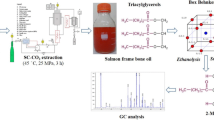
Abstract
In this study, a biphasic system combining oil and ionic liquid was utilized for lipase-catalyzed transesterification of salmon oil and alcohol to concentrate n-3 PUFAs, notably EPA and DHA. Various process variables, such as enzyme type, quantity, alcohol chain length, temperature, reactant proportions, and ionic liquid selection, were systematically assessed to optimize the process and enhance the yield of these valuable fatty acids. It was found that the Novozym 435 and Lipolase 100L Type EX emerged as the most effective enzymes. The impact of varying alcohol chain lengths (C1–C8) was examined, revealing that the Novozym 435 enzyme displayed its peak synthetic activity with 2-propanol. The results revealed a substantial increase in the overall activity during the transesterification reaction when employing ILs featuring hydrophobic cations and anions with low nucleophilicity. Specifically, the [omim+][NTf2−] ionic liquid exhibited the highest level of activity. This research holds promise for more efficiently and sustainably obtaining concentrated n-3 PUFAs from fish oil while reducing environmental impact relative to other existing concentration processes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, there has been a growing interest in the pharmaceutical and food industries regarding omega-3 polyunsaturated fatty acids (n-3 PUFAs) due to their numerous health benefits. Among the various n-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are of particular importance to human nutrition. These fatty acids are well known for their advantageous properties, which include their cardioprotective effects through the reduction of triacylglycerol levels, their anti-inflammatory and anti-cancer properties, as well as their potential to slow the progression of Alzheimer's disease [1]. Notably, DHA is the most abundant n-3 PUFA found in the central nervous system, including the brain and retina, making it vital for consumption during childhood and pregnancy [2, 3].
The primary natural sources of n-3 polyunsaturated fatty acids (n-3 PUFAs) are marine organisms, including fish, seafood, and algae. These organisms are directly or indirectly nourished by phytoplankton, the primary producers of n-3 PUFAs in the marine food chain [4]. While fish oils are the most commonly recognized source of n-3 PUFAs, most fish oils have relatively low contents of EPA and DHA, typically around 30% wt.% [5]. Some fish species from families like Scombridae, Clupeidae, and Salmonidae are known for having higher concentrations of these beneficial fatty acids [4]. The demand for nutraceuticals and dietary supplements, designed to enhance the health benefits of fish oils, has grown significantly. As a result, there has been a surge of interest in enriching fish oil products with EPA and DHA, not only for their potential health benefits but also to increase profitability within the fishing industry [1]. Traditional methods for obtaining fish oil involve a two-stage process: oil extraction from raw materials and subsequent refining. However, more recent processes for producing omega-3 concentrates for pharmaceutical or nutritional purposes include chromatography, vacuum or molecular distillation, low-temperature crystallization, formation of urea complexes, and cutting-edge techniques such as supercritical liquid fractionation, supercritical fluid chromatography, and enzymatic methods [4].
The application of enzymes in industrial processes, while relatively recent, has emerged as an attractive alternative to conventional methods. Enzymatic processes offer advantages in terms of simplicity, cost-effectiveness, and reduced energy consumption. When it comes to concentrating n-3 PUFAs, these processes primarily rely on specific enzymes, often lipases, known for their ability to catalyze various reactions involving triglycerides. The process of n-3 PUFAs concentration through enzymatic means frequently involves the transesterification reaction of triglycerides found in fish oil. This reaction converts these triglycerides into fatty acid ethyl esters and glycerol. Traditional transesterification methods usually employ acid or alkaline catalysts, which can result in low selectivity and undesirable side reactions. Additionally, the high temperatures required for these catalysts may lead to the oxidation of fish oil [5]. Consequently, the use of enzymatic catalysis in the transesterification of fish oils has emerged as an alternative to conventional techniques. Enzymatic processes offer the advantage of operating at milder conditions, minimizing the deterioration of n-3 PUFA concentrates [6,7,8,9]. In the literature, various enzymatic methods followed by membrane filtration separation processes have been proposed to obtain EPA and DHA, either in the form of free fatty acids, ethyl or methyl esters, or 2-acylglycerides [10]. Enzymatic esterification and transesterification processes are particularly valuable in the production of lipid derivatives derived from n-3 PUFAs, as they often possess superior properties when compared to the corresponding free fatty acids.
The primary challenge associated with enzyme-based processes is the limited miscibility of substrates, which hinders effective contact and reduces the conversion of n-3 PUFAs. As a result, enzymatic transesterification often requires the inclusion of traditional organic solvents, such as hexane or tertiary alcohols, to create a homogeneous reaction medium [11]. However, these solvents typically have characteristics that are less desirable. They are frequently toxic, flammable, or volatile, thereby posing environmental pollution risks and potential health hazards. Moreover, the use of these solvents contributes to higher synthesis costs, as they necessitate proper disposal of residual organic solvents and are often not amenable to recycling [12].
Ionic liquids (ILs) have emerged as highly promising solvents in various chemical and biochemical processes. Their negligible vapor pressure distinguishes ILs as environmentally friendly solvents compared to conventional organic solvents [13]. Moreover, ILs possess excellent chemical and thermal stability and can be tailored for specific applications by choosing the appropriate anion and cation components. This ability to custom-design ILs has earned them the moniker "designer solvents" and presents a significant advantage over traditional organic solvents [14]. Beyond their environmental appeal, ILs have found utility as solvents or reaction media in enzyme-catalyzed reactions. Enzymes can catalyze reactions that are challenging to perform in aqueous media, such as transesterification reactions [15, 16]. ILs can stabilize enzymes and enhance selectivity in transesterification reactions by preventing undesirable side reactions, like hydrolysis. The hydrophobicity and nucleophilicity of ILs are crucial factors influencing the behavior of lipases in IL media. De los Ríos et al. [17] demonstrated that more hydrophobic and less nucleophilic ILs promote higher synthetic activity in enzymatic transesterification reactions catalysed by the Candida antarctica lipase B.
ILs containing aromatic rings such as imidazolium or pyridinium have shown potential to extract and enrich n-3 PUFAs by forming reversible π-bonding [18]. Recently, the use of ILs in lipid extraction from microalgae has shown the potential to overcome common drawbacks [19]. However, to the best of our knowledge, only Fu et al. [12] have carried out the lipase-catalyzed transesterification reaction of fish oils using imidazolium-based ionic liquids to obtain a higher concentration of n-3 PUFAs. They studied the action of three lipases and three ILs and the thermal stability and oxidative kinetics of the products obtained. However, in our opinion, the optimization of the process variables has not been studied in depth and a wider range of enzymes and ILs with different cations is also preferred in order to select the most favorable experimental conditions to have the highest yield. This optimization is especially remarkable to scale up the process of fish oil refining to industry.
In this work, six different enzymes and fifteen imidazolium-based ILs containing different cations and anions (see Fig. 1) have been used to carry out the transesterification reaction of salmon oil with several alcohols. Up to date, this is the first time that the effect of the main process variables (enzyme, IL, quantity of enzyme, length of the chain of the alcohol, reaction temperature and substrate molar ratio) on the effectiveness of the enrichment of n-3 PUFAs from salmon oil has been profoundly analyzed.
Materials and methods
Chemicals and materials
Salmon oil was purchased from Luposan Ibérica (Alicante, Spain). All the enzymes (Novozym® 435, Lipozyme RM IM, Lipozyme TL IM, Lipolase 100L Type EX, Palatase 20000L and Novoshape) were a gift from Novo España S.A. (Spain). Novozym® 435 is a Candida antarctica lipase (CaLB) immobilized on acrylic resin by adsorption. Lipozyme RM IM is a Rhizomucor Miehei lipase immobilized on a macroporous anion-exchange resin. Lipozyme TL IM is a Thermomyces Lanuginosa lipase immobilized on porous silica granules. Lipolase 100L Type EX (from Thermomyces lanoginosus) and Palatase 20000L (from Aspergillus oryzae) are liquid-free lipase and Novoshape is a free pectin methylesterase. The ILs 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim+][PF6−]), 1-hexyl-3-methylimidazolium hexafluorophosphate ([hmim+][PF6−]), 1-methyl-3-octylimidazolium hexafluorophosphate ([omim+][PF6−]), 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim+][BF4−]), 1-methyl-3-octylimidazolium tetrafluoroborate ([omim+][BF4−]), 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([emim+][NTf2−]), 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([bmim+][NTf2−]), 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([hmim+][NTf2−]), 1-methyl-3-octylimidazolium bis(trifluoromethylsulfonyl)imide ([omim+][NTf2−]), 1-butyl-2,3-dimethylimidazolium bis(trifluoromethylsulfonyl)imide ([bdmim+][NTf2−]), 1-ethyl-3-methylimidazolium triflate ([emim+][TfO−]) and 1-ethyl-3-methylimidazolium ethylsulphate ([emim+][EtSO4−]) were purchased from IoLiTec (Heilbronn, Germany) (purity > 99%). The Ils 1-butyl-3-methylimidazolium methylsulphate ([bmim+][MeSO4−]), 1-butyl-3-methylimidazolium 2-(2-methoxiethoxi) ethylsulphate ([bmim+][MDEGSO4−]), and 1-butyl-3-methylimidazolium octylsulphate ([bmim+][OcSO4−]) were purchased from Solvent Innovation GmbH (Cologne, Germany) (purity > 99%). Figure 1 shows the constituents of the ILs used for the transesterification reactions. Solvents and other chemicals were purchased from Sigma Aldrich (Madrid, Spain) and were of the highest purity available.
Determination of acid value and peroxide value of the salmon oil
The acid value is an indicator of the acidity of fish oil, defined as the amount of potassium hydroxide in milligrams required to neutralize 1 g of the oil. It is directly proportional to the free fatty acid content of the oil. On the other hand, the peroxide value is an indicator of the quality and stability of oils. It quantifies the total hydroperoxides produced in the oil during primary oxidation [20].
The acid value of the salmon oil was determined according to the standard procedure [21]. First, 50 mL of a 1:1 ethanol: ethyl ether mixture and 0.5 mL of phenolphthalein indicator were added to a conical flask and neutralized with 0.1 M potassium hydroxide until a permanent pink color was achieved. Then, 10 g of salmon oil was dissolved in 50 mL of the neutralized solvent mixture. Titration was carried out with 0.1 M potassium hydroxide until a permanent pink color was reached. The acid value, expressed in mg KOH/g, was calculated using Eq. (1)
where V is the volume in mL of 0.1 M potassium hydroxide used for the titration of the sample and m is the sample mass in g.
The peroxide value of the salmon oil was also determined according to the standard procedure [21]. 5 g of salmon oil was weighed in a volumetric flask. Next, 30 mL of a 3:2 v/v glacial acetic acid: chloroform mixture was added to dissolve the oil. Afterward, 500 µL of saturated potassium iodide solution was added to the flask. The flask was vigorously shaken for 60 s, followed by the addition of 30 mL of water. The resulting yellowed solution was titrated with 0.01 M sodium thiosulfate solution until it became almost colorless.
To complete the titration, 5 mL of a 1% w/w starch solution was added to the mixture, and the resulting solution was titrated until it became completely colorless with 0.01 M sodium thiosulfate solution. A blank titration under the same conditions was also conducted as a control. The peroxide value, expressed in milliequivalents per gram (meq/g), was calculated using Eq. (2)
where V is the volume in mL of 0.01 M sodium thiosulfate used for the titration of the sample, Vcontrol is the volume in mL of 0.01 M sodium thiosulfate used for the titration of the blank and m is the sample mass in g.
Determination of density of the salmon oil
The density of the oil was determined using an Anton Paar Oscillating U-tube densitometer, specifically the DMA 4500 M model. This measurement was conducted across a range of temperatures from 293.15 to 313.15 K. The associated standard uncertainties for this measurement process were as follows: u(T) = 0.03 K, and u(ρ) = 0.00005 g/cm3. To ensure the reliability of the apparatus, it was calibrated using Millipore quality water and ambient air.
During the measurements, approximately 1 mL of the salmon oil was carefully transferred to the apparatus using a syringe. Particular attention was given to avoid the introduction of bubbles into the tube, as this could lead to errors in the density measurements. Each measurement was conducted in triplicate.
Chemical composition of the salmon oil
Thin-layer chromatography (TLC)
The chemical composition of the salmon oil used was determined by thin-layer chromatography (TLC). First, the silica gel plate was activated in an oven at 100 °C for 1 h. TLC was carried out using a hexane: diethyl ether: acetic acid solution (75:25:2 v:v:v) as the mobile phase. 3 µL of salmon oil was injected onto the TLC plates, which were then immersed in the mobile phase placed in a separation chamber. After approximately 1 h, the plates were removed from the chamber, and any remaining mobile phase was allowed to evaporate.
Subsequently, the plates were visualized by spraying with 50% sulfuric acid (using nitrogen as the gas propellant) and then placing the plates in the oven at 100 °C for 15 min. Finally, the plates were scanned, and the images were analyzed using the UN-SCAN-IT Gel Analysis Software. The analysis of the shaded areas led to the calculation of the composition in percentage. Each area corresponded to a specific component: triglycerides, methyl esters, free fatty acids, monoglycerides, and diglycerides.
Gas chromatography analysis
The content of the majority fatty acids in the salmon oil was determined by prior methylation of the sample followed by subsequent analysis using gas chromatography (GC). In summary, 1 mL of a 1 mg/mL methyl tricosanoate in isooctane solution was added to a screw-capped tube, and the solvent was evaporated under vacuum. Then, 25 mg of salmon oil and 4 mL of a methanol: sulfuric acid solution (96:4 v:v) were added to the tube. The tube was sealed under a nitrogen atmosphere and stirred for 1 h at 100 °C. Afterward, the tube was cooled, and 2 mL of distilled water and 1 mL of heptane were added. The tube was vigorously shaken and left until phase separation occurred. The upper phase (heptane) was collected in a GC vial, and the solvent was removed under vacuum. To the first tube, 1 mL of heptane was added, shaken again, and left until phase separation. Once more, the upper phase was collected and added to the GC vial for analysis.
GC analysis was performed with an Agilent 6890N instrument equipped with FID detector and a Supelco-2560 ™ capillary GC column (30 m × 250 µm × 0.20 µm) based on a poly(biscyanopropyl siloxane). Methyl tricosanoate served as the internal standard. The chromatographic conditions were as follows: carrier gas (He) at 23.86 psi (19.489 mL/min total flow); temperature program: 170 ºC, 1 ºC/min, 220 ºC, hold 20 min; split ratio: 10/1. Injector and detector temperatures were set at 250 and 260 ºC, respectively. The retention times of the PUFA standards were as follows: γ-linolenic acid ethyl ester (C18:3n6), 21.6 min; linolenic acid methyl ester (C18:3n3), 23.6 min; cis-11,14,17 eicosatrienoic acid methyl ester (C20:3n3 or ETA), 35.3 min; cis-5,8,11,14,17 eicosapentanoic acid methyl ester (C20:5n3 or EPA), 36.8 min; tricosanoic acid methyl ester (C23:0 used as internal standard), 51.4 min and cis-4,7,10,13,16,19 docosahexaenoic acid methyl ester (C22:6n3 or DHA), 51.6 min. Methyl ester concentrations in the reaction mixture were calculated from calibration curves using stock solutions of pure compounds.
Enzyme-catalyzed transesterification reaction
Reactions were carried out under two different modes. For the evaluation of the reaction profiles to obtain the specific activity of the enzymes, the required mass of the enzyme (ranging from 5 to 45 mg) was placed in a 10 mL screw-capped vial equipped with a stirring bar. Then, 3 mL of salmon oil, 3 mL of an IL, and 1.3 mL of methanol (molar ratio methanol:oil of 10.1) were added to start the transesterification reaction. The vial was placed in a thermostatic bath at the desired temperature (from 30 to 50 ºC) and stirred at 900 rpm, and samples were taken at 30 min, 1, 1.5, 2, 3, 4, 5, 6, 7, 24, and 48 h. For this, at each time point, the vial was removed from the bath, stirred in a vortex, and then centrifuged for 5 min at 2500 rpm. 100 µL from the organic upper phase were mixed with 250 µL of hexane and 250 µL of distilled water in a 2 mL vial to extract n-3 PUFAs into the hexane phase. Afterward, the 10 mL vial was vortexed and placed again in the thermostatic bath. For the analysis of the samples, the 2 mL vial was stirred and left until phase separation was observed. Then, 150 µL from the upper phase was diluted with 1 mL of the internal standard solution (2.71 mM methyl tricosanoate in hexane). Finally, 2 µL of the resulting solution were analyzed by GC.
On the other hand, for the study of the influence of the variables, the reactions were carried out in 1.5 mL vials. Briefly, the required mass of enzyme, 0.5 mL of salmon oil, 0.5 mL of IL, and 250 µL of alcohol (methanol, ethanol, 2-propanol, butanol, or octanol) was added to the vial, which was immediately placed in the shaker located in the oven at the desired temperature. Samples were withdrawn at 24 h and 48 h. For this, the vial was centrifuged at 7000 rpm for 5 min and 100 µL of the upper phase were diluted with 250 µL of hexane and 250 µL of distilled water in a 2 mL vial. The vial with the reaction was vortexed and placed again in the oven. The vial with the sample was stirred and left until phase separation was observed. Then, 150 µL from the upper phase were diluted with 1 mL of the internal standard solution. Finally, 2 µL of the resulting solution were analyzed by GC. All the experiments were carried out in duplicate, and mean values were reported. The efficiency of the enzymatic action was determined by the calculation of two parameters: n-3 PUFAs production and synthetic activity. Synthetic activity was calculated from the initial slope of the concentration profile of n-3 PUFAs.
Determination of enzyme transesterification activity
The specific activity of an enzyme reflects its ability to convert a particular substrate into a product per unit of time. Enzyme activity is quantified in micromoles of substrate transformed per minute (U). The activity is determined from the initial slope (m) obtained from the concentration versus time curve of the corresponding ester. The individual activities are calculated from the slopes of each alkyl ester, and the total activity is calculated from the slope of the total concentrations.
In this case, the specific activity, Aspecific, is calculated using Eq. (3).
where malkyl ester is the response factor or initial slope of the alkyl ester synthesis curve, mol [L h]−1; Vreaction is the total reaction volume, L; and massenzyme is the mass of enzyme, mg.
To calculate the synthetic activity and the quantity of n-3 PUFAs, the reaction profiles were obtained for all the assays performed. As an example, the reaction profiles of each n-3 PUFA and the total n-3 PUFA values are shown in Fig. 2 for an assay under the following conditions: 3 mL of salmon oil, 50 ºC, 150 mg of Novozym 435 enzyme, 1.3 mL of methanol and 3 mL of [emim+][NTf2−] as reaction medium.
Statistical analysis
Data were presented as mean ± SD (standard deviation), calculated from three independent samples per condition using GraphPad Prism 8.0.1 software (GraphPad Software, San Diego, CA, USA). As normality (Kolmogorov–Smirnov, p > 0.05) and homoscedasticity (Levene, p > 0.05) were met, the statistical significance was determined using the parametric tests of Tukey (p < 0.05) and ANOVA (p < 0.05) for the comparisons of two or more groups, respectively.
Results and discussion
Characterization of the salmon oil
As stated in Sect. "Chemical composition of the salmon oil", the composition of the salmon oil was analyzed by TLC obtaining a content of triglycerides of 93 wt. % and a content of diglycerides of 7 wt. %. In addition, the salmon oil was analyzed by GC to identify the main n-3 PUFAs (as methyl esters) that are forming the triglycerides. Table 1 shows the position of the peaks and weight % of the main n-3 PUFAs in the chromatogram.
The acid value and peroxide value of the studied salmon oil were 3.05 mg KOH/g and 4.8 meq/kg, respectively. Global Organization for EPA and DHA Omega-3 (GOED) International guidelines recommend values for acid value and peroxide value of ≤ 3 mg KOH/g [22] and ≤ 5 meq/kg [23], respectively. Therefore, we can conclude that the original salmon oil meets the international recommendations for fish oils intended for human consumption.
The values of salmon oil density obtained at different temperatures are shown in Table 2.
Influence of the length of the alcohol chain
With the objective to obtain a n-3 PUFAs concentrate, the influence of length of the alkyl chain of the alcohol used for the transesterification reaction has been studied. With this purpose, five alcohols (from C1 to C8) and the ionic liquid [bmim+][PF6−] and Novozym 435 were chosen because they have previously shown promising results acting as reaction medium in biocatalytic reactions [17]. The following conditions were used: 30 ºC, 0.5 mL of salmon oil, 0.5 mL of [bmim+][PF6−], 25 mg of Novozym 435 and 215 µL of alcohol. The molar ratio alcohol:oil was 10.1, 7, 5.5, 4.5, and 2.6 for methanol, ethanol, propanol, butanol, and octanol, respectively. Figures 3 and 4 show the influence of the alcohol chain length on the synthetic activity of the enzyme and on the total n-3 PUFAs obtained after 24 and 48 h of transesterification reaction, respectively. As can be seen, both parameters, synthetic activity and total n-3 PUFAs, show a bell curve with a maximum with 2-propanol. This behavior has been previously described by de los Ríos et al. [24] during the synthesis of esters in ILs ([bmim+][PF6−] and [omim+][PF6−]) and using another lipase (Lipozyme CalB L). They concluded that 1-butanol showed the best results, and that this behavior could be explained by the differences on the affinity of the lipase toward the different substrates, although steric hindrance and other effects, including denaturalization by small alcohol molecules, should also be considered.
Figure 5 shows the DHA and EPA percentages obtained with the different alcohols used for the transesterification reaction. On the one hand, the highest percentages of DHA were obtained when octanol (10.5%) and methanol (8.7%) were used. On the other hand, the highest percentage of EPA was obtained with methanol (11.5%), and, for the rest of alcohols, the EPA percentage was approximately halved. According to these results, methanol was selected for the rest of assays due to its high capacity of producing high percentages of DHA and EPA. In the case of using 2-propanol, which showed a good total production of n-3 PUFAs, the concentration of EPA was much lower.
Enzyme selection
In this study, the most suitable enzyme to carry out the transesterification reaction and the subsequent concentration of DHA and EPA in an ionic liquid were selected. The ionic liquid [hmim+][NTf2−] was chosen because it has previously shown promising results acting as reaction medium in biocatalytic reactions [17], and the medium was much less viscous than using [bmim+][PF6−] (at 303.15 K, the viscosity of [hmim+][NTf2−] is 0.0549 Pa s, while [bmim+][PF6−] has a viscosity of 0.207 Pa s). For these reaction assays, a temperature of 30 ºC, 25 mg or 50 µL of enzyme, 0.5 mL of salmon oil, 0.5 mL of ionic liquid, and 3.6 of methanol were used (molar ratio methanol: oil of 10.1). Figures 6 and 7 show the synthetic activity of the different enzymes in the transesterification reaction and the total µmol of n-3 PUFAs obtained after 24 and 48 h of reaction, respectively.
As observed in Figs. 6 and 7, the most effective enzymes were Lipolase 100L Type EX and Novozym 435, achieving synthetic activity values of 270 and 104 µmol/(h·g), respectively. Lipozyme TL showed a moderate activity of 34 µmol/(h·g), while the remaining enzymes did not show significant activity. As expected, the enzymes demonstrated higher synthetic activity at 48 h of reaction than at 24 h, except Lipolase 100L Type EX which exhibited the approximately the same activity. Figure 8 shows the percentages of DHA and EPA obtained in the transesterification reaction relative to the total n-3 PUFAs using the studied enzymes. As can be seen, the proportion of EPA was higher than that of DHA with all the enzymes. From the results, it can be inferred that Novozym 435 provided a better (DHA + EPA) proportion. For this reason, this enzyme was selected for the rest of experiments.
Influence of the enzyme concentration
After selecting Novozym 435 enzyme for the transesterification reaction, the concentration of the enzyme was optimized over a range of 5–45 mg. The reaction conditions for these experiments remained consistent with those used for enzyme selection: 30 ºC, 0.5 mL of salmon oil, 0.5 mL of [hmim+][NTf2−] and a methanol:oil molar ratio of 10.1. Figures 9 and 10 depict the influence of the quantity of Novozym 435 enzyme on the synthetic activity and the total µmol of n-3 PUFAs obtained after 24 and 48 h of reaction, respectively. As can be seen, the synthetic activity of Novozym 435 enzyme and the total of n-3 PUFAs increases with the quantity of enzyme up to 35 mg. From this value, they remain approximately constant. The total n-3 PUFAs substantially increased from 24 to 48 h of reaction doubling the value.
Figure 11 illustrates the percentages of DHA and EPA obtained in the transesterification reaction in relation to the total n-3 PUFAs using different quantities of enzymes. As can be seen, the proportion of EPA was consistently higher than that of DHA in all cases, and the difference between two n-3 PUFA become smaller as the quantity of enzyme increased. The DHA percentage increased from 5 to 25 mg of enzyme, reaching a constant value up to 45 mg. Conversely, EPA exhibited a decreasing trend as the quantity of enzyme increased. However, the (DHA + EPA) proportion remained similar in all cases, hovering around 15–17%. Based on the results, 25 mg of Novozym 435, which corresponds to a (DHA + EPA) proportion of 16%, was selected for the remainder of the assays.
Ionic liquid selection
The influence of the nature of the ionic liquid used as reaction medium for the transesterification reaction of salmon oil has also been studied. For this, 15 ILs with different anions and cations were employed under the following conditions: 30 ºC, 0.5 mL of salmon oil, 0.5 mL of ionic liquid, 25 mg of Novozym 435, and a methanol:oil molar ratio of 10.1. Figures 12 and 13 display the synthetic activity and the total n-3 PUFAs obtained after the experiments with the different ILs, respectively. The results can be explained based on three parameters: i) cation hydrophobicity, ii) anion nucleophilicity, and iii) viscosity of the ionic liquid [16].
In this context, it was observed that an increase in the ionic liquid hydrophobicity led to an increase in synthetic activity. This behavior was proved by comparing the series of the following ionic liquids: [emim+][NTf2−], [bmim+][NTf2−], [hmim+][NTf2−] and [omim+][NTf2−]. It was observed that the synthetic activity and the total n-3 PUFAs increased as the length of the alkyl chain of the imidazolium cation increased, indicating higher hydrophobicity of the cation. The increase in the hydrophobicity allows the enzyme to maintain water molecules in its microenvironment and thus preserve its active conformation.
The synthetic activity was lower when ILs containing nucleophilic anions such as ethylsulfate, methylsulfate, and triflate were used. These anions can strongly interact with the positively charged groups of the enzyme provoking its denaturalization.
Finally, the viscosity of the ionic liquid also plays a crucial role in the synthetic activity of the enzyme. Considering cation hydrophobicity and anion nucleophilicity, one might expect that the synthetic activity obtained with [omim+][BF4−] or [omim+][PF6−] should be higher than that found with [bmim+][BF4−], given the higher hydrophobicity of the [omim+] cation and the higher nucleophilicity of the [BF4−] anion. However, the opposite was observed, which can be explained by the lower viscosity of the [bmim+][BF4−] ionic liquid. This lower viscosity allows for better contact between the oil and the enzyme in the ionic liquid medium.
Figure 14 shows the percentages of DHA and EPA obtained with the different ILs used in the transesterification reaction. A clear distinction can be observed in DHA and EPA percentages between the ILs containing highly nucleophilic anions (i.e., ethyl sulfate, methyl sulfate, or triflate) and the ILs containing the rest of the anions (i.e., hexafluorophosphate, tetrafluoroborate, or bis(trifluoromethylsulfonyl)imide). When highly nucleophilic anions were used, the proportion of DHA and EPA obtained was very low, whereas it was higher with the rest of the anions. The differences cannot be attributed to the hydrophobicity of the ionic liquid, but rather to the specific interactions between the enzyme, the substrate, and the ionic liquid used. According to the results, the ionic liquid [emim+][NTf2−] provided the highest (DHA + EPA) yields from salmon oil.
Influence of the temperature reaction
The reaction temperature is also an important parameter during the optimization of the transesterification reaction. In the case of reactions catalyzed by enzymes, the temperature range which can be used is limited due to its low thermal stability. However, as was shown in [24], the use of ILs allows to increase the temperature. With this purpose, a temperature range from 30 to 50 ºC was evaluated using the following conditions: 3 mL of salmon oil, 3 mL of [emim+][NTf2−], 150 mg of Novozym 435 and a methanol:oil molar ratio of 10.1. Figures 14 and 15 show the influence of temperature on the synthetic activity of the enzyme and on the total n-3 PUFAs obtained after 24 and 48 h of transesterification reaction, respectively. As can be seen in Fig. 15, the synthetic activity of the enzyme was approximately the same at 30 and 35 ºC, then greatly increased from 35 to 40 ºC and, from this value, it remained constant until 50 ºC. The Fig. 16 showed an important increase of the total n-3 PUFAs obtained from 30 to 35 ºC and then the value was slightly increased to 50 ºC. These results corroborate the high thermal stability of the enzyme in the ionic liquid medium.
Figure 17 presents the DHA and EPA percentages obtained with the different temperature values used for the transesterification reaction. It can be clearly seen that this parameter does not significantly affect the DHA and EPA percentages, being around 10% in all experiments. This demonstrates that the DHA and EPA proportions are not influenced by the variations of temperature.
Conclusions
In this study, the production of omega-3 fatty acids, specifically DHA and EPA, from salmon oil by a biocatalytic process in two-phase oil/ionic liquid systems has been successfully achieved.
The most suitable enzymes for the enzymatic transesterification reaction were the lipases Novozym 435 and Lipolase 100L Type EX, as they yielded the highest reaction yields and a greater proportion of (DHA + EPA) in the reaction product.
The influence of the alcohol chain length (C1–C8) has been studied and a maximum synthetic activity of the Novozym 435 enzyme was obtained for 2-propanol, although the highest percentage of (DHA + EPA) in the reaction product was obtained using methanol.
It was found that the total activity for the transesterification reaction was significantly higher when ILs with hydrophobic cations and low nucleophilicity anions were used, the highest activity being achieved with the [omim+][NTf2−] ionic liquid. In the production of DHA and EPA, significant differences were found between ILs composed of strongly nucleophilic cations, such as triflate, in which the proportion of DHA and EPA was practically nil, and those composed of less nucleophilic anions, such as hexafluorophosphate, in which the proportion of both fatty acids was found to be between 4 and 13%. In the latter group, no relationship was observed between the hydrophobicity of the ionic liquid and the proportion of DHA and EPA found in the reaction mixture.
The process described in this work has not yet been tested with other types of oils with different initial concentrations of EPA and DHA and oils from different fish species could have different initial levels of these omega-3 fatty acids. This variability could affect the efficiency and performance of the biocatalytic process. Hence, the application of this process to other oils will require a tailored approach.
Nevertheless, the successful biocatalytic conversion of omega-3 fatty acids from salmon oil is a promising achievement and it can be concluded that ILs are an efficient reaction medium for the production of omega-3 fatty acids, specifically DHA and EPA, and a promising alternative for the development of environmentally friendly technologies. In conclusion, ILs are an efficient reaction medium for the production of omega-3 fatty acids, specifically DHA and EPA, and the biocatalytic processes in two-phase systems is a promising alternative for the development of environmentally friendly technologies. The results of this work open the door to future advances. Future research could focus on refining the process to improve its efficiency, yield, and cost-effectiveness. This could involve adjusting reaction conditions, enzyme selection and designing new biocatalysts to increase productivity. On the other hand, industrial scale-up is crucial. In the future, process parameters will have to be optimized for large-scale production while ensuring sustainability and cost-effectiveness. Another future prospect is the exploration of less toxic and more environmentally friendly ILs, which could improve sustainability and overall process efficiency.
Data availability
The data used to support the finding of this study are included within the article.
References
Zhang Z, Liu F, Ma X, Huang H, Wang Y (2018) Two-stage enzymatic preparation of eicosapentaenoic acid (EPA) And docosahexaenoic acid (DHA) enriched fish oil triacylglycerols. J Agric Food Chem 66:218–227. https://doi.org/10.1021/acs.jafc.7b04101
Dyall SC (2015) Long-chain omega-3 fatty acids and the brain: a review of the independent and shared effects of EPA, DPA and DHA. Front Aging Neurosci 7:52. https://doi.org/10.3389/fnagi.2015.00052
Ward OP, Singh A (2005) Omega-3/6 fatty acids: alternative sources of production. Process Biochem. 40:3627–3652. https://doi.org/10.1016/j.procbio.2005.02.020
Rubio-Rodríguez N, Beltrán S, Jaime I, de Diego SM, Sanz MT, Carballido JR (2010) Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innov Food Sci Emerg Technol 11:1–12. https://doi.org/10.1016/j.ifset.2009.10.006
Melgosa R, Sanz MT, Benito-Román Ó, Illera AE, Beltrán S (2019) Supercritical CO2 assisted synthesis and concentration of monoacylglycerides rich in omega-3 polyunsaturated fatty acids. J CO2 Util 31:65–74. https://doi.org/10.1016/j.jcou.2019.02.015
Melgosa R, Sanz MT, Solaesa ÁG, de Paz E, Beltrán S, Lamas DL (2017) Supercritical carbon dioxide as solvent in the lipase-catalyzed ethanolysis of fish oil: kinetic study. J CO2 Util 17:170–179. https://doi.org/10.1016/j.jcou.2016.11.011
Melgosa R, Sanz MT, Solaesa ÁG, Bucio SL, Beltrán S (2015) Enzymatic activity and conformational and morphological studies of four commercial lipases treated with supercritical carbon dioxide. J Supercrit Fluids 97:51–62. https://doi.org/10.1016/j.supflu.2014.11.003
Nagao T, Watanabe Y, Maruyama K, Momokawa Y, Kishimoto N, Shimada Y (2011) One-pot enzymatic synthesis of docosahexaenoic acid-rich triacylglycerols at the sn-1(3) position using by-product from selective hydrolysis of tuna oil. N Biotechnol 28:7–13. https://doi.org/10.1016/j.nbt.2010.07.021
Liu S, Zhang C, Hong P, Ji H (2007) Lipase-catalysed acylglycerol synthesis of glycerol and n−3 PUFA from tuna oil: optimisation of process parameters. Food Chem 103:1009–1015. https://doi.org/10.1016/j.foodchem.2006.08.037
Carvalho P, Bueno C, Noffs M, O. de, S. Tsunezi, S. da, (2003) Aplicação de lipases microbianas na obtenção de concentrados de ácidos graxos poliinsaturados. Quim Nova. https://doi.org/10.1590/S0100-40422003000100014
Solaesa G, Sanz MT, Melgosa R, Bucio SL, Beltrán S (2015) Glycerolysis of sardine oil catalyzed by a water dependent lipase in different tert-alcohols as reaction medium. Grasas y Aceites. https://doi.org/10.3989/gya.0238151
Fu H, Li M, Ni R, Lo YM (2018) Enzymatic catalysis for sustainable production of high omega-3 triglyceride oil using imidazolium-based ionic liquids. Food Sci Nutr 6:2020–2027. https://doi.org/10.1002/fsn3.733
Montalbán MG, Trigo R, Collado-González M, Díaz-Baños FG, Víllora G (2016) Liquid–liquid equilibria for ternary mixtures of 1-alkyl-3-methyl imidazolium bis{(trifluoromethyl)sulfonyl}imides, n-hexane and organic compounds at 303.15 K and 0.1 MPa. J Chem Thermodyn. https://doi.org/10.1016/j.jct.2016.08.033
Montalbán MG, Collado-González M, Lozano-Pérez AA, Díaz Baños FG, Víllora G (2018) Extraction of organic compounds involved in the kinetic resolution of rac-2-pentanol from n-hexane by imidazolium-based ionic liquids: Liquid–liquid equilibrium. J Mol Liq. https://doi.org/10.1016/j.molliq.2017.12.157
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246. https://doi.org/10.1038/35051719
de los Ríos AP, Hernández Fernández FJ, Gómez D, Rubio M, Víllora G (2011) Biocatalytic transesterification of sunflower and waste cooking oils in ionic liquid media. Process Biochem 46:1475–1480. https://doi.org/10.1016/j.procbio.2011.03.021
De Los Ríos AP, Hernández-Fernández FJ, Martínez FA, Rubio M, Víllora G (2007) The effect of ionic liquid media on activity, selectivity and stability of Candida antarctica lipase B in transesterification reactions. Biocatal Biotransform 25:151–156. https://doi.org/10.1080/10242420701379213
Cheong L-Z, Guo Z, Yang Z, Chua S-C, Xu X (2011) Extraction and enrichment of n-3 polyunsaturated fatty acids and ethyl esters through reversible π–π complexation with aromatic rings containing ionic liquids. J Agric Food Chem 59:8961–8967. https://doi.org/10.1021/jf202043w
Motlagh SR, Harun R, Biak DRA, Hussain SA, Ghani WAWAK, Khezri R, Wilfred CD, Elgharbawy AAM (2019) Screening of suitable ionic liquids as green solvents for extraction of eicosapentaenoic acid (EPA) from microalgae biomass using COSMO-RS model. Molecules. https://doi.org/10.3390/molecules24040713
Haq M, Park S-K, Kim M-J, Cho Y-J, Chun B-S (2018) Modifications of Atlantic salmon by-product oil for obtaining different ω-3 polyunsaturated fatty acids concentrates: an approach to comparative analysis. J Food Drug Anal 26:545–556. https://doi.org/10.1016/j.jfda.2017.05.006
Kirk RS, Sawyer R, Pearson D (1991) Pearson’s composition and analysis of foods, 9th edn, Longman Scientific and Technical Harlow, Harlow SE—708 s: illustrations. https://worldcat.org/title/476173425
FAO/WHO (2017) Standard for fish oils CODEX STAN 329–2017 Adopted in 2017, pp 1–6
Global Organisation for EPA and DHA Omega-3 (2019) Voluntary monograph, GOED Volunt. Monogr
de los Ríos AP, Hernández-Fernández FJ, Tomás-Alonso F, Gómez D, Víllora G (2008) Synthesis of esters in ionic liquids: The effect of vinyl esters and alcohols. Process Biochem 43:892–895. https://doi.org/10.1016/j.procbio.2008.04.012
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. This work has been partially supported by grants ref. TED2021-130389B-C21 funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and by the European Union “NextGenerationEU”/PRTR”, ref. PID2020-113081RB-I00 funded by MCIN/AEI/https://doi.org/10.13039/501100011033 and ref. 22129-PI-22 funded by the research support program of the Seneca Foundation of Science and Technology of Murcia, Spain, and by “ESF Investing in your future” Imane Moulefera acknowledges support from Spanish Ministry of Universities under “Margarita Salas program”, financed by Next Generation EU.
Author information
Authors and Affiliations
Contributions
M.G.F: conceptualization, methodology, investigation, writing—original draft. I.M: investigation, writing—review and editing. M.G.M: writing—review and editing. G.V: conceptualization, supervision, project administration, funding. All authors discussed the results and commented on the manuscript. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not involve any studies conducted on animal or human subjects. We affirm our commitment to upholding ethical principles in research and publication. Our work is conducted in accordance with established guidelines and regulations to maintain integrity and trust in scientific inquiry.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fuster, M.G., Moulefera, I., Montalbán, M.G. et al. Biocatalytic transesterification of salmon oil in ionic liquid media to obtain concentrates of omega-3 polyunsaturated fatty acids. Eur Food Res Technol 250, 1707–1719 (2024). https://doi.org/10.1007/s00217-024-04484-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-024-04484-1