019) Evaluations of physicochemical and biological properties of pullulan-based films incorporated with cinnamon essential oil and Tween 80. Int J Biol Macromol 122:388–394
AlOmar MK, Hayyan M, Alsaadi MA, Akib S, Hayyan A, Hashim MA (2016) Glycerol-based deep eutectic solvents: physical properties. J Mol Liq 215:98–103
Wany A, Kumar A, Nallapeta S, Jha S, Nigam VK, Pandey DM (2013) Extraction and characterization of essential oil components based on geraniol and citronellol from Java citronella (Cymbopogon winterianus Jowitt). Plant Growth Reg 73:133–145
Haghighatpanah N, Mirzaee H, Khodaiyan F, Kennedy JF, Aghakhani A, Hosseini SS, Jahanbin K (2020) Optimization and characterization of pullulan produced by a newly identified strain of Aureobasidium pullulans. Int J Biol Macromol 152:305–313
Luís Â, Ramos A, Domingues F (2020) Pullulan films containing rockrose essential oil for potential food packaging applications. Antibiotics 9:681
Rose J (2015) Chapter 7 - Surface properties (physical and chemical) and related reactions: characterization via a multi-technique approach. Frontiers of Nanoscience, vol 8. Elsevier, pp 217–243
Ruckenstein E, Lee SH (1987) Estimation of the equilibrium surface free energy components of restructuring solid surfaces. J Coll Interface Sci 120:153–161
Encinas N, Pantoja M, Abenojar J, Martínez MA (2010) Control of wettability of polymers by surface roughness modification. J Adhes Sci Technol 24:1869–1883
Gomes PB, Mata VG, Rodrigues AE (2005) Characterization of the Portuguese-grown Cistus ladanifer essential oil. J Essent Oil Res 17:160–165
Ramos ÓL, Fernandes JC, Silva SI, Pintado ME, Malcata FX (2012) Edible films and coatings from whey proteins: a review on formulation, and on mechanical and bioactive properties. Crit Rev Food Sci Nutr 52:533–552
Siracusa V, Rocculi P, Romani S, Rosa MD (2008) Biodegradable polymers for food packaging: a review. Trends Food Sci Technol 19:634–643
Shih FF, Daigle KW, Champagne ET (2011) Effect of rice wax on water vapour permeability and sorption properties of edible pullulan films. Food Chem 27:118–121
Majhi S, Tyagi A, Mishra A (2019) Bio-polymeric packaging material for packaging of raw food. Reference module in materials science and materials engineering. Elsevier. https://doi.org/10.1016/B978-0-12-803581-8.10841-0
Kuorwel KK, Cran MJ, Sonneveld K, Miltz J, Bigger SW (2011) Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J Food Sci 76:R164–R177
Maifreni M, Frigo F, Bartolomeoli I, Buiatti S, Picon S, Marino M (2015) Bacterial biofilm as a possible source of contamination in the microbrewery environment. Food Cont 50:809–814
Miquel S, Lagrafeuille R, Souweine B, Forestier C (2016) Anti-biofilm activity as a health issue. Front Microbiol 7:592
Khan F, Pham DTN, Oloketuyi SF, Manivasagan P, Oh J, Kim Y (2020) Chitosan and their derivatives: antibiofilm drugs against pathogenic bacteria. Colloids Surf B Biointerfaces 185:110627
Santos C, Ramos A, Luís Â, Amaral ME (2023) Production and characterization of k-Carrageenan films incorporating Cymbopogon winterianus essential oil as new food packaging materials. Foods 12:2169
Gutiérrez TJ, Guzmán R, Jaramillo CM, Famá L (2016) Effect of beet flour on films made from biological macromolecules: native and modified plantain flour. Int J Biol Macromol 82:395–403
Evangelho JA, Silva GD, Biduski B, Halal SLM, Kringel DH, Gularte MA, Fiorentini AM, Zavareze ER (2019) Antibacterial activity, optical, mechanical, and barrier properties of corn starch films containing orange essential oil. Carbohyd Pol 222:114981
Nunes MR, Castilho MCM, Veeck APL, Rosa CG, Noronha CM, Maciel MVOB, Barreto PM (2018) Antioxidant and antimicrobial methylcellulose films containing Lippia alba extract and silver nanoparticles. Carbohydr Polym 192:37–43
Lai HM, Padua GW (1997) Properties and microstructure of plasticized zein films. Cereal Chem 74:771–775
López P, Sánchez C, Batlle R, Nerín C (2005) Solid-and vapor-phase antimicrobial activities of six essential oils: susceptibility of selected foodborne bacterial and fungal strains. J Agr Food Chem 53:6939–6946
Acknowledgements
Authors would also like to thank Ana Paula Gomes from the Optical Center of the University of Beira Interior for her help in the acquisition of SEM images and DSC analysis.
Funding
Open access funding provided by FCT|FCCN (b-on). This work was developed within the scope of the CICS-UBI projects UIDB/00709/2020 and UIDP/00709/2020, financed by national funds through the Portuguese Foundation for Science and Technology (FCT). FibEnTech-UBI is also financed by FCT under the scope of the project UIDB/00195/2020. Ângelo Luís acknowledges the Scientific Employment contract (DL57/2016/CP1354/CT0010) funded by FCT.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
In this study no humans or animals were used.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simões, A., Ramos, A., Domingues, F. et al. Pullulan-Tween 40 emulsified films containing geraniol: production and characterization as potential food packaging materials. Eur Food Res Technol 250, 1721–1732 (2024). https://doi.org/10.1007/s00217-024-04514-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04514-y