Abstract
In this study, we evaluated Electrical Impedance Spectroscopy (EIS) as a monitoring tool of the physiological state of Bryopsis, Cystoseira, Stypopodium, Cladophora, Taonia, Padina, Ulva and Sargassum tissues. We analyzed the electrical response differences in the EIS between species and in the same seaweed tissue before and after electroporation. Electroporation using high voltage pulsed electric field (PEF) treatment was used as a model for cell disruption affecting the tissue physiology without being noticeable to the naked-eye. Significant differences in all the seaweeds were observed before and after electroporation. We found that seaweed species with smaller and rounder cells have a clearer dispersion profile (around a frequency of 10–100 kHz) compared to the dispersion profile of seaweed with larger cells with unround form. Those results suggest that EIS could be used as a fast non-invasive monitoring technique of the changes in the physiology of seaweeds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweed is an alternative biomass resource offering growing opportunities for human ever-increasing need in food, animal feed, biomaterial, energy, or drugs [1,2,3,4,5,6,7]. This untapped resource is now getting increased attention after being mostly neglected compared to their land cousins. Indeed, the development of seagriculture and the use of seaweed products are booming, new species are being discovered and those under-studied organisms are slowly getting “hacked”, as we did for microorganisms, plants, or animals.
Yet, we are far from mastering those peculiar marine organisms, and even species that were studied, grown, and used for more than hundreds of years, remain full of secrets. For example, the life cycle of most seaweed is still out of our grasps, hindering our capacity to use and study them [8]. The understanding of their physiology, their biochemistry, their mechanical properties, and their responses to abiotic and biotic stresses, human activities, or their processing are all crucial. Knowing what to look at when studying seaweed also depends on our capacity to measure, identify, and quantify relevant observations and changes.
New analytical tools for seaweed are thus required [9]. From an industrial and academic point of view, easily and quickly obtaining information about their physiology is a requisite in its study in laboratory or natural habitat (ecology, algal bloom monitoring, etc.), upstream processes (culture, hatching, strain selection, …) as well as downstream processes (harvesting, spoilage, the efficiency of preservation or disruption operations, …) [9]. However, there is a lack of knowledge in using classic and emerging physiological monitoring technologies on seaweed [8,9,10]. One of such technologies, which are widely used for physiological measurements of many organisms is Electrical Impedance Spectroscopy (EIS).
From an electrical point of view, tissues can be represented as an equivalent circuit containing resistors and capacitors integrated into various ways, depending on the tissue structure and physiological state [11, 12]. EIS is an analytical method that is used to characterize tissues based on their hindrance to the flow of the electrical current and is related to the material absorption of electromagnetic waves [13]. The approach consists of applying an electric stimulus, current or voltage, and measuring the resulting current or voltage [13]. Thus, by forcing a weak electrical current to pass through a biological sample at various frequencies, one can monitor and observe changes in its electrical properties, themselves related to the changes of the tissue physico-chemical features, notably the cell membrane integrity [11, 12, 14].
In biology and medicine, EIS, applied to organisms and organic matters, allows observing profound changes in the physico-chemical properties of tissues in a matter of few minutes [11, 15, 16]. Since EIS is sensitive to changes in ions concentration in or outside cells, fat content, membranes integrities and amount, cell sizes and shapes, extracellular matrix properties, etc. EIS has been used successfully to monitor physiological states of microorganisms, microalgae, animals, and human tissues and cells, plant tissues and cells or biomolecules [11, 12, 15,16,17,18,19,20,21,22], and is a growing academic field with its own communities and journals [23]. This has led to gain knowledge used both in and outside academia in medicine, agriculture, food industry, and ecology [15,16,17,18,19, 21]. Yet only one report of the use of EIS on a single seaweed species is available [24].
The goal of this study is to assess EIS as a rapid non-invasive monitoring tool of the physiological states of marine macroalgae tissue. For this, we harvested 8 different seaweed genus from 2 different classes: Phaeophyceae and Chlorophyta, from the Israeli Mediterranean Coast. We then measured their impedance in a wide frequencies range (from 100 Hz to 1 MHz) before and after the application of high-voltage pulsed electric field treatment (PEF).
PEF induces electroporation of the cell membranes, leading to profound changes at the cellular level and tissue level [25]. However, PEF has limited effects on the cell wall and extracellular matrices [26], thus leaving the seaweed tissue’s macrostructure mostly intact. Notably, no visual differences could be observed between electroporated and non-treated seaweed in opposition to other common physical methods to treat seaweeds such as cutting, drying, or crushing. Thus, we chose PEF as a good model treatment that induces profound physiological changes in the macroalgal tissue that are not visually obvious. Moreover, PEF has been proposed and proved as a versatile tool for seaweed processing from genetic engineering to extraction of valuables materials [24, 27,28,29,30,31,32,33]. This work will also help to advance the knowledge on the effect of PEF on seaweed tissues with the use of EIS.
Here, we report on the use of EIS for seaweed tissue changes monitoring. First, we show the use of EIS to classify seaweed species. We show that some of the eight tested seaweeds have their own unique EI spectra. Second, we show that EIS can differentiate between PEF treated and untreated seaweed biomass, a cell disintegration index was included. Third, we relate the changes observed in the EI spectrum to seaweed cell shape and size and the relative fraction of cell volume of the seaweed tissue.
Materials and methods
Experimental set up description
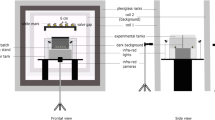
The experimental setup (Fig. 1) has three main parts. (1) Pulsed electric fields (PEF) generator, (2) electroporation cell (EPC) and (3) impedance meter. The PEF device was designed and developed in the laboratory to generate specific PEFs protocols at preprogrammed parameters [24, 31]. The EPC is integrated by a chamber to hold the biomass sample in gravity press-electrode space during the electroporation process. A commercial impedance meter (ScioSpec, ISX-3, Germany) was used for EIS measurements. The current was measured using a PicoScope 4224 Oscilloscope with a Pico Current Clamp (60A AC/DC), (Pico Technologies Inc., UK).
Macroalgae biomass preparation and electroporation
Eight seaweed species were collected on the Israeli north coast (Rosh Hanikra, Israel) in March 2019. The seaweeds were identified and classified according to taxonomical divisions. Samples were gently washed with deionized water and centrifuged in a manual kitchen centrifuge to remove the surface water 3 times for 2 min. For PEF treatment, 1 g of the fresh weight (FW) biomass was loaded in the chamber with 0.5 ml of deionized water and pressure in the upper electrode weight was released (1.6 kg). The distance between the two electrodes was monitored using a displacement sensor (optoNCDT, Micro-Epsilon, NC). Once the distance had stabilized by the applied pressure of the upper electrode, the multifrequency impedance was measured, then PEF was applied, and then the impedance was measure again. The used PEF protocol was 100 ± 10 V mm−1 field strength, pulse duration 50 µs, pulse number 100, pulse repetition frequency 4 Hz. Cystoseira sp. received the same treatment with the exception of the electric field intensity being 50 ± 10 V mm−1, due to its very high conductivity. The experiment was repeated in triplicate for each species.
Impedance measurement and disintegration index
Multi-frequency electrical impedance was measured by injecting a 100 mV peak potential difference in the frequency range of 100 Hz–1 MHz and then measuring the current to estimate the impedance of the system. A PC (HP mini 110-1150LA PC, HP Inc.) was used to program the Impedance meter module (ScioSpecTM, ISX-3, Germany) and store the data. Three measurements were made before and after the PEF treatment for each sample. Thus, each algae impedance is calculated as the average of three values for the three technical replicates, themselves being the average of three measurement replications.
The cell disintegration index (Zp) was calculated on the basis of the measurement of the absolute impedance value of control (Zc) and PEF-treated tissue (Ztr) in the low (1 kHz) and high (150 kHz) frequency ranges accordingly with [34, 35], as follows:
where the value of Zp varies between 0 for intact tissue and 1 for fully permeabilized tissue.
EIS fitting electrical model
The experimental impedance data were heuristically fitted to a mathematical model. The model used to fit the experimental data consists in a Constant Phase Element (CPE), to model the impedance behavior of the electrode–electrolyte interface, in series with a Cole impedance equation to model the impedance behavior of the sample [36]:
where \(\omega\) is the frequency (in rads/s) and \(j\) is the unit imaginary number. In the Cole equation (\({Z}_{Cole}\left(\omega \right)\)), \({R}_{\infty }\) would correspond to the sample impedance at infinite frequency, \({R}_{0}\) would correspond to the sample impedance at zero frequency, \(\tau\) would be the characteristic time constant of the sample and \(\alpha\) is a dimensionless parameter with a theoretical value between 0 and 1; in practice between 0.5 and 1. The equation employed to model impedance behavior of the electrode–electrolyte interface (\({Z}_{CPE}\left(\omega \right)\)) would be equivalent to that of a capacitance with n = 1 and \({Q}_{0}\) would correspond to the capacitance value. Similarly, to the case of the \(\alpha\) parameter, the \(n\) parameter can have a theoretical value between 0 and 1 but, in practice, it lies between 0.5 and 1. Fitting was performed for the frequency range from 100 Hz to 100 kHz.
Confocal microscopy imaging for tissue characterization
A Leica SP5 confocal microscope was used for imaging of the seaweed tissue sample. Frozen seaweed samples were defrosted, place on a slide and observe directly using the natural autofluorescence of the pigments at a magnification power of ×60 or ×100. No excitation with laser was used. Images were taken using different channels (gray and red) and the best picture was used for subsequent image analysis.
The analysis was performed using the open-source software ImageJ. First, pictures were selected according to their quality and the ability to clearly observe the cells and their outline. At least 4 pictures per algae type were analyzed. Each cell was selected manually and analyzed as regions of interest (ROI). Parameters related to their size (cell area (µm2), cell perimeter (µm)) and shape (major and minor axis of the best fit ellipse (µm)), angle, circularity, aspect ratio, roundness, and solidity) were obtained for each cell (for more details see: https://imagej.nih.gov/ij/docs/guide/146-30.html). The number of selected cells selected was between 16 and 115 depending on the algae type.
In addition, the total cell area on the tissue (the surface covered by the cells without including the extracellular matrix in the ROI) was measured by manually drawing the outline of each cell, then create a « mask » of the outline of the selected cells, and measure the selected area surface as % of the total surface of the tissue. A minimum of 4 pictures per algae were analyzed in such a way.
Data processing and statistical analysis
Impedance measurement data were analyzed by the non-parametric Mann–Whitney U test to compare differences in macroalgae species between control and PEF treatment conditions at every frequency, and one way ANOVA test and Tukey’s Post Hoc test. Data from ImageJ analysis were analyzed using ANOVA and Tukey’s Post Hoc test or non-parametric tests when assumptions were not met with their relevant post-hoc test. Significant differences were considered at p < 0.05. Data processing and analysis were performed using Excel (Microsoft Excel®, version 16.42), R (version 3.6.3, with RStudio version 1.3.959), and/or MATLAB (R2012b).
The raw data obtained from EIS provided for each frequency a complex number composed of a real number “R” associated with an imaginary number “X”. The data from triplicate measurement of 3 biological replicates were collected and for each frequency measurement, the mean and standard deviation of “R” and “X” were measured for each species.
Magnitude and phase were calculated for each species based on the average values of real and imaginary, and then normalized based on the highest value for each species. Bode plots of magnitude and phase were drawn for each species are presented below and in supplementary materials.
Real and imaginary parameters were normalized based on the highest values for each one, before being used to plot Nyquist plots presented below and in supplementary materials.
Results
The PEF parameters used for the electroporation protocol in the eight experimental species as well as the change in the volume occupied by the sample between the electrode and the current at the first and last pulses, respectively, are shown in Table 1. The applications of PEF induced profound changes in the seaweed biomass electromechanical properties. First, a drop in the volume between the electrode (in average for the 8 species: 19.73 ± 9.81%) was observed, as well as an increase in the electrical current flowing through the chamber (on average the increase of current between the first and last pulse was 20.39 ± 9.11 A, from 5.85 to 33.63 A) after each pulse for all species. The current of the first and last pulse applied to each species followed a typical exponential decay waveform (see Fig. S1 in Supplementary materials).
Bioimpedance spectra present noise throughout the analyzed bandwidth, such condition could be associated to the non-uniform contact of the tissue with the surface of the electrodes, where there may even be porous spots, nevertheless, a clear change in the impedance signal before and after PEF was noticed (Fig. 2, Figs. S2 and S3). EIS measurement system shows noise artefact effects starting around 100 kHz; thus, data analysis was developed below such frequency. EIS data were normalized based on its corresponding higher value point to keep the same dynamic range between species samples. Figure 2 shows the normalized magnitude and phase EIS (Bode plots), magnitude and phase changes due to PEF treatment are evident in the whole explored bandwidth. Statistically significant differences (p-value < 0.05) were observed in the whole frequency range above 0.1 kHz as well as in the range of 0.1–20 kHz for magnitude and phase parameters, respectively. For the magnitude parameters, non-treated algae showed a sharp decrease between 0.1 and 1 kHz, followed by stabilization or slower decrease in the frequency range of 1–100 kHz. PEF-treated algae showed in contrast a steeper decrease of magnitude in the range of 0.1–10 kHz, followed by a slow decrease at frequencies higher than 10 kHz and stabilization around a minimum value at the 100 kHz region. That minimum value is reached for the non-treated algae impedance magnitude, but at much higher frequency, thus the difference between the two curves is maximum between 1 and 100 kHz. This is common in plant biomass and it is used for example to calculate the cell disintegration index [35]. Concerning the impedance phase, the phases of non-treated and PEF-treated algae exhibit different behaviors across most of the frequency range. Notably, all PEF-treated algae have an impedance phase signal that is smaller than the non-treated phase at frequencies lower than ~ 10 kHz, before crossing it and becoming higher at higher frequencies. Non-normalized values of magnitudes and phase (see Fig. S2, Supplementary data) also showed clear differences before and after treatments, but also some inter-species differences. Magnitude in all algae had an inflection point around 10–100 kHz (log 4 to log 5 Hz) and it´s clearer for Bryopsis (algae n°1) Cystoseira (algae n°2), Padina (algae n°6), and to a lesser extent sample n°7 (Ulva) and n°8 (Sargassum) (see Fig. 2, Fig. S2A). For those three algae (n°1, n°2, and n°6), the impedance magnitude drops with increasing frequency and was observed throughout all the frequency range (0.1–100 kHz) from values above 2900 Ω to value below 1000 Ω. The total magnitude drop from minimum to maximum frequency was the highest for those three species compared to the other 5 algae. Those differences in the magnitude of the impedance of the 8 algae species disappeared entirely after PEF treatment but for Bryopsis (Fig. 2, Fig. S2B). Concerning impedance phase, most algae show a phase profile of a sigmoid, but for Bryopsis (algae n°1) Cystoseira (algae n°2), Padina (algae n°6), who have a different phase profile than the other 5 algae, as mentioned above this difference was also visible from the magnitude spectra (Fig. S2A,C). Only three algae showed a different pattern before PEF with the line of their Nyquist plot starting to curve at high frequency (sometimes even forming a half-circle), namely Bryopsis (algae n°1), Cystoseira (algae n°2) and Padina (algae n°6), which is the typical shape of Nyquist plot of biological tissues (Fig. 3, Fig. S3). However, the Nyquist plot of those three species after the PEF treatment lost that curvy feature entirely (Fig. 3, Fig. S3). In general, the Nyquist plots showed similar patterns than other biomass such as banana, bottle gourd, marine egg, or chicken tissues [37,38,39,40]. Table 2 shows the disintegration index estimated for every experimental species. The average disintegration index is 74.05 ± 4.01%, confirming the impact of PEF treatment on the seaweed tissues at similar indexes of disintegration then plant biomass [34, 35]. Bryopsis and Taonia had the lowest values of disintegration (52.9 ± 3.4% and 50.2 ± 14.2% respectively) while Padina had the highest (90.1 ± 1.7%). Bioimpedance spectroscopy changes were observed in PEF biomass in relation to non-electroporated biomass (Figs. 1, Figs. S3 and S4). The untreated biomass displays the common features of a so called \(\beta\) dispersion in the frequency range from about 10–100 kHz. The parameters obtained by fitting the data to the above referred mathematical model are indicated in Table S2 (in supplementary materials) and shown alongside experimental data in Fig. S4. At lower frequencies (< 10 kHz) the impedance of the electrode–electrolyte interface dominates. The occurrence of a \(\beta\) dispersion is more obviously manifested in Figs. S3 and S4 where the presence of a semicircle is observed in the imaginary part versus real part plot of the impedance.
Normalized bioimpedance magnitude and phase spectra (measured by EIS) before and after Pulsed Electric Field treatment, macroscopic and microscopic pictures of the 8 seaweed samples. Bioimpedance data are normalized based on its corresponding higher value point, they are average of triplicate measurements of triplicate samples per algae species, error bars are standard deviation. Microscopic images for Bryopsis were done with light microscopy (magnification ×40), all the others were taken from confocal fluorescence microscope (magnification ×100)
Since EIS measures the behavior of the electrical signal passing through the sample at various frequencies, they are links between the EIS data, and the tissue features from where its electrical properties originate. We chose to explore such links with raw morphological data related to cell size, shape, and their prevalence over extracellular matrix. Morphological data show clear differences between all algae as expected and seen in selected images for each species in the column “Micro-image” from Fig. 2. For all algae but Bryopsis, it was possible to observe a typical tissue image of cells delimited by a cell wall and bound together by an extracellular matrix. In Bryopsis, not such tissue organization was observed. At first, cell-like structures of various shapes were observed by light and confocal microscopy inside the surface of the algae. However, there were identified as diatoms, and it became clear that there was not the same multiple-cell organization and structure as observed in other samples [41,42,43]. This unique feature will be detailed in the Discussion part below, but as there were no cells, no morphological data could be obtained, and thus all morphological quantitative data only concerned algae n°2 to n°8. All morphological features selected (see Materials & Methods) but “angle” showed significant differences between some of the algae species in terms of size and shape (Table S1 in Supplementary materials). Only three features are presented and discussed as they convey more and complementary information: cell area (relating cell size), total cell area (in %, relating to tissue organization), and cell roundness (relating to cell shape). Figure 4 shows the distribution of cell areas among algae, showing that all algae were significantly different from each other but Cladophora and Padina. The algae order from the biggest to the smallest cell size was Cladophora & Padina > Taonia >> Stypopodium > Sargassum > Ulva > Cystoseira. The 7 species can be separated into two groups: those with heterogeneous cell area above 500 µm2 (Cladophora, Taonia and Padina) and those with relatively homogenous below 500 µm2. Figure 5 shows the distribution of cell roundness among the 7 seaweed species. Cell roundness is a value calculated using both parameters describing an elliptic shape and how close the shape is from a circle. The closest the roundness of a shape is to one, the closest to a circle with a more homogenous shape it is, while smaller values describe a more angular shape (such as a rectangle) with a heterogeneity of shape in one of the plane direction compared to the other (for example, an ellipse with a high ratio between the major and the minor axis). Significant differences between species were found although many couples of species did not have significantly different roundness (see Fig. 5 for details). Algal species arranged in order of highest to lowest roundness yielded the following: Ulva > Cystoseira > Sargassum > Stypopodium > > Padina > Taonia > Cladophora. Stypopodium exhibited the highest inhomogeneity in terms of cell roundness (and thus shape) among all species. Interestingly, the trio of species Cladophora, Taonia and Padina that were distinguishable from the other 4 for their cell sizes distribution, also stands out in terms of roundness. Figure 6 shows the ratio of surface occupied by cells and the total tissue surface area. Significant differences between the macroalgae species were found. Arranging the species from the highest to smallest total cell area ratio yields the following: Padina > Stypopodium > Cladophora > Sargassum > Taonia > Ulva > Cystoseira. However, many species did not have significant differences in total cell area as shown in Fig. 6. Cladophora and Ulva exhibited higher heterogeneity than the other species. Padina and Cystoseira are the two extremes among the species being the ones with the highest and lowest total cell area, respectively.
Boxplot of cell area (µm2) for the algae species n°2 to 8. Welch test showed statistically significant differences between algae (p value < 0.05). Games Howell Post Hoc Test indicated all algae are different from each other but 4 and 6 (designated by the green stars and line). Two algae with stars of different colors have significantly different cell areas. Boxes are delimited by the first and third quartile, while the median is the thicker horizontal line inside. Lines extend from the boxes to the minimum and maximum values (but for outsiders shown as dot). (2 = Cystoseira, 3 = Stypopodium, 4 = Cladophora, 5 = Taonia, 6 = Padina, 7 = Ulva, 8 = Sargassum). Bryopsis (algae n°1) morphological data could not get collected due to its unique morphology as a coenocytic algae (see Discussion about that feature in the article)
Boxplot of cell roundness for the algae species n°2 to 8. Welch test showed statistically significant differences between algae (p-value < 0.05). Games Howell Post Hoc Test indicated significant differences between algae but for 5 couples (2&7, 2&8, 3&8, 4&5, 5&6). Letters of colors serve as guide for identifying pair-wise statistical comparison (there is no significant differences between two algae sharing a common letter and/or color). Boxes are delimited by the first and third quartile, while the median is the thicker horizontal line inside. Lines extend from the boxes to the minimum and maximum values (but for outsiders shown as dot. (2 = Cystoseira, 3 = Stypopodium, 4 = Cladophora, 5 = Taonia, 6 = Padina, 7 = Ulva, 8 = Sargassum). Bryopsis (algae n°1) morphological data could not get collected due to its unique morphology as a coenocytic algae (see Discussion about that feature in the article)
Boxplot of total cell area (as % if the total seaweed tissues surface) for the algae species n°2 to 8. One way ANOVA test showed statistically significant differences between algae (p value < 0.05). Tukey’s Post Hoc Test indicated significant differences between all algae but for several subgroups (3&4,5,8; 4&5&8; 7&4,5,8; 3&6). Letters and colors serve as guide for identifying pair-wise statistical comparisons (there is no significant differences between two algae sharing a common letter and/or color). Boxes are delimited by the first and third quartile, while the median is the thicker horizontal line inside. Lines extend from the boxes to the minimum and maximum values. (2 = Cystoseira, 3 = Stypopodium, 4 = Cladophora, 5 = Taonia, 6 = Padina, 7 = Ulva, 8 = Sargassum). Bryopsis (algae n°1) morphological data could not get collected due to its unique morphology as a coenocytic algae (see Discussion about that feature in the article)
Discussion
All magnitude and phase spectra obtained from EIS showed different signatures in control with respect to PEF conditions for all seaweed species (Fig. 2), the average of the disintegration index is 0.7405 ± 0.04, thus tissue disintegration is evident by signature changes as shown in Bode and Nyquist plots (Figs. 2, 3, Figs. S2, S3). Even for the algae with the lowest disintegration index (Bryopsis and Taonia), the difference in Bode and Nyquist plots was appreciable (Figs. 2, 3, Figs. S2, S3). Such changes caused by the PEF treatment are characterized by a drop in the impedance, visible in the magnitude and the Nyquist plots as well as a stronger change in the profile of the curve at frequencies above 1 kHz, as typically reported for other electroporated biomass [35, 44, 45]. Indeed, PEF treatments induced permeabilization of the membrane of the cells, releasing intracellular material in the treatment media, decreasing its resistance (and thus its impedance) [44]. In addition, the loss of the membrane integrity impacts the electrical behavior of the sample as bilipid membranes are dielectrics playing the role of capacitors in biological samples. The loss of the capacitance in the sample induces changes in the profile of the Bode and Nyquist plots [11, 12, 36]. The reduction or disappearance of inflection points (change in the curve direction and slope) between 10 and 100 kHz is observed in most samples notably sample n°1 (Bryopsis), 2 (Cystoseira) and 6 (Padina) and to a lesser extent sample n°7 (Ulva) and n°8 (Sargassum). Thus, EIS can be used to monitor the effect of pulsed electric field treatment on seaweed biomass, as it has been shown with other types of land and aquatic biomass [34, 35, 44]. This could be useful for optimizing PEF treatment for seaweed biomass notably to find the threshold of electropermeabilization of seaweed cell membranes or irreversible treatments. Although differences are visible throughout most of the frequency spectra used in that work, specific frequencies where such differences are at their maximum could be selected. Typical such frequencies at 1 kHz and 150 kHz used for the calculation of the disintegration index were chosen from literature and previous works [34, 35]. However, depending on the species and goals, other frequency values could be selected for increased sensitivity and robustness. As stated in the introduction, we used PEF as a model for profound physiological changes that cannot be easily observed with simple observation (naked eyes, microscopy, etc.). These findings support the use of EIS as a fast and non-destructive monitoring tool for physiological change in seaweed. It is not in the scope of this manuscript to screen the effectiveness of EIS for monitoring other physiological states (such as disease, nutrient depletion, abiotic stress, life-cycle stage, etc. [15,16,17,18,19,20,21,22, 34]) however we hope this work will inspire such future study in that regard.
Concerning the EIS different spectra of the 8 seaweed species before PEF treatment, only a few differences between species were visible. It was not possible to determine a clear pattern unique to each seaweed species. However, as mentioned previously, the seaweed could be separated into two groups: a general group (species n° 3 Stypopodium, 4 Cladophora, 5 Taonia, 7 Ulva, and 8 Sargassum) with similar behavior, and another group with peculiar behaviors (species n°1 Bryopsis, 2 Cystoseira, and 6 Padina). The profiles of magnitude, phase, and Nyquist plots of the species of this second group were unique compared to the other 7 species. Interestingly those three species, Bryopsis, Cystoseira, and Padina, exhibit in their Nyquist plot (Fig. 3, Fig. S3) a semi-circular pattern at high frequency (above 10 kHz) with a strong inflection point, typical of the behavior of biological tissues following a Cole–Cole model [11, 18]. It is not clear why such a feature was not found in other seaweeds. Among the potential explanations, we suggest that potential differences in their cell structure could be playing a role (this point will be discussed later), but also the quality of the sample and of the set up during measurement (notable the contact between the sample and the electrode) [44]. The role of the setup quality for EIS measurement is crucial, and in our case, the electrode and chamber were designed for applying PEF treatment, not for EIS measurement, and that has affected the quality of our data (notably at low frequencies below 1 kHz and at high frequencies above 200 kHz) probably due to the electrode capacitive effect and resonance effect from the setup.
In a previous work by Levkov et al. (2020) who used a similar chamber and protocol, but with a different instrument (Vector Network Analyzer), was measured the impedance of a green seaweed Ulva sp. before and after PEF [24]. Similar trends were found and the semi-circular pattern on the Nyquist plot was visible for the Ulva sp. biomass before PEF treatment, while this could not be visible in this work. It is possible that the sample quality (although freshly harvested), preparation steps, or the instrument affected the quality and results of the measurement. Special care should thus be applied when preparing samples, choosing and using the apparatus, and selecting the thallus, and works is needed to screen protocols for high-quality EIS on seaweed biomass.
Concerning modeling, we mentioned previously that some algae seemed to show a typical behavior of a circuit following a Cole–Cole Model [18, 39, 45]. The application and use of equivalent circuit model are precious tools to simulate and extract more information from complex tissues using EIS [16,17,18, 21, 24, 45]. The presence of the beta-dispersion as mentioned previously is indicative of a dense assembly of living cells (Fig. S4, in that figure the electrode–electrolyte interface produces the straight line of about 45°.). In the treated samples the \(\beta\) dispersion disappears. In those cases, the behavior is equivalent to that of two electrodes in a saline medium. This indicates that the cell membranes, which because of their dielectric nature are responsible for the \(\beta\) dispersion, have been destroyed or severely permeated. Change in the mathematical model parameters can be found in Table S2, however, their further processing is not in the scope of this study, although interesting [46, 47]. For example, such modeling allows for the extraction of valuable quantitative parameters from the equivalent circuit, such as cytoplasm resistance, membrane resistance and capacitance, and extracellular matrix resistance [16,17,18, 21, 22, 35, 36, 44]. All of them being potential indicators of the physiological states of seaweed. This is also interesting for the field of Bioimpedance and EIS since seaweed differed from usual studied organisms notably due to their marine origin. Thus, attempts to model their impedance behavior could yield new sorts of models and their respective equivalent circuits, offering additional relations between living tissues, EIS spectra, and their simulated equivalent circuits.
Observation of the inter-species difference of changes in the EIS spectra before and after PEF revealed a surprising absence of variations. Indeed, looking at the Bode and Nyquist plots of seaweed after PEF, their profile all look alike (Figs. 2, 3-PEF, Figs. S2, S3). This is particularly visible in the combined normalized Nyquist plots of the 8 species (Fig. 3-PEF), which are all very similar apart from the algae n°1 Bryopsis, probably for the reason explained in the next paragraph. It is possible that this is a consequence of the setup used in that paper, where the electrode (with such a high surface) contribution plays a significant role in the EIS measurement compared to the electroporated samples. This homogeneity of the EIS spectra of PEF-treated seaweed is quite remarkable considering the diversity of species and morphology of the studied samples. So, although the EIS spectra are all different to each other to a certain extent before PEF treatment, high-degree of electroporation removed those differences.
The last aim of this paper was to try to find relations between morphological data and EIS spectra, in an attempt to reveal patterns that could be used for species identification or other potential uses. Profound morphological disparities between species were revealed but their actual link to EIS spectra was not clear, if existent. For example, we observed similar profiles in Bode plots for the species n°3, 4, 5, 7, and 8, while they have different cell sizes, cell shapes and total cell areas. On the other way around, algae with similar cell size, shape, or total cell area do not show the same behavior in their Bode or Nyquist plots (Fig. 2, Figs. S2 and S3). For example, the algae 4, 5, and 6 (Cladophora, Taonia, and Padina) had similar cell size and shape yet their EIS spectra are nowhere to show similarity, Padina having a completely different Nyquist plot than the other two (Fig. S3). Interestingly extreme morphology could be linked to special patterns in Bode and Nyquist plots. Padina has the biggest cell size, among the lowest roundness and highest total cell area, and showed a typical semi-circular feature on its Nyquist plot at high-frequency above 10 kHz. Also, Cystoseira, with the lowest cell size, high roundness, and lowest total cell area showed such a feature. Heterogeneity of the morphological data (standard deviation) did not seem to explain such results either, as those two algae have both homogenous and heterogenous morphological features. Could those extreme morphological features be linked to peculiar results under EIS? Perhaps it should be investigated in depth.
Interestingly, the missing algae n°1 Bryopsis, which could not be included in the morphological data, tell a similar story. Bryopsis is a coenocytic alga with few giant multinuclear cells [41,42,43]. Thus, its cellular structure is not based on microscopic cells bound together as other algae, but giant cells connected to a huge central vacuole space in the algae. This vacuole is even known to host its own endophytes (microbiota), that could encompass the diatoms we observed by microscopy and that we first identified as Bryopsis’s cell [41,42,43]. This unique morphology seemed to be visible by EIS as this algae show a very peculiar pattern in its Bode and Nyquist plots (Fig. 2, Figs. S2 and S3). Although the cell size, the cell shape, the cell fraction of the tissues, and potential heterogeneities in those 3 features have been shown to affect EIS spectra in other organisms [11, 12, 16,17,18, 36], we could not draw any such pattern from those seaweed samples. It seems that EIS could not serve as an identifying tool for seaweed species or extracting clear morphological information (such as cell size and shape). However, the morphological data were obtained from specific parts of the thalli and only on one plan (2D). Thus, the morphological data obtained here might not represent well-enough the ones of the samples. By a more exhaustive morphological characterization, it could possible to find links between structure and EIS spectra. This should be taken into consideration in future works.
The only exception regarding potential links between morphological data and EIS spectra are concerning the species Cystoseira (n°2) and Ulva (n°7), and to a lesser extent Sargassum (n°8). Those three species are the species with their cell being the closest to a round shape (Fig. 5), and the smallest cell area (and thus size, Fig. 4). Control magnitude spectra before the application of PEF showed clear dispersion regions in the low part of the beta frequency range (10–100 kHz) as long as cell micro-structures follow a round shape of fewer than 400 µm2 in cell surface area (Figs. 4 and 5), see spectra and micro-image of species Cystoseira (n°2), Ulva (n°7), and Sargassum (n°8), and to a much lesser extent in algal species Taonia (n°5) and Padina (n°6) (Fig. 2). Those observations are in agreement with theoretical predictions for ideal tissue structure [36]. In contrast, such dispersion regions were not evident in spectra of samples n°1, 3, and 4 (Figs. 2 and 3) where the cell shapes and size were different. In particular, the sample n°1 showed a clearly different magnitude signature, and it´s associated with a particular tissue structure considered as giant multinuclear cells as described previously.
Conclusion
We applied EIS on the tissues of 8 seaweed species from two classes Phaeophyceae and Chlorophyta. As a sham for potential physiological changes in seaweed tissues, we used electroporation to induce profound physiological changes hardly visible with naked eyes. EIS was able to successfully reveal changes induced by electroporation in all 8 species tissues. Comparing the EIS data of each species we described the profound differences in their impedance behavior. We related them to their morphology and identified the potential source of those differences. After electroporation, those differences disappeared entirely. EIS could thus become a tool to monitor physiological changes in seaweed, such as the effectiveness of cell disruption techniques (such as PEF treatment as done in this work), and more. One of the next steps required to expand the application of EIS on seaweed would be to develop impedance models for seaweed tissues and efforts in this sense are ongoing.
Data availability
All data are available upon requests, including R-Code for data processing.
References
Smit AJ (2004) Medicinal and pharmaceutical uses of seaweed natural products: a review. J Appl Phycol 16(4):245–262
Skjermo J, Aasen I, Arff J, Broch O, Carvajal A (2014) A new Norwegian bioeconomy based on cultivation and processing of seaweeds: opportunities and R&D needs. SINTEF Fisheries and Aquaculture Report A25981
van Hal JW, Huijgen WJJ, López-Contreras AM (2014) Opportunities and challenges for seaweed in the biobased economy. Trends Biotechnol 32(5):231–233
Buschmann AHAH, Camus C, Infante J, Neori A, Israel Á, Hernández-González MCMC, Pereda SVSV, Gomez-Pinchetti JLJL, Golberg A, Tadmor-Shalev N et al (2017) Seaweed production: overview of the global state of exploitation, farming and emerging research activity. Eur J Phycol 52(4):391–406
Seghetta M, Hou X, Bastianoni S, Bjerre A-B, Thomsen M (2016) Life cycle assessment of macroalgal biorefinery for the production of ethanol, proteins and fertilizers – a step towards a regenerative bioeconomy. J Clean Prod 137:1158–1169
Fernand F, Israel A, Skjermo J, Wichard T, Timmermans KR, Golberg A (2017) Offshore macroalgae biomass for bioenergy production: environmental aspects, technological achievements and challenges. Renew Sustain Energy Rev 75:35–45
Bikker P, Krimpen MM, Wikselaar P, Houweling-Tan B, Scaccia N, Hal JW, Huijgen WJJ, Cone JW, López-Contreras AM, van Krimpen MM et al (2016) Biorefinery of the green seaweed Ulva lactuca to produce animal feed, Chemicals and Biofuels. J Appl Phycol 28:3511–3525
Charrier B, Abreu MH, Araujo R, Bruhn A, Coates JC, De Clerck O, Katsaros C, Robaina RR, Wichard T (2017) Furthering knowledge of seaweed growth and development to facilitate sustainable aquaculture. New Phytol 216(4):967–975
Peleg Y, Shefer S, Anavy L, Chudnovsky A, Israel A, Golberg A, Yakhini Z (2019) Sparse NIR optimization method (SNIRO) to quantify analyte composition with visible (VIS)/near infrared (NIR) spectroscopy (350 nm-2500 nm). Anal Chim Acta 1051:32–40
Charrier B, Wichard T, Reddy CRK (2018) Protocols for macroalgae research. CRC Press, Boca Raton
Ivorra A (2003) Bioimpedance monitoring for physicians: an overview. Cent Nac Microelectron 2(July):1–35
Martinsen OG, Grimnes S, Schwan HP (2002) Interface phenomena and dielectric properties of biological tissue. Encycl Surf Colloid Sci 20:2643–2653
Bertemes-Filho P (2018) Electrical impedance spectroscopy. In: Simini F, Bertemes-Filho P (eds) Bioimpedance in biomedical applications and research. Springer International Publishing, Cham, pp 5–27
Golberg A, Laufer S, Rabinowitch HD, Rubinsky B (2011) In vivo non-thermal irreversible electroporation impact on rat liver galvanic apparent internal resistance. Phys Med Biol 56(4):951–963
Grossi M, Riccò B (2017) Electrical impedance spectroscopy (EIS) for biological analysis and food characterization: a review. J Sens Sens Syst 6:303–325
Jócsák I, Végvári G, Vozáry E (2019) Electrical impedance measurement on plants: a review with some insights to other fields. Theor Exp Plant Physiol 31(3):359–375
Prasad A, Roy M (2020) Bioimpedance analysis of vascular tissue and fluid flow in human and plant body: a review. Biosyst Eng 197:170–187
Borges E, Sequeira M, Cortez AF, Pereira HC, Pereira T, Almeida V, Cardoso J, Correia C, Vasconcelos TM, Duarte IM (2014) Bioimpedance parameters as indicators of the physiological states of plants in situ. Int J Adv Life Sci 6:74–86
El Khaled D, Castellano NN, Gazquez JA, Salvador RMG, Manzano-Agugliaro F (2017) Cleaner quality control system using bioimpedance methods: a review for fruits and vegetables. J Clean Prod 140:1749–1762
Sui J, Foflonker F, Bhattacharya D, Javanmard M (2020) Electrical impedance as an indicator of microalgal cell health. Sci Rep 10(1):1251
Bera TK, Bera S, Chowdhury A, Ghoshal D, Chakraborty B (2017) Electrical impedance spectroscopy (EIS) based fruit characterization: a technical review. In: Guha D, Chakraborty B, Dutta HS (eds) Computer, communication and electrical technology. CRC Press, pp 279–288
Chen B-Y, Lee C-H, Chang J-S, Hsueh C-C (2015) Impedance fingerprint selection of DHA-producing photoautotrophic microalgae. J Taiwan Inst Chem Eng 57:36–41
Grimnes S, Martinsen ØG (2019) Journal of Electrical Bioimpedance (JEB)–a new, open access, scientific journal. J Electr Bioimpedance 1(1):1
Levkov K, Linzon Y, Mercadal B, Ivorra A, González CA, Golberg A (2020) High-voltage pulsed electric field laboratory device with asymmetric voltage multiplier for marine macroalgae electroporation. Innov Food Sci Emerg Technol 60:102288
Kotnik T, Kramar P, Pucihar G, Miklavcic D, Tarek M (2012) Cell membrane electroporation – part 1: the phenomenon. IEEE Electr Insul Mag 28(5):14–23
Delsart C (2017) Plant cell wall: description, role in transport, and effect of electroporation. In: Miklavcic D (ed) Handbook electroporation. Springer International Publishing, Cham, pp 489–509
Robin A, Sack M, Israel A, Frey W, Müller G, Golberg A (2017) Deashing macroalgae biomass by pulsed electric field treatment. Bioresour Technol 2018(255):131–139
Prabhu M, Levkov K, Levin O, Vitkin E, Israel A, Chemodanov A, Golberg A (2020) Energy efficient dewatering of far offshore grown green macroalgae Ulva sp. biomass with pulsed electric fields and mechanical press. Bioresour Technol 295:122229
Robin A, Golberg A (2016) Pulsed electric fields and electroporation technologies in marine macroalgae biorefineries. Handb Electroporation 4:2923–2938
Robin A, Kazir M, Sack M, Israel A, Frey W, Mueller G, Livney YD, Golberg A (2018) Functional protein concentrates extracted from the green marine macroalga Ulva sp., by high voltage pulsed electric fields and mechanical press. ACS Sustain Chem Eng 6(11):13696–13705
Levkov K, Vitkin E, González CA, Golberg A (2019) A laboratory IGBT-based high-voltage pulsed electric field generator for effective water diffusivity enhancement in chicken meat. Food Bioprocess Technol 12(12):1993–2003
Polikovsky M, Fernand F, Sack M, Frey W, Müller G, Golberg A (2016) Towards marine biorefineries: selective proteins extractions from marine macroalgae Ulva with pulsed electric fields. Innov Food Sci Emerg Technol 37:194–200
Prabhu MS, Levkov K, Livney YD, Israel A, Golberg A (2019) High-voltage pulsed electric field preprocessing enhances extraction of starch, proteins, and ash from marine macroalgae Ulva ohnoi. ACS Sustain Chem Eng 7(20):17453–17463
Angersbach A, Heinz V, Knorr D (2002) Evaluation of process-induced dimensional changes in the membrane structure of biological cells using impedance measurement. Biotechnol Prog 18(3):597–603
Angersbach A, Heinz V, Knorr D (1999) Electrophysiological model of intact and processed plant tissues: cell disintegration criteria. Biotechnol Prog 15(4):753–762
Grimnes S, Martinsen OG (2011) Bioimpedance and bioelectricity basics. Academic Press, Cambridge, MA
Singh G, Singh V, Anand S, Lall B (2017) Study of variations in electrical impedance measurements with different impurities in vegetable phantoms. In: 2017 IEEE long island systems, applications and technology conference (LISAT), IEEE, pp 1–7
Bera TK, Nagaraju J (2011) Electrical impedance spectroscopic studies on broiler chicken tissue suitable for the development of practical phantoms in multifrequency EIT. J Electr Bioimpedance 2(1):48–63
Cole KS (1937) Electric impedance of marine egg membranes. Trans Faraday Soc 33:966–972
Bera TK, Bera S, Kar K, Mondal S (2016) Studying the variations of complex electrical bio-impedance of plant tissues during boiling. Procedia Technol 23:248–255
Hollants J, Leroux O, Leliaert F, Decleyre H, De Clerck O, Willems A (2011) Who is in there? Exploration of endophytic bacteria within the siphonous green seaweed Bryopsis (Bryopsidales, Chlorophyta). PLoS ONE 6(10):e26458
Hollants J, Decleyre H, Leliaert F, De Clerck O, Willems A (2011) Life without a cell membrane: challenging the specificity of bacterial endophytes within Bryopsis (Bryopsidales, Chlorophyta). BMC Microbiol 11(1):255
La Claire JW (1987) Microtubule cytoskeleton in intact and wounded coenocytic green algae. Planta 171(1):30–42
Castellví Q, Mercadal B, Ivorra A (2016) Assessment of electroporation by electrical impedance methods. In: Miklavčič D (ed) Handbook of electroporation. Springer-Verlag, Cham, pp 671–690
Trainito CI, Français O, Le Pioufle B (2015) Analysis of pulsed electric field effects on cellular tissue with Cole-Cole model: monitoring permeabilization under inhomogeneous electrical field with bioimpedance parameter variations. Innov Food Sci Emerg Technol 29:193–200
Ivorra A (2010) Tissue electroporation as a bioelectric phenomenon: basic concepts BT. In: Rubinsky B (ed) Irreversible electroporation. Springer, Berlin Heidelberg, pp 23–61
Rubinsky B (2009) Irreversible electroporation. Springer Science & Business Media, Berlin, Heidelberg
Acknowledgements
The authors thank Dr Bénédicte Charrier (Roscoff Marine Station, France) and Dr Phillipe Andrey (INRA, France) for their advice and helps with image handling.
Funding
Open access funding provided by Tel Aviv University.
Author information
Authors and Affiliations
Contributions
AR: conceptualization, software, formal analysis, investigation, writing-original draft, visualization. KL: formal analysis, investigation, resources. CAG-D: software, validation, formal analysis, investigation, resources, writing-original draft, visualization. NPL-S: formal analysis, investigation. AG: conceptualization, formal analysis, investigation, writing-original draft, supervision, funding acquisition.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical approval
This article does not require any ethical approval.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Robin, A., Levkov, K., González-Díaz, C.A. et al. Electrical bioimpedance spectroscopy as a non-invasive monitoring tool of physiological states of macroalgae tissues: example on the impact of electroporation on 8 different seaweed species. Eur Food Res Technol (2024). https://doi.org/10.1007/s00217-024-04510-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00217-024-04510-2