Abstract
This study aimed to evaluate the mineral content, and content of bioactive compounds in fruits of Cornelian cherry (Cornus mas L.). Neochlorogenic acid, chlorogenic acid, caffeic acid, and rutin were determined in the samples. Vitamin C content ranged from 610.36 to 1344 mg kg−1 FW. Content of K, Ca, P, Mg, and Na in samples ranged from 1750.8 to 2645.7 mg kg−1 FW, from 281.14 to 561.62 mg kg−1 FW, from 180.38 to 294.95 mg kg−1 FW, from 68.19 to 115.43 mg kg−1 FW, and from 2.48 to 71.33 mg kg−1 FW respectively. Content of Fe, Zn, Cu, Mn, Ni, Cr, and Co in samples ranged from 2.77 to 4.88 mg kg−1 FW, from 0.49 to 0.99 mg kg−1 FW, from 0.25 to 0.53 mg kg−1 FW, from 0.07 to 0.17 mg kg−1 FW, from 0.02 to 0.13 mg kg−1 FW, from 0.01 to 0.02 mg kg−1 FW, and from 0.01 to 0.08 mg kg−1 FW respectively. Variations across cultivars were found to be statistically significant, indicating that genetics is a key factor influencing the concentration of bioactive compounds and minerals in Cornelian cherry fruits. Understanding the genetic factors influencing mineral and bioactive compound content in cornelian cherry cultivars is essential for targeted breeding programs, crop improvement, and the development of cultivars with enhanced nutritional and health-promoting attributes. This knowledge contributes to sustainable agriculture and supports the production of crops that align with consumer preferences and health trends.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Numerous minor species of small fruits, whether they are harvested from their natural habitats or deliberately cultivated, are frequently used for both direct human consumption and the production of food products [1]. Cornelian cherry (Cornus mas L.) belongs to the Cornaceae family and is a very valuable plant that contains health-promoting compounds not only in its fruits but also in its anatomical parts (roots, leaves, flowers). It was already known in ancient times, it is grown in Romania, Bulgaria, southern Europe, and Asia [2]. Cornelian cherry is an undemanding plant that can easily adapt to unfavorable environmental conditions. It can be consumed fresh as well as dried and is a suitable raw material for the food industry. It can be used to make various drinks, syrups, gels, liqueurs, purees, and jams [3]. It has a unique taste, nutritional properties, and several health benefits.
The main carbohydrate of cornelian cherry fruits is glucose, followed by fructose and sucrose [4]. The fruits are rich in the essential mineral elements such as potassium, calcium, magnesium, and sodium. Other minerals such as phosphorus, iron, zinc, copper, and manganese are represented in smaller amounts. Variability is noted depending on the location of the collection [5]. Its delicious fruits could be used more often in the food industry because they are a rich source of chemoprotective substances such as flavonoids (anthocyanins, flavonols), phenolic acids, terpenes, carotenoids, vitamins, organic acids [3]. Ellagic acid is found in fruits in small amounts [6]. It is characterized by a high content of vitamin C and iridoids [7]. In addition to vitamin C, other vitamins found in these fruits include α-tocopherol, biotin, and riboflavin. Their concentration varies depending on the variety as well as climatic conditions. Among flavonoids, the main components are flavonol glycosides, especially kaempferol and quercetin. Myricetin can be attached as another aglycon component. The high content of secondary metabolites, mainly anthocyanins, indicates their use as a functional food. Among the anthocyanins in the fruits, mainly cyanidin 3-O-galactoside, cyanidin 3-O-glucoside, delphinidin-3-O-galactoside, and peonidin 3-O-glucoside were identified [8, 9]. Due to the presence of phytochemicals, the consumption of cornelian cherry has protective effects against atherosclerosis, and cardiotonic and antiobesity effects have also been reported [10]. It is used in folk medicine to treat a wide range of diseases such as indigestion, diabetes, and liver and kidney diseases [11, 12]. Individual fruits contain different content and different groups of bioactive substances, which can manifest in the antioxidant, anti-inflammatory, anticarcinogenic, and antidiabetic effects of fruits [13]. Extracts from cornelian cherry fruits are good sources of antioxidants, showing antioxidant activity [14]. The hepatoprotective effect of C. mas has also been proven in animals by improving the levels of aspartate transaminase (AST), and alanine transaminase (ALT) [15]. Even despite the mentioned health benefits, the fruits of cornelian cherry are still not sufficiently used as ingredients in various foods, in some regions of the country, especially among organic growers, there is growing interest in processing fruits already at the level of family farms.
The chemical composition of the fruits is diverse and depends to a large extent on the variety, as well as on cultivation and environmental and climatic conditions [16]. Progress in the cultivation of Cornus mas L. also leads to the development of varieties with more attractive colors and larger fruits. Therefore, this work aims to determine the effect of the variety on the content of monitored minerals, bioactive substances and the antioxidant activity of the fruits.
Materials and methods
Plant material
The fruits of ten cultivars and three genotypes of Cornus mas L. were collected in the state of full ripeness in the M.M. Gryshko National Botanical Garden of National Academy of Sciences of Ukraine (50.415092897763536, 30.562754581478206).
Aproximately 1500 g of each cultivar was harvested by hand to obtain average sample. For the analyses, 500 g of each average sample was washed with distilled water and homogenized.
Extract preparation
Twenty five gram of homogenized fresh samples were weighted and poured by 50 ml of 80% methanol. The mixture was shaken on the Unimax 2010 horizontal shaker (Heidolph Instrument GmbH, Germany) for 12 h. The solution was then filtered via Munktell No. 390 filtrating paper (Munktell & Filtrac, Germany), and put into sealed 50 mL vial tubes.
Total polyphenol content
Folin–Ciocalteau colorimetry assay was used to determine the total polyphenol content [17]. Distilled water, 20% Na2CO3 (Sigma Aldrich, USA), and the Folin-Ciocalteu phenol reagent (Merck, Germany) were used. A 50 mL volumetric flask was filled with 0.1 mL of extract. Following the addition of 0.85 mL of Folin–Ciocalteau reagent, 5 mL of 20% Na2CO3 was added after 3 min. The mixture was stirred, and the flask was filled with distilled water to the mark. Flasks were kept at laboratory temperature for two hours. Shimadzu UV-1800 UV/Visible Scanning Spectrophotometer (Shimadzu, Kyoto, Japan) was then used to analyze the sample against a blank solution at 765 nm. Based on the calibration curve, the total polyphenol content was expressed as mg of gallic acid equivalent in 1 kg of fresh matter (mg GAE kg−1). Analyses were performed in 4 replicates.
Total anthocyanin content
The pH differential method was used to determine the total anthocyanin content [18]. 1 ml of extract was pipetted into two centrifuge tubes and 1 ml of 0.01% HCl in 80% ethanol was added. Subsequently, 10 ml of a 2% aqueous solution of HCl was added to the first centrifuge tube, and 10 ml of solution with pH = 3.5 (prepared from 0.2 M Na2HPO4 and 0.1 M citric acid) was added to the second centrifuge. Shimadzu UV- 1800 UV/ Visible Scanning Spectrophotometer Shimadzu, Kyoto, Japan) was then used to analyze the sample against a blank solution at 520 nm. Analysis were performed in 4 replicates.
Antioxidant activity
DPPH radical scavenging assay
The DPPH radical scavenging assay was used to determine antioxidant activity [19]. A working DPPH solution was prepared using methanol and the DPPH• + radical (2,2-diphenyl-1-picrylhydrazyl) (Sigma Aldrich, USA). 3.9 mL of working DPPH solution and 0.1 mL of extract were mixed, stirred, and left in the dark for 10 min. The solution was measured at 515.6 nm against a blank solution, using a Shimadzu UV- 1800 UV/ Visible Scanning Spectrophotometer (Shimadzu, Kyoto, Japan). Based on the calibration curve, antioxidant activity was expressed as the mmol of Trolox equivalents per kilogram of fresh matter (mmol TE kg−1 FW). Analysis were performed in 4 replicates.
ABTS radical scavenging assay
The ABTS radical scavenging assay was used to determine antioxidant activity [20]. Working ABTS solution was created using the ABTS• + radical cation (2,2′-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) (Sigma Aldrich, USA), potassium persulfate (K2S2O8) (Sigma Aldrich, USA), and acetate buffer (pH = 4.3). 3 ml of ABTS solution and 0.05 ml of extract were mixed, stirred, and left in the dark for 20 min. The solution was measured at 734 nm against a blank solution, using a Shimadzu UV-1800 UV/Visible Scanning Spectrophotometer (Shimadzu, Kyoto, Japan). Based on the calibration curve, antioxidant activity was expressed as the mmol of Trolox equivalents per kilogram of fresh matter (mmol TE kg−1 FW). Analysis were performed in 4 replicates.
FRAP assay
The FRAP assay was used to determine antioxidant activity [21]. Working FRAP solution was created using TPTZ—(2,4,6-tris(2-pyridyl)-S-triazine) (Sigma Aldrich, USA), ferric chloride (FeCl3) (Sigma Aldrich, USA), and acetate buffer (pH = 3.5). 3 ml of working FRAP solution and 0.05 ml of extract were mixed, stirred, and left in the dark for 20 min. The solution was measured at 593 nm against a blank solution, using a Shimadzu UV-1800 UV/Visible Scanning Spectrophotometer (Shimadzu, Kyoto, Japan). Based on the calibration curve, antioxidant activity was expressed as the mmol of Trolox equivalents per kilogram of fresh matter (mmol TE kg−1 FW). Analysis were performed in 4 replicates.
Vitamin C analysis
For Vitamin C analysis, two grams of homogenized fresh samples were weighted and then stabilized with 20 mL of 3% meta-phosphoric acid in ultrapure water, to stabilize and precipitate proteins in fruit samples. The mixture was thoroughly vortexed for 1 min. This solution was filtered through 0.45 μm PVDF Millipore syringe filter (Millipore Corporation, Bedford, MA, USA). Vitamin C was determined by HPLC Agilent 1260 Infinity II (Agilent Technologies, Waldbronn, Germany) with quaternary solvent manager coupled with degasser, sample manager, column thermostat and DAD detector. All analyses were performed on C-18 column with reverse phase Cortex (2.7 mm × 150 mm × 3.5 µm) (Waters, USA). Mobile phases consisted of methanol and 0.1% H3PO4 in ddH2O (v/v). The isocratic elution was as follows: 0–10 min (20% methanol and 80% acidified water). The mobile phase flow was 0.6 mL/min and the sample injection was 10 µL. Column thermostat was set to 30 °C and the samples were kept at 8 °C the sampler manager. The detection and quantification wavelength was set at 256 nm. The spectral data were collected and processed using Agilent CDS software for LC 3D Systems.
Individual phenolics analysis
Prior to HPLC analysis, the extracts were filtered through a Q-Max syringe filter, (0.22 µm, 25 mm, PVDF) (Frisenette ApS, Knebel, Denmark). The content of individual phenolics was determined by HPLC Agilent 1260 Infinity II (Agilent Technologies, Waldbronn, Germany) with quaternary solvent manager coupled with degasser, sample manager, column thermostat and DAD detector. All analyses were performed on C-18 column with reverse phase Cortex (2.7 mm × 150 mm × 3.5 µm) (Waters, USA). The mobile phase consisted of gradient acetonitrile with phosphoric acid (pH = 3.5) (A) and 0.1% (v/v) phosphoric acid in deionized H2O (B). The gradient elution was as follows: 0–1 min isocratic elution (20% of A), 1–5 min linear gradient elution (20–25% of A), 5–15 min (25–30% of A), and 15–25 min (30–40% of A). The post-run was 3 min. The flow rate was 1 mL/min, and the injection volume was 5 μL. The column thermostat was set to 30 °C, and the samples were kept at 4 °C in the sampler manager. The detection and quantification wavelength was set at 320 nm for neochlorogenic acid, chlorogenic acid, and caffeic acid, and at 372 nm for rutin. The spectral data were collected and processed using Agilent CDS software for LC 3D Systems.
Macroelement analysis
To determine K, Ca, P, Mg, and Na content, 1 g of homogenized samples were mineralized in a mixture of 10 ml of concentrated HNO3 and 5 ml of concentrated HCLO using a closed microwave digestion system Mars Xpress 5 (CEM Corp., Matthews, NC, USA). After mineralization, cooled samples were filtered through quantitative filter paper Filtrak 390 (Munktell, GmbH, Bärenstein, Germany). For the K, Ca, Mg, and Na determination, 2 ml of filtered sample was diluted with redistilled water to the volume of 50 ml, and measured against a blank solution using atomic absorption spectrophotometer VARIAN AASpectra DUO 240FS (Varian, Ltd., Mulgrave, VIC, AUS). For the P determination, 1 ml of filtered sample was diluted with 8 ml of solution ((C6H8O6, H2SO4, (NH4)2MoO4, and C4H4KO7Sb,0 × 5H2O)) and deionized water to the volume of 50 ml, and measured against blank solution using a UV/Visible Scanning Spectrophotometer Shimadzu UV-1800 (Shimadzu, Kyoto, Japan). Analyses were performed in 4 replicates.
Microelement analysis
To determine Fe, Zn, Cu, Mn, Ni, Co and Cr content, 1 g of homogenized samples were mineralized in a mixture of 5 ml of concentrated HNO3 and 5 ml of deionized water using a closed microwave digestion system Mars Xpress 5 (CEM Corp., Matthews, NC, USA). After mineralization, cooled samples were filtered through quantitative filter paper Filtrak 390 (Munktell, GmbH, Bärenstein, Germany), and filled with deionized water to the volume of 50 ml. Samples were measured against a blank solution using atomic absorption spectrophotometer VARIAN AASpectra DUO 240FS (Varian, Ltd., Mulgrave, VIC, AUS). Analyses were performed in 4 replicates.
Statistical analysis
The Shapiro–Wilk test showed no normal distribution of the analysed quantitative variables; therefore, the non-parametric ANOVA test (Kruskal–Wallis) and Dunn pairwise test with Holm correction was used for the comparison between the tested variables. Principal component analysis was usedto examine the interrelations among a set of variables [22]. Spearman correlation was used to determine the relationships between individual analysed parameters [23, 24].
Results and discussion
Fruits, as a rich source of various phytochemicals and nutrients, make up a significant part of human nutrition. Studies have shown a negative correlation between the consumption of fruits and vegetables and chronic illnesses, such as cardiovascular and neurological disorders, and several types of cancers [25]. According to FAOSTAT, average amount of fruit consumed in year 2021 was 86.95 kg per capita, with apples, bananas, oranges and mandarines making up the majority of this number [26]. Non-conventional, or minor fruit species, such as Cornelian cherry, are also rich sources of essential nutrients and bioactive phytochemicals, while also playing a significant part in the economic and social perspectives of local communities [27].
Content of minerals, bioactive polyphenols, and antioxidant activity of Cornelian cherry fruits are discussed below.
Total polyphenol content, total anthocyanin content, and antioxidant activity
Total polyphenol content, total anthocyanin content, and antioxidant activity of monitored cultivars are presented in the Table 1. Total polyphenol content in analysed samples ranged from 583 to 1877 mg GAE kg−1 FW, with the highest value found in the cultivar 'Svitliachok' and the lowest in the cultivar 'Nizhnyi'. Kazimierski et al. [16] reported values of TPC from 2170 to 6140 mg GAE kg−1 FW. The content of total polyphenols was also discussed by Moldovan et al. [13], who determined 4899.4 mg GAE kg−1 FW. Antolak et al. [28] reported the content of total polyphenols to be 2330 mg GAE kg−1 FW. De Biaggi et al. [11] determined 1970 mg GAE g−1 FW, which corresponds with our values. Gąstoł et al. [29] reported the total content of polyphenols to be 45.6 mg GAE g−1. It is well established that several factors, including variety, location, and environmental conditions, influence the polyphenol content [30]. The obtained results indicate that fruits of Cornus mas L. are a rich source of total polyphenols, which exhibit a range of biological effects.
According to previous scientific works, anthocyanins obtained from Cornus mas L. can show a strong protective effect against atherosclerosis [12]. They are natural antioxidants with strong antioxidant and anti-inflammatory properties. Anthocyanins form complexes with DNA fibres and thereby protect the genetic material from oxidative stress [31]. They are unstable and reactive molecules, and their colour depends on pH, temperature, exposure to light, and the presence of metals [32]. The total content of anthocyanins is also strongly influenced by the genotype, the colour, and the degree of maturity [33]. In addition, the storage temperature of fruits also plays an important role. Total anthocyanin content in the analysed samples ranged from 50 to 549 mg kg−1 FW. Hassanpour et al. [34] determined a higher content of anthocyanins in the monitored varieties. Kucharska et al. [35] reported a wider range of values from 5.59 to 134.57 and 341.18 mg 100 g−1 FW. Güzel [36] also reported a higher content of anthocyanins (69–200 mg 100 g−1 FW). Cornus mas L. shows a higher content of anthocyanins than common berries, but as the results show, the content is strongly influenced by the variety.
Antioxidant activity was determined by DPPH (2.44–2.81 mMol Trolox kg−1 FW), ABTS (5.53–6.25 mMol Trolox kg−1 FW) and FRAP (3.31–4.89 mMol Trolox kg−1 FW) assays. The highest AA was determined in the variety Cm 01, and in cultivar 'Svitliachok'. Rop et al. [37] determined similar values. On the other hand, our values were lower than the values determined by Klymenko et al. [38]. The antioxidant activity of Cornus mas L. also strongly depends on the variety [39].
HPLC analysis
Cornus mas L. is a rich source of vitamin C, which as an antioxidant protects food from enzymatic and non-enzymatic browning. In the process of non-enzymatic browning, it reduces the oxidation of quinones, and the color of the products remains unchanged [40]. Vitamin C and phenolic contents of monitored cultivars are presented in the Table 2. Vitamin C content in analysed samples ranged from 733 to 1344 mg kg−1 FW. Similar values are given by Rop et al. [37], Kostecka et al. [41] and Szczepaniak et al. [42]. Vidrih et al. [43] indicate that the vitamin C content is also significantly influenced by the variety. The variable content of vitamin C can be affected by the influence of the year, degree of maturity as well as handling [12].
Three phenolic acids were determined in the analyzed samples: neochlorogenic (2.67–8.66 mg kg−1 FW), chlorogenic (1.33–7.32 mg kg−1 FW), and caffeic (1.33–6.02 mg kg−1 FW). The content of rutin in analyzed samples ranged from 6.67 to 20.67 mg kg−1 FW. These values are lower than that determined by Moldovan et al. [13] and De Biaggi et al. [11]. Cosmulescu et al. [44] also determined these phenolic acids in their samples but at higher amounts, the content of the rutin corresponded with our results. Many authors reported the presence of chlorogenic as well as neochlorogenic acid in the fruits of Cornus mas L. The composition and quantity of specific phenolic compounds determined vary in a wide range, depending on genetic, various abiotic (agrochemical and climatic conditions, geographical origin, availability of water and light) and biotic (herbivores, microorganisms) conditions [45]. The synthesis and accumulation of bioactive compounds, such as anthocyanins, flavonoids, and phenolic acids, are regulated by specific biochemical pathways within plant cells. Genetic variation among cornelian cherry cultivars can affect the activity and regulation of these pathways, leading to differences in the types and concentrations of bioactive compounds produced [46,47,48]. Therefore, regarding data on the content of specific phenolic substances, the authors are rather inconsistent.
Mineral content in analyzed samples
Mineral contents of monitored cultivars are presented in the Tables 3 and 4. Content of K, Ca, P, Mg, and Na in analysed samples ranged from 1750.8 to 2645.7 mg kg−1 FW, from 281.14 to 561.62 mg kg−1 FW, from 180.38 to 294.95 mg kg−1 FW, from 68.19 to 115.43 mg kg−1 FW, and from 2.48 to 71.33 mg kg−1 FW respectively.
Karaaslan et al. [49] reported similar content of K, Ca, and Mg in fruits of Cornelian cherry (2090.82, 425.92 and 104.23 mg kg−1 respectively.) Gozlekci et al. [50] reported that the content of K, Ca, P, Mg, and Na varies significantly between individual genotypes. They reported similar content of K and Ca (2780–3340, 342–540 mg kg−1 respectively), and higher content of P, Mg, and Na (280–350, 210–254, and 50–65 mg kg−1 respectively) in Cornelian cherry fruits. Dokoupil and Reznicek (2012) [51] reported similar content of Ca, and Na (301–656 and 58–82 mg kg−1 respectively), and higher contents of K and Mg (3411–3798; 237–292 mg kg−1 respectively) in Cornelian cherry fruits. Content of K, Mg and Na showed significant differences among cultivars, in cultivar 'Vydubetsky' the contents were higher than our results (3411, 261.6 and 81.89 mg kg−1 respectively).
Cetkovská et al. [52] reported higher content of Ka and Ca (4225–9729, 517–1164 mg kg−1 respectively) and similar to higher content of Mg (72–430 mg kg−1.) Contents varied significantly depending on the cultivar. The content of Ka, Ca in cultivar 'Vydubetsky' was higher than our results (4225 and 858 mg kg−1 respectively), while the content of Mg was lower (72 mg kg−1). According to Krośniak et al. (2010) [53], juice from Cornelian cherry is also great source of minerals, content of K, Ca, and Na was 1639, 323.4, and 324.2 mg l−1 respectively.
Content of Fe, Zn, Cu, Mn, Ni, Cr, and Co in analysed samples ranged from 2.77 to 4.88 mg kg−1 FW, from 0.49 to 0.99 mg kg−1 FW, from 0.25 to 0.53 mg kg−1 FW, from 0.07 to 0.17 mg kg−1 FW, from 0.02 to 0.13 mg kg−1 FW, from 0.01 to 0.02 mg kg−1 FW, and from 0.01 to 0.08 mg kg−1 FW respectively. Karaaslan et al. [49] reported slightly lower content of Fe (2.78 mg kg−1) and higher content of Zn (1.34 mg kg−1) in Cornelian cherry fruits. Gozlekci et al. [50] reported that the content of Fe and Mn varies significantly between individual genotypes. They reported higher content of Fe, Mn, Zn and Cu content in fruits (23–34, 3–12, 2–4, and 1–3 mg kg−1 respectively). Dokoupil and Reznicek [51] reported higher content of Fe, Zn, Cu and Mn in fruits (41–49, 2.65–3.52, 1.09–1.74, and 24–29 mg kg−1 FW respectively). Content of Fe showed significant differences among cultivars, in cultivar 'Vydubetsky' it was 41.98 mg kg−1 which is significantly higher than our results. Cetkovská et al. [52] reported lower content of Fe (0.46–1.62 mg kg−1), and similar to higher content of Mn, Cu, Zn and Cr (0.71–1.92, 0.53–3.6, 0.48–4.42, and 0.025–0.23 mg kg−1 respectively) in Cornelian cherry fruits. Contents varied significantly depending on the cultivar. The content of Cu in the cultivar 'Vydubetsky' (0.67 mg kg−1) was similar to our results, while the content of Fe was lower (0.48 mg kg−1) and content of Zn, Mn and Cr was higher (1.83, 2.89, and 0.073 mg kg−1 respectively). The mineral composition of fruits is influenced by various factors, including genetic factors, soil conditions, climate, and agricultural practices. The mineral content of fruits is strongly influenced by the mineral composition of the soil in which the plants are grown. Soil pH, nutrient availability, and other environmental factors, type and amount of fertilizer used, irrigation methods, and crop management practices can affect mineral uptake by plants. [54,55,56,57,58].
Based on the results of our study and results reported by other authors, it can be stated that the content of monitored parameters are influenced by genetic factors, as well as agroenvironmental factors. Genetic factors influence the expression of genes involved in the synthesis of minerals and bioactive compounds. Variability in gene expression can result in different levels of these compounds among cultivars. Environmental factors, including soil composition, climate, and cultivation practices, interact with the genetic makeup of cornelian cherry plants. Genotype-environment interactions can influence the expression of genes involved in nutrient uptake and the synthesis of bioactive compounds.
Relationships between analysed parameters
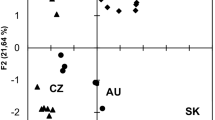
Relationships between bioactive compounds and antioxidant activity of monitored cultivars are presented in the Table 5. Based on the correlation matrix, we can suggest that vitamin C, total polyphenols, total anthocyanins, caffeic acid and rutin mainly contribute to the antioxidant activity of the monitored cultivars. Principal component analysis showed that cultivars 'Koralovyi', 'Yantarnyi' and 'Nizhnyi' could be characterized by higher content of chlorogenic acid, cultivars 'Olena' and 'Svitliachok' by their content of anthocyanins, cultivar 'Ekzotychnyi' by the content of caffeic acid and variety Cm 01 by the content of Vitamin C.
Relationships between mineral contents of monitored cultivars are presented in the Table 6. Based on the correlation matrix, we can suggest that cultivars with higher content of Mg have also higher content of P, and lower content of Zn and Co. Cm 02 could be characterized by their Mg content, and cultivars 'Koralovyi', 'Yantarnyi', and 'Nizhnyi' by their Co and Zn content.
Conclusions
We can conclude that the composition of Cornus mas L. is diverse and depends on the variety. Fruits of Cornelian cherry could contribute valuable bioactive compounds and minerals to the human diet. The results showed that there are significant differences in the content of minerals and bioactive compounds among different cultivars even when the growing conditions are identical, which highlights the importance of cultivar on the chemical composition of Cornus mas L. The obtained results could serve as a basis for further investigation of the influence of the genetics and cultivation practices on the content of bioactive substances and minerals, and can be also used in the field of plant breeding, to ensure an optimal combination of suitable cultivars and growing conditions.
The results of the study also emphasize the role of non-conventional fruit species as sources of mineral and bioactive substances in human nutrition. Due to its mineral content and profile of bioactive compounds with beneficial effects on human health, use of the Cornelian cherry in the food industry and pharmaceutical industry could be further investigate.
Data availability
All data supporting the findings of this study are available from the corresponding author upon request.
References
Vollmannova A, Musilova J, Toth T, Arvay J, Bystricka J, Medvecky M, Daniel J (2014) Phenolic compounds, antioxidant activity and Cu, Zn, Cd and Pb content in wild and cultivated cranberries and blueberries. Int J Environ Anal Chem 94(14–15):1445–1451. https://doi.org/10.1080/03067319.2014.974588
Czerwinska ME, Melzig MF (2018) Cornus mas and cornus officinalis-analogies and differences of two medicinal plants traditionally used. Front Pharmacol 9:894. https://doi.org/10.3389/fphar.2018.00894
Dinda B, Kyriakopoulos AM, Dinda S, Zoumpourlis V, Thomaidis NS, Velegraki A, Markopoulos Ch, Dinda M (2016) Cornus mas L. (cornelian cherry), an important European and Asian traditional food and medicine: Ethnomedicine, phytochemistry and pharmacology for its commercial utilization in drug industry. J Ethnopharmacol 193:670–690. https://doi.org/10.1016/j.jep.2016.09.042
Perova IB, Zhogova AA, Poliakova AV, Eller KI, Ramenskaia GV, Samylina IA (2014) Biologically active substances of cornelian cherry fruits (Cornus mas L.). Vopr Pitan 83:86–94
Juranovic-Cindric I, Zeiner M, Krpetic M, Stingeder G (2012) ICP-AES determination of minor and major elements in cornelian cherry (Cornus mas L.) after microwave assisted digestion. Microchem J 105:72–76. https://doi.org/10.1016/j.microc.2012.03.011
Milenkovic-Andjelkovic AS, Andjelkovic MZ, Radovanovic AN, Radovanovic BC, Nikolic V (2015) Phenol composition, DPPH radical scavenging and antimicrobial activity of Cornelian cherry (Cornus mas) fruit and leaf extracts. Hemijska Industrija 69:331–337. https://doi.org/10.2298/HEMIND140216046M
Martinović A, Cavoski I (2020) The exploitation of cornelian cherry (Cornus mas L.) cultivars and genotypes from Montenegro as a source of natural bioactive compounds. Food Chem 318:126549. https://doi.org/10.1016/j.foodchem.2020.126549
Pawlowska AM, Camangi F, Braca A (2010) Quali-quantitative analysis of flavonoids of Cornus mas L. (Cornaceae) fruits. Food Chem 119:1257–1261. https://doi.org/10.1016/j.foodchem.2009.07.063
Tenuta MC, Deguin B, Loizzo MR, Cuyamendous C, Bonesi M, Sicari V, Trabalzini L, Mitaine-Offer AC, Xiao J, Tundis R (2022) An overview of traditional uses, phytochemical compositions and biological activities of edible fruits of European and Asian cornus species. Foods 11(9):1240. https://doi.org/10.3390/foods11091240
Salejda AM, Kucharska AZ, Krasnowska G (2018) Effect of cornelian cherry (Cornus mas L.) juice on selected quality properties of beef burgers. J Food Qual 2018:1563651. https://doi.org/10.1155/(2018/1563651
De Biaggi M, Donno D, Mellano MG, Riondato I, Rakotoniaina EN, Beccaro GL (2018) Cornus mas (L.) fruit as a potential source of natural health-promoting compounds: physico-chemical characterisation of bioactive components. Plant Foods Human Nutr 73(2):89–94. https://doi.org/10.1007/s11130-018-0663-4
Bayram HM, Ozturkcan A (2020) Bioactive components and biological properties of cornelian cherry (Cornus mas L.): a comprehensive review. J Funct Foods 75:104252
Moldovan B, Filip A, Clichici S, Suharoschi R, Bolfa P, David L (2016) Antioxidant activity of Cornelian cherry (Cornus mas L.) fruits extract and the in vivo evaluation of its anti-inflammatory effects. J Funct Foods 26:77–87
Behrangi N, Ghafoori H, Farahmand Z, Khani EM, Sanati MH (2015) Comparison among cornelian cherry and Prunus cerasus according to phenolic content and antioxidant capacity by three various methods of extraction. Food Nutr Sci 6:1166–1173. https://doi.org/10.4236/fns.(2015.612122
Alavian SM, Banihabib N, Haghi ME, Panahi F (2014) Protective effect of Cornus mas fruits extract on serum biomarkers in CCl4—induced hepatotoxicity in male rats. Hepat Mont 14:e10330
Kazimierski M, Regula J, Molska M (2019) Cornelian cherry (Cornus mas L.)—characteristics, nutritional and pro-health properties. Acta Sci Pol Technol Aliment 18(1):5–12
Lachman J, Hamouz K, Čepl J, Pivec V, Šulc M, Dvořák P (2006) The effect of selected factors on polyphenol content and antioxidant activity in potato tubers. Chem Listy 100:522–527
Lapornik B, Prošek M, Wondra AG (2005) Comparison of extracts prepared from plant by-products using different solvents and extraction time. J Food Eng 71(2):214–222. https://doi.org/10.1016/j.jfoodeng.(2004.10.036
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28(1):25–30. https://doi.org/10.1016/S0023-6438(95)80008-5
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26(9–10):1231–1237. https://doi.org/10.1016/S0891-5849(98)00315-3
Pedersen CB, Kyle J, Jenkinson A, Gardner PT, McPhail DB, Duthie GG (2000) Effects of blueberry and cranberry juice consumption on the plasma antioxidant capacity of healthy female volunteers. Eur J Clin Nutr 54(5):405–408. https://doi.org/10.1038/sj.ejcn.1600972
Addinsoft XLSTAT (2014) Analyse de donneés et statistique avec MS Excel. Addinsoft, New York
R Core Team (2021) R: A language and environment for statistical computing (Version 4.1) [Computer Software] Available online: https://cran.r-project.org
The jamovi project (2022) jamovi (Version 2.3) [Computer Software]. Available online: https://www.jamovi.org
Yahia EM, García-Solís P, Celis MEM (2019) Contribution of fruits and vegetables to human nutrition and health. Postharvest physiology and biochemistry of fruits and vegetables. Woodhead Publishing, pp 19–45
FAOSTAT. Food and Agriculture Organization of the United Nations. https://www.fao.org/faostat/en/#data/FBS
Hossain MM, Rahim MA, Haque MR (2021) Biochemical properties of some important underutilized minor fruits. J Agric Food Res 5:100148. https://doi.org/10.1016/j.jafr.2021.100148
Antolak H, Czyzowska A, Sakač M, Mišan A, Đuragić O, Kregiel D (2017) Phenolic compounds contained in little-known wild fruits as antiadhesive agents against the beverage-spoiling bacteria asaia spp. Molecules 22(8):1256. https://doi.org/10.3390/molecules22081256
Gąstoł M, Krośniak M, Derwisz M, Dobrowolska-Iwanek J (2013) Cornelian cherry (Cornus mas L.) juice as a potential source of biological compounds. J Med Food 16(8):728–732. https://doi.org/10.1089/jmf.2012.0248
Bystricka J, Musilova J, Tomas J, Vollmannova A, Lachman J, Kavalcova P (2014) Changes of polyphenolic substances in the anatomical parts of buckwheat (Fagopyrum esculentum Moench.) during its growth phases. Foods 3(4):558–568
Sarma DA, Sharma R (1999) Anthocyanin-DNA copigmentation complex: mutual protection against oxidative damage. Phytochem 52(7):1313–1318. https://doi.org/10.1016/s0031-9422(99)00427-6
Li D, Zhang X, Xu Y, Li L, Aghdam MS, Luo Z (2019) Efect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem 289:112–120. https://doi.org/10.1016/j.foodchem.2019.03.042
Dzydzan O, Bila I, Kucharska AZ, Brodyak I, Sybirna N (2019) Antidiabeticeffects of extracts of red and yellow fruits of cornelian cherries (Cornus mas L.) on rats with streptozotocin-induced diabetes mellitus. Food Funct 16(10):6459–6472. https://doi.org/10.1039/C9FO00515C
Hassanpour H, Yousef H, Jafar H, Mohammad A (2011) Antioxidant capacity and phytochemical properties of cornelian cherry (Cornus mas L.) genotypes in Iran. Sci Hortic 129(3):459–463
Kucharska AZ, Szumny A, Sokół-Łętowska A, Piórecki N, Klymenko SV (2015) Iridoids and anthocyanins in cornelian cherry (Cornus mas L.) cultivars. J Food Compos Anal 40:95–102. https://doi.org/10.1016/j.jfca.2014.12.016
Güzel N (2021) Morphometric and physico-chemical properties of Cornelian cherry (Cornus mas L.) grown in Çorum, Turkey. Akademik Gıda 19(4):373–380. https://doi.org/10.24323/akademik-gida.1050750
Rop O, Mlcek J, Kramarova D, Jurikova T (2010) Selected cultivars of cornelian cherry (Cornus mas L.) as a new food source for human nutrition. Afr J Biotechnol 9(8):1205–1210
Klymenko S, Kucharska AZ, Sokół-Łętowska A, Piórecki N (2019) Antioxidant activities and phenolic compounds in fruits of cultivars of Cornelian cherry (Cornus mas L.). Agrobiodivers Improv Nutr Health Life Qual 3:484–499. https://doi.org/10.15414/agrobiodiversity.2019.2585-8246.484-499
Spychaj R, Kucharska AZ, Szumny A, Przybylska D, Pejcz E, Piórecki N (2021) Potential valorization of Cornelian cherry (Cornus mas L) stones: roasting and extraction of bioactive and volatile compounds. Food Chem 358:129802. https://doi.org/10.1016/j.foodchem.2021.129802
Roidoung S, Dolan KD, Siddiq M (2016) Gallic acid as a protective antioxidant against anthocyanin degradation and color loss in vitamin-C fortified cranberry juice. Food Chem 210:422–427. https://doi.org/10.1016/j.foodchem.2016.04.133
Kostecka M, Szot I, Czernecki T, Szot P (2017) Vitamin C content of new ecotypes of cornelian cherry (Cornus mas L.) determined by various analytical methods. Acta Sci Pol Hortorum Cultus 16(4):53–61. https://doi.org/10.24326/asphc.2017.4.6
Szczepaniak OM, Kobus-Cisowska J, Nowosad K, Stuper-Szablewska K, Markowska J, Szulc P (2021) Relationship of colour with the phytocompounds present in Cornus mas cultivars. Int J Food Prop 24(1):400–414. https://doi.org/10.1080/10942912.2021.1898420
Vidrih R, Čejić Ž, Hribar J (2012) Content of certain food components in flesh and stones of the cornelian cherry (Cornus mas L.) genotypes. Croat J Food Sci Technol 4:64–70
Cosmulescu S, Trandafir I, Nour V (2017) Phenolic acids and flavonoids profiles of extracts from edible wild fruits and their antioxidant properties. Int J Food Prop 20(12):3124–3134. https://doi.org/10.1080/10942912.2016.1274906
Bajić-Ljubicić J, Popović Z, Matić R, Bojović S (2018) Selected phenolic compounds in fruits in wild growing Cornus mas L. Indian J Tradit Knowl 17(1):91–96
Falcone Ferreyra ML, Rius SP, Casati P (2012) Flavonoids: biosynthesis, biological functions, and biotechnological applications. Front Plant Sci 3:222. https://doi.org/10.3389/fpls.2012.00222
Marchiosi R, dos Santos WD, Constantin RP, de Lima RB, Soares AR, Finger-Teixeira A, Ferrarese-Filho O (2020) Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem Rev 19:865–906. https://doi.org/10.1007/s11101-020-09689-2
Khusnutdinov E, Sukhareva A, Panfilova M, Mikhaylova E (2021) Anthocyanin biosynthesis genes as model genes for genome editing in plants. Int J Mol Sci 22(16):8752. https://doi.org/10.3390/ijms22168752
Karaaslan MG, Karaaslan NM, Burhan ATES (2018) Investigation of mineral components and antioxidant properties of a healthy red fruit: Cornelian cherry (Cornus mas L.). J Turkish Chem Soc 5(3):1319–1326. https://doi.org/10.18596/jotcsa.449593
Gozlekci S, Esringu A, Ercisli S, Eyduran SP, Akin M, Bozovic D, Sagbas HI (2017) Mineral content of cornelian cherry (Cornus mas L.) fruits. Oxid Commun 40(1):301–308
Dokoupil L, Řezníček V (2012) Production and use of the cornelian cherry–Cornus mas L. Acta Univ Agric Silvic Mendelianae Brun 60(8):49–58. https://doi.org/10.11118/actaun201260080049
Cetkovská J, Diviš P, Vespalcová M, Pořízka J, Řezníček V (2015) Basic nutritional properties of cornelian cherry (Cornus mas L.) cultivars grown in the Czech Republic. Acta alim 44(3):357–364. https://doi.org/10.1556/aalim.2014.0013
Krośniak M, Gąstoł M, Szałkowski M, Zagrodzki P, Derwisz M (2010) Cornelian cherry (Cornus mas L.) juices as a source of minerals in human diet. J Toxicol Environ Health A 73(17–18):1155–1158. https://doi.org/10.1080/15287394.2010.491408
Santoni F, Paolini J, Barboni T, Costa J (2014) Relationships between the leaf and fruit mineral compositions of Actinidia deliciosa var. Hayward according to nitrogen and potassium fertilization. Food Chem 147:269–271. https://doi.org/10.1016/j.foodchem.2013.09.154
Joy EJ, Broadley MR, Young SD, Black CR, Chilimba AD, Ander EL, Watts MJ (2015) Soil type influences crop mineral composition in Malawi. Sci Tot Environ 505:487–595. https://doi.org/10.1016/j.scitotenv.2014.10.038
Kim EH, Lee SY, Baek DY, Park SY, Lee SG, Ryu TH, Oh SW (2019) A comparison of the nutrient composition and statistical profile in red pepper fruits (Capsicums annuum L.) based on genetic and environmental factors. App Biol Chem 62:1–13. https://doi.org/10.1186/s13765-019-0456-y
Devarajan R, Jayaraman JK, Somasundaram SM, Ragupathy S, Raman P, Sathiamoorthy K, Subbaraya U (2021) Genetic diversity in fresh fruit pulp mineral profile of 100 Indian Musa accessions. Food Chem 361:130080. https://doi.org/10.1016/j.foodchem.2021.130080
Mota M, Martins MJ, Policarpo G, Sprey L, Pastaneira M, Almeida P, Oliveira CM (2022) Nutrient content with different fertilizer management and influence on yield and fruit quality in apple cv. Gala Hortic 8(8):713. https://doi.org/10.3390/horticulturae8080713
Funding
Open access funding provided by The Ministry of Education, Science, Research and Sport of the Slovak Republic in cooperation with Centre for Scientific and Technical Information of the Slovak Republic. This research was funded by the scientific grant APVV-22-0255.
Author information
Authors and Affiliations
Contributions
Conceptualization, JL and JB; methodology, JA; software, MŠ, JA; validation, JB, AB; formal analysis, MŠ; investigation, JA, ĽH; resources, OG; data curation, VK; writing—original draft preparation, JL, NČ; writing—review and editing, NČ, MŇ; visualization, NČ; supervision, MB, AB; project administration, VK; funding acquisition, MB. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lidiková, J., Čeryová, N., Grygorieva, O. et al. Cornelian cherry (Cornus mas L.) as a promising source of antioxidant phenolic substances and minerals. Eur Food Res Technol 250, 1745–1754 (2024). https://doi.org/10.1007/s00217-024-04513-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04513-z