Abstract
The main physicochemical characteristics of novel artisanal chocolates (both dark and milky) intended for vegan consumers or for those requiring assumption of fewer simple sugars, were analysed. Replacement of milk (with coconut copra, almonds, and soy protein isolates), and sucrose (with coconut sugars, stevia and erythritol, respectively) in dark chocolate, were accounted for by means of texture analysis, rheology, water activity, fatty acid composition, differential scanning calorimetry (DSC) and fast field cycling (FFC) nuclear magnetic resonance (NMR) relaxometry. The vegan sample (i.e., the milk-less one) showed lower values of hardness and adhesiveness as well as a larger peak in the melting behavior at the calorimetric evaluation (DSC). Moreover, the absence of milk resulted in the halving of the yield stress and a decrease in both the apparent and Casson’s viscosity. In the sample of chocolate with less sucrose, the peak temperatures measured at the DSC indicate crystallization of cocoa butter in its best form (Vβ2), unlike in dark chocolate, due to the different sugar composition. Similarly, the Casson yield stress (τ0), increased significantly (almost 70%), with the substitution of sugar. Finally, the results of NMR FFC relaxometry made it possible to identify aggregates of different sizes, laying the basis for its use as a rapid, non-destructive method for chocolate analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chocolate is valued more for its sensory characteristics and polyphenol content than for its nutritional properties. For this reason, confectionery companies, to broaden the consumer audience, are looking for new chocolate formulations that can satisfy dietary styles associated with cultural choices or intolerances and pathologies. Chocolate contains about 30% sucrose or even more. This is needed not only to favor sweetness, but also to increase volume and mass, as well as to ameliorate chocolate texture, aroma and mouthfeel [1]. Consumers increasingly demand a reduction in simple sugar content in food, including diabetics who must limit or avoid the consumption of mono- and disaccharides. Stevia is intensely sweet natural compound, approximately 300 times as sweet as sucrose on weight basis [1]. Erythritol, is a viable alternative for replacing sugars in chocolate, since its sweetness is 60% that of sucrose, with a glycemic response and energy value of 0 and 0.2 kcal g−1 respectively, compared to 100 and 4 kcal g−1 of sucrose: due to its lower absorption in the body [2, 3]. However, a very recent study revealed that erythritol could be associated with the risk of major adverse cardiovascular events [4], although, to this day, the main food safety agencies (i.e., FDA and EFSA) have not yet ruled on the matter. Less used is, instead, coconut sugar, a natural sweetener obtained by evaporation of the sap of Cocos nucifera, is less unhealthy than sucrose, due to the presence of minerals and vitamins, proteins, phenols, and low glycaemic index [5]. Moreover, lactose-intolerant or even vegan subjects are unable to consume milk-based chocolates due to the presence of powdered cow milk which is added during chocolate production. As a general remark, addition of milk allows production of sweeter chocolate with more malleability and thermolability than dark chocolate [6,7,8]. It is therefore desirable to study new formulations for producing both milk-free chocolates with vegetable substitutes and dark chocolate with sweeteners and few added simple sugars (hereafter referred to as sugar substitutes), to increase the number of consumers. The structure of chocolate, a very important parameter for the consumer’s perception of its quality, is correlated with the forms of crystallization resulting from the cocoa butter components used during chocolate preparation. The crystalline arrangement of the (V) form crystals in the cocoa butter allows a melting point between 33 and 34 °C [9]. This means that a well-structured chocolate can be stored at room temperature for a sufficiently long time, thereby making easier logistics operations without the use of refrigerated containers. Chocolate flow properties of chocolate are other important parameters for evaluating its structure. Moreover, the taste of chocolate in the mouth is directly influenced by viscosity. Therefore, chocolate bad taste can be perceived when wrong flow/viscosity characteristics are achieved [10, 11]. In addition, final chocolate texture, appearance and flavour can be considered as key attributes for consumer choice and acceptability [12,13,14]. All the keys are related to the phase transitions of polymorphic forms in fat systems by their melting points which, in turn, are monitored by differential scanning calorimeter (DSC) [15]. Chocolate characteristics can be associated also with the molecular dynamics of the complex mixtures making this food product. Molecular dynamics can be explored by low field NMR relaxometry. This is a fast, reproducible, accurate and non-invasive technique, which can be applied to expand knowledge of chocolate stabilization characteristics [16, 17]. The aim of this study was to evaluate the main chemical-physical properties of novel vegan and substituted-sugar chocolates. Therefore, two different chocolates were handcrafted. In milk-less products, viable for lactose-intolerant and vegan people, milk powder was replaced with dried coconut copra, roasted almonds, and isolated soy proteins. In the case of substituted-sugar dark chocolate, sucrose was replaced with stevia, erythritol and coconut sugar. Comparison with traditional products (milk- and sucrose-containing chocolates) was carried out by analyzing water activity, fatty acids content, texture, melting point by differential scanning calorimetry (DSC), rheology and fast field cycling (FFC) NMR relaxometry.
Materials and methods
Materials
The chocolate samples were made in an artisan confectionery laboratory (Pasticceria Cappello, Palermo, Italy) using, for both the control and the experimental samples, a refiner with counter-rotating porphyry rollers for mixing (Ing. Polin EC. S.p.A., Verona, Italy) and obtaining the paste of cocoa, and a bench robot set at 60 °C for 4 h of conching. The chocolate obtained was manually tempered on marble and molded into circular shapes in silicone molds with a weight of 7–8 g, 4.5 mm of thickness and 35 mm of diameter for each shape obtained; cooled for 10 min at 10 °C then left at room temperature (18–20 °C).
Sample coding is as follows:
-
MiC: milk-containing chocolate
-
VeC: milk-less vegan chocolate
-
DaC: sucrose-containing dark chocolate
-
SuSDC: substituted-sugar dark chocolate
Raw materials
Cocoa mass was purchased by Valhrona (France), Manjari Pur Madagascar 100%, for milk chocolate samples production, and Araguani Pur Venezuela 100% cocoa mass for dark chocolate, were used. The cocoa butter and whole milk powder were purchased from ICAM Professional SPA, (Lecco, Italy). Dehydrated coconut (Pearls of Samarkand, Sri Lanka), powdered isolated soy proteins (Natural Soy Isolate, ProLabs, Eros, EuroSup), pure stevia powder (UOP Durante, Italy), and crystal coconut sugar (Monte Nativo, Sri Lanka), were purchased on online marketplace. Almonds were purchased by Musumeci company, (Bronte, CT, Italy). Natural Bourbon vanilla powdered by Vanilla Gourmet (Pescara, Italy); soy lecithin by Nutrition&Santè (Lecinova, Italy); by Chimpex (Caivano, NA, Italy). The percentage composition of the ingredients in the different samples is shown in Table 1. The formulation of the SuSDC sample results in an about 8% reduction in calorific value: with about 2333.4 kJ 100 g−1, compared to 2531.7 kJ g−1 in DaC sample. All samples produced and the analyses below were performed in triplicate.
Water activity and dry matter
The analysis of the water activity was carried out with the HygroPalm-23 instrument (Rotronic, Basserdorf, Germany) following the ISO 21807:2004 method. The samples were all analysed in triplicate. Percentage of dry matter was analysed by using official analytical method (AOAC 930.15, 1990). Samples were dried in oven at 105 °C for 3 h, then weight loss was measured.
Fatty acids groups
Fatty acids were identified and quantified, after methylation [18], by using a gas chromatograph (HRGC 5300; Carlo Erba, Milano, Italy) fitted with a 30 m × 0.25 mm, 0.20 µm i.d. Rtx-2330 (Restek, Bellefonte, PA) capillary column and a FID detector.
Texture analysis
Penetration test was made using a TaXT2 texture analyzer (Stable Microsystem) equipped with a cylindrical probe of 5 mm diameter (P5). The area of the probe was ten times smaller than the area of the sample. The hardness of the sample was the maximum force registered during the test; the adhesiveness was the negative force recorded during the probe withdrawal from the sample after the penetration. The operative parameters used for the test were as follows: pre-test speed 2 mm s−1; test speed 2 mm s−1; post-test speed 5 mm s−1; distance 50%; load cell 25 kg. The test was conducted at 18 ± 0.5 °C.
Differential scanning calorimetry
The melting profiles of chocolate samples were determined using Differential Scanning Calorimetry (DSC) (Q Series DSC, TA Instruments, New Castle, Delaware, USA). 4–5 mg of samples were loaded in an aluminium pan and nitrogen was used as transport inert gas at a flow rate of 50 ml min−1. An empty pan was used as a reference, while indium was used for instrument calibration. Samples were initially equilibrated at 20 °C and then heated to 60 °C at 5 °C min−1 rate. Thermograms were analysed by TA Universal Analysis software (TA Instruments, New Castle, Delaware, USA), defining the onset temperature (Tonset), melting peak temperature (Tm), end temperature (Tend) and the melting enthalpy (ΔHm). Melting index (Tindex) was calculated as (Tend -Tonset) and measures the melting range [19].
Rheological analysis
The rheological properties of chocolate were studied using a Haake Mars III rheometer (Thermo Scientific) equipped with Couette geometry (with coaxial cylinders) with an outer cylinder diameter of 43 mm, the inner one of 41 mm, and a vertical distance (from the bottom) between the two cylinders equal to 3 mm. Chocolate flow parameters were determined by following the official method viscosity (ICA, 2000) of cocoa and chocolate products (Analytical method 46. CAOBISCO, Brussels). Chocolate was placed in a closed glass container. It was heated in a benchtop oven at 52 °C for at least 1 h before evaluating the flow properties at 40 °C. In detail, the test was programmed in 4 steps: 1) pre-shear at a fixed shear rate (\(\dot{\gamma }\)) of 5 s −1 for 5 min; 2) ascending ramp with (\(\dot{\gamma }\)) from 2 to 50 s −1 in 3 min, 3) fixed shear rate at (\(\dot{\gamma }\)) = 50 s −1 for 1 min; 4) descending ramp with (\(\dot{\gamma }\)) from 50 to 2 s −1 in 3 min.
The data recorded in the upward flow curve section were then interpolated using Casson’s model:
where τ0 is the Casson yield stress, i.e., the stress required for the fluid to start flowing, and ηc is the Casson viscosity indicating the force required to maintain chocolate flow during the test. The apparent viscosity, measured at 40 s−1, relates to the viscosity of the chocolate at high shear rates and represents the behavior of the chocolate during pumping and mixing processes. Apparent viscosity values were taken from the ascending curve [20]. Thixotropy was measured by the hysteresis area formed by the difference between the upward and downward curves. The larger the hysteresis area, the longer the time needed for the fluid to recover its structure.
Fast-field-cycling (FFC) NMR relaxometry
Details about the technique have been already reported elsewhere [21]. Here, only a brief report on the used experimental conditions is reported. All the experiments were conducted on a Stelar Smartracer Fast-Field-Cycling Relaxometer (Stelar s.r.l., Mede, PV–Italy) set at a constant temperature of 25 °C. The proton spins were polarized at a polarization field (BPOL) corresponding to a proton Larmor frequency (νL) of 10 MHz for a period (TPOL) of about five times the T1 estimated at this frequency. After each BPOL, the magnetic field intensity (indicated as BRLX) was systematically changed in the proton Larmor frequency νL comprised in the range 0.01–10 MHz. The period τ, during which BRLX was applied, has been varied on 32 logarithmic spaced time sets, each of them adjusted at every relaxation field to optimize the sampling of the decay/recovery curves. Free induction decays were recorded following a single 1H 90° pulse applied at an acquisition field (BACQ) corresponding to νL of 7.2 MHz. A time domain of 100 μs sampled with 512 points was applied. Field-switching time was 3 ms, while spectrometer dead time was 15 μs. For all the experiments, a recycle delay of 2 s was used. A non-polarized FFC sequence was applied when the relaxation magnetic fields were in the range of the proton Larmor frequencies comprised between 20 and 10 MHz. A polarized FFC sequence was applied for BRLX values ranging between 3 and 0.01 MHz [21].
All the decay/recovery curves acquired by applying the aforementioned experimental runs were exported to OriginPro 7.5 SR6 (Version 7.5885, OriginLab Corporation, Northampton, MA, USA) to apply the stretched exponential function (also known as Kohlraush–Williams–Watts function) reported in Eq. (1) [22].
Here, I(τ) is the magnetization intensity at a given τ value; I0 is the magnetization intensity at the asymptote of the decay/recovery curve; τ is the period of time during which BRLX is applied; T1 is the longitudinal relaxation time; k is a parameter accounting for the relaxometry complexity of the samples; y0 is the experimental offset. Equation (1) accounts for the large sample heterogeneity resulting in a multiexponential behavior of the decay/recovery curves. In particular, this equation can be considered as a superposition of exponential contributions, which describes the likely physical picture of some distributions in T1. Its application has the advantage that it can handle a wide range of relaxometry behaviors within only one single model. For this reason, any assumption about the number of exponentials to use for modelling the FFC NMR relaxometry data is not necessary [21].
The NMRD profiles (i.e., R1 = 1/T1–vs–νL curves) were modelled according to the free-model analysis [23] to obtain the distribution of correlation times from which information about the dynamic domains in chocolates were obtained.
Statistical analysis
The means and standard deviations of experimental results were calculated from three replicate trials. To determine their differences, one-way ANOVA and Tukey’s test were used. Differences at p < 0.05 were considered significant and were indicated with different letters. All statistical analyses were done via the SPSS Version 20.0 statistic software package (SPSS Inc., Chicago, IL, USA).
Results and Discussion
Fatty acids groups
Table 1 reports the ingredients used to produce the different artisanal chocolates. All the fatty acid groups (i.e., the saturated fatty acids, SFA, the mono- and polyunsaturated fatty acids, MUFA, and PUFA, respectively) significantly varied between VeC and MiC samples (Table 2). This difference can be explained by considering the different formulation (Table 1) and the chemical composition of the newly added vegetable ingredients. As expected, the DaC and SuSDC samples revealed similar contents of the three fatty acid groups (Table 2). Fat crystallization in chocolate can be understood by studying the molecular interactions between different species of saturated and unsaturated fatty acids, which are differently bound to glycerol carbons. Such analysis is beyond the scope of this research; however, the fatty acid data will be discussed to interpret some of the following results.
Water activity and dry matter
Water activity (aw) refers to the water capacity to be bound to different components in a food matrix. In particular, the lower the aw value, the more bound are the water molecules. In this study, MiC and VeC samples showed similar aw values (Table 3), thereby revealing that replacement of milk with milk-substitutes does not affect water binding. The same results were obtained for DaC and SuSDC. In fact, as for VeC and MiC, DaC and SuSDC showed the same aw values. However, it must be noticed that the water activities for MiC-VeC were higher than those measured for DaC- SuSDC. This can be explained by considering that milk powder (in MiC) and coconut sugar (in VeC) contain highly hygroscopic saccharides such as inulin in coconut [24, 25]. The latter, indeed, lacks in both DaC and SuSDC. Finally, all the samples (i.e., Mic-Vec, and DaC-SuSDC) revealed the same dry matter content. This is very important because in the paragraphs below the FFC NMR relaxometry results are discussed. All the differences are not due to aw and dry matter values, but to the chemical nature of the organic chocolate components.
Differential Scanning Calorimetry
The main quality parameters of chocolate, which are good mouth meltability, snapping properties at room temperature, and glossiness, depend on the crystallization form of cocoa butter used as fat phase. In particular, the (V) is the preferred polymorph for commercial chocolate production, because it guarantees the proper reaching of quality parameters cited above. DSC is widely used for the evaluation of fat polymorphism in processed food products and for the evaluation of extent of fat crystal network organization during the chocolate manufacturing. Figure 1, 2, 3 shows the melting profiles registered during the heating of four chocolate samples. Each thermogram has been evaluated measuring the onset peak corresponds to the temperature at which a specific crystalline form begins to melt, the maximum peak corresponding to the temperature at which the melting curve reaches its peak maximum, the end of melting and the enthalpy related to the whole melting peak (Table 4). The DaC and SuSDC samples show a single peak, more sharply defined in the SuSDC chocolate, in contrast to MiC and VeC, for which a double melting transition is visible (inset of Fig. 4). The melting temperatures of cocoa butter polymorphs are reported as follows: I sub-α (17.3 °C), II α (23.3 °C), III β’2 (25.5 °C), IV β’1 (27.5 °C), V β2 (33.8 °C), VI β1 (36.3 °C) [26]. The maximum peak temperatures detected for chocolate samples, suggest the crystallization of cocoa butter in its (V β2) form, the best, only in SuSDC sample. The observed differences among the melting profiles (Fig. 1) and the registered temperatures (Table 4) can be ascribed by the various ingredients employed for the formulation of chocolates. The double melting transition in Mic and VeC sample could be caused by the triglyceride’s composition of milk and coconut copra fat, that affect crystallization behavior of cocoa butter CB [27]. Moreover, the differences between melting peaks as well as the enthalpy values in DaC and SuSDC samples can be due to the different sugar composition, too. In fact, sugars and their particle sizes affect the CB crystallization because they represent nuclei which provide the “seed” around which fat crystals grow [28, 29]. The melting enthalpy and T index for DaC chocolate was significantly higher than those of SuSDC, (p < 0.05) indicating a higher extent of fat crystallization in systems, probability due to the simultaneous presence of high amount of CB and homogeneous sugar phase in systems.
Correlation times obtained by inverse transformation of data resulted by relaxation rate (Fig. 3) for milk chocolate (MiC); vegan chocolate (VeC); dark chocolate (DaC), and sugar-substituted dark chocolate (SuSDC)
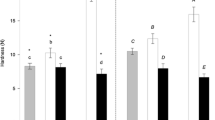
Texture analysis
Penetration test was used to determine hardness of the chocolate disks and adhesiveness, defined as the work required to pull the sample away from a surface [30,31,32]. Hardness of chocolate is a good parameter that points out proper control of temperature and stability of the fat crystal network formed during tempering process [33, 34]. Treated samples (VeC and SuSDC) showed hardness values lower to the control samples (MiC and DaC), respectively (Table 5). These data suggested that samples with lower melting enthalpy tend to have a softer structure at the same temperature, i.e., when subjected to the stress of texture analysis, showing a lower resistance to probe penetration [35]. DSC results also showed a lower Tpeak for VeC sample, related to the lower point of fusion. Moreover, VeC sample had the lowest value of adhesiveness. Hardness value was highest in DaC, because of the absence of any other substances that can affect the typical structure of the dark chocolate. SuSDC had the lower hardness value than (DaC) sample, owing to the presence of coconut sugar, highly hygroscopic sweetener containing a significant amount of inulin (about 5 g 100 g−1). These data confirmed that the substitution of sucrose by other sugars provides low hardness values compared to reference chocolate [10].
Rheology
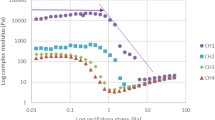
Chocolate can be considered as a suspension of solid particles in a Newtonian fluid (cocoa butter). It is well known that chocolate does not usually have Newtonian behavior, which was also confirmed for the samples analysed, all of which exhibited the characteristics of Casson’s plastic fluid. Chocolate rheology is usually quantified using parameters like yield stress (τ0) and apparent viscosity (measured at shear rate 40 s−1). Yield stress is a material property and is the stress corresponding to the yield point at which the material begins to deform plastically [36]. Furthermore, water contents may have a negative effect on flow properties resulting in higher yield value and viscosity. Values of apparent viscosity reported in Table 6 showed that the vegan chocolate had a lower viscosity at high shear rate compared to the parent milk product and that the sugar replacing in dark chocolate resulted in a slower increase of η app compared to the control. Results of flow curves data fitting to the Casson equation and the measurement of the hysteresis area (thixotropy) are shown in Table 620. For all the samples, the shear rate applied resulted in a non-linear response in terms of shear stress (Fig. 2) indicating that chocolate aggregates aligned to the flow as the shear rate increase thus opposing less resistance, which is the reason why chocolate samples showed shear thinning behavior. As for τ0 values, the new formulations showed an opposite trend. In fact, in vegan chocolate, the substitution of milk powder caused more than a halving of the yield stress and a similar decrease in apparent and Casson viscosity. These data, consistent with the texture value, showed a variation in the rheological properties of vegan chocolate compared to traditional one. The differences between dark and sugar-substituted chocolate showed an inverse trend in τ0 values, which increased significantly (almost 70%) with sugar substitution, while the increase in viscosity was smaller (about 9%). This last was consistent other results [37], in which it was reported that dark sweetened with isomalt presented higher Casson viscosity than that of the sucrose-containing chocolate, confirming that the chocolate formulations comprising high levels of sugar substitutes had higher apparent and Casson viscosity, and yield stress than the those of the control [38]. Thixotropy is a function of time-dependent fluid and can be evaluated through apparent viscosity or shear stress decreasing with the time of shear at constant rate. Thixotropy is calculated from the area of loop or a specific point on the ramp curves of shear stress or apparent viscosity at a specific shear rate [39]. Values reported in Table 6 were calculated as the area of loop formed between the upward and downward flow curves and as can be observed all samples showed thixotropic behavior. However, thixotropy indicates the role of time in the process of re-structuring after shearing.
FAST-FIELD-CYCLING (FFC) NMR relaxometry
In Fig. 3, Nuclear Magnetic Resonance Dispersion (NMRD) profiles, reporting the longitudinal relaxation rate as a function of the Larmor frequency of the applied electromagnetic field, is shown. VeC sample (the vegan chocolate) with fat replacers of milk powder, shows the lowest relaxation rate as compared to the other samples. This is justified by the different molecular motion affecting the fluctuation of the local electromagnetic field. A faster molecular motion is related to longer relaxation times T1 and slower relaxation rate values [40]. In this case, it is possible to suggest that the different fat composition that characterizes VeC sample, affects the interaction of molecules of cocoa butter that serve for the crystallization process. This is because of the interaction between cocoa butter and milk powder replacers’ fats generating aggregates with higher molecular mobility as compared to the other chocolate samples. Nothing else can be qualitatively deduced by the visual inspection of Fig. 3. For this reason, the model described elsewhere [23] has been applied.
The inverse transformation of the function related to the relaxometry NMRD showed a time domain graph where different correlation times, each referred to the dynamic components of the chocolate matrixes, are reported (Fig. 4). The longest correlation times can be associated to larger aggregates moving slowly. Conversely, as correlation time values decrease, molecular dynamics increases because of the reduction of the molecular aggregates. Therefore, starting from the left-hand side of Fig. 4, it is possible to state that the first proton population (at around 0.016 ms for MiC, VeC, and SuSDC, respectively, while at 0.031 ms for DaC) is due to the presence of substances with higher proton mobility and faster correlation time. According to the preparation procedure of the different chocolates, the band at the shortest τC value can be associated to the presence of lactose and sucrose, that is two small-sized disaccharides. Moreover, the second correlation time at around 0.12 ms is conceivably due to proteins. In particular, in MiC, a net peak related to proteins is visible. This is explained by considering that this type of chocolate contains the largest number of proteins due to the use of cow milk, whereas the other products are made by cow milk surrogate proteins which enable a lower and broader band centered at 0.12 ms. Finally, the correlation time at the longest values (2.2 ms for MiC, 3.1 ms for VeC, 1.4, and 1.6 ms for DaC and SuSDC, respectively) is related to non-polar substances such as the lipid fraction. The correlation time for this component is the longest in all the graphs, thereby corresponding to a high interaction of this component in the matrix. In fact, the cocoa butter is the main component of the continuous phase and responsible of the crystalline state of chocolate. MiC showed a high and large peak because of presence of both cocoa butter and milk powder fats. Both are mainly composed by saturated fatty acids that make them solid with the lowest proton mobility.
According to the molecular mobility within the chocolate, the first peak of VeC sample (the one with milk powder replacers) is related to sucrose with lowest correlation time. A shorter and less net peak is related to the presence of isolated soybean proteins that were added in the mixture to replace the amount of milk powder proteins. Then, the range of signals related to lipids present in the VeC mixture was more variegated, with short and jagged population of peaks, and it was not possible to distinguish each peak related to a single component. But it was possible to identify solid fat components (related to cocoa butter and all the saturated fatty acids of coconut copra) with higher correlation times, while liquid fat components (oils, mainly unsaturated and polyunsaturated fatty acids from almond and coconut) have lower correlation times compared to solid fats, because of the slightly higher mobility of their protons compared to the solid fat components [17]. This different composition of the fat content in the sample is possibly the main reason of the interference of the different lipids in the crystallization process of cocoa butter. Results of NMR analysis are comparable with DSC results, where MiC and VeC sample showed a lower Tpeak of melting (compared to the dark chocolate samples) and a wider peak during the transition phase of chocolates. Sucrose is present also in the third sample (DaC), but at a lower concentration (25% of added sucrose). The corresponding band is slightly shifted to the right, compared to the first two samples. The explanation can be found in the Fig. 3, where the DaC sample shows higher Longitudinal Relaxation Rate compared to SuSDC dark chocolate sample. Higher relaxation rates and lower relaxation times are related to a deeper solid–solid or sugar–solid interaction in food [41]. That means that sucrose may be more embedded within the cocoa butter continuous phase compared to the other chocolate samples. The proton population related to proteins in this sample is related to the proteins of cocoa mass, generally bound within tannins complexes, but visible in DaC and SuSDC. The last net peak in the DaC graph is referred to cocoa butter, the highest peak with the highest correlation time, because of the high level of aggregation that is responsible for the crystallinity of chocolate. Alternative sugars as sucrose replacers were used for the formulation of the fourth sample (SuSDC). One of these was the coconut sugar (a low-absorbable sugar containing sucrose, and inulin, that is a β-D-fructose polymer), then, erythritol and stevia sweetener. Figure 4 reveals a sucrose band that is less intense than that present in the other samples. This is in line with the sucrose concentration (about 50%) in coconut sugar [41], so SuSDC contains about 6.0% of sucrose (Table 1). That is, about one quarter of the DaC sample, so it is visible as a very small peak in the band of molecules with faster molecular movement at the beginning of Fig. 4 in SuSDC. The relaxation rate of the SuSDC sample shown in Fig. 3 is lower than that of DaC, due to less interaction between the alternative sugars within the cocoa butter network. This is consistent with the sugar-solid interactions elsewhere explained [42]. The cocoa mass protein was included in the last peak in both the DaC and SuSDC samples (Fig. 3).
Conclusion
The substitution of milk powder with vegetable ingredients showed several chemical-physical differences with milk chocolate. In particular, the mixture of coconut copra, almonds and soy protein isolates, added instead of milk powder, affected the consistency, with lower hardness and adhesiveness values, and the melting behavior at DSC, with a smaller enthalpy peak. Partial substitution of sucrose with other sugars in dark chocolate changed the texture due to the presence of a hygroscopic ingredient (such as inulin in coconut sugar), which reduced the hardness values in the sugar-substituted sample. Substitution of sucrose by sweeteners, on the other hand, showed that the crystallization of cocoa butter was closer to the best form (V β2) in the new chocolate, while the Casson yield stress (τ0) increased significantly (almost 70%) with sugar substitution. Finally, NMR FFC relaxometry proved to be a promising tool for the rapid and non-destructive assessment of chocolate quality. In fact, the application of the free-model analysis provides useful chocolate fingerprints that can be used to recognize in only one shot the origin and nature of different chocolates, provided that a large number of samples is analyzed. Therefore, more analyses are needed to better address FFC NMR investigations.
References
Aidoo RP, Depypere F, Afoakwa EO, Dewettinck K (2013) Industrial manufacture of sugar-free chocolates - Applicability of alternative sweeteners and carbohydrate polymers as raw materials in product development. Trends Food Sci Technol 32:84–96. https://doi.org/10.1016/j.tifs.2013.05.008
Nastaj M, Sołowiej BG, Stasiak DM, Mleko S, Terpiłowski K, Łyszczek RJ, Tomasevic IB, Tomczyńska-Mleko M (2022) Development and physicochemical properties of reformulated, high-protein, untempered sugar-free dark chocolates with addition of whey protein isolate and erythritol. Int Dairy J 134:105450. https://doi.org/10.1016/j.idairyj.2022.105450
Homayouni Rad A, Konar N, Rasouli Pirouzian H, Mirtajeddini SB, Toker OS, Rasouli Pirouzian R (2023) Polyols and chocolate technology: recent developments and advances. Int J Food Sci Technol 58:1–8. https://doi.org/10.1111/ijfs.16175
Witkowski M, Nemet I, Alamri H et al (2023) The artificial sweetener erythritol and cardiovascular event risk. Nat Med 29:710–718. https://doi.org/10.1038/s41591
Nurhayati R, Zulfa N, Herawati ERN, Laila U (2022) Physicochemical, and microbiological characteristics of probiotic dark chocolate bar sweetened with palm sugar and coconut sugar. Food Res 6:97–107. https://doi.org/10.26656/fr.2017.6(5).591
Franke K, Heinzelmann K (2008) Structure improvement of milk powder for chocolate processing. Int Dairy J 18:928–931. https://doi.org/10.1016/j.idairyj.2008.03.013
Glicerina V, Balestra F, Dalla Rosa M, Romani S (2016) Microstructural and rheological characteristics of dark, milk and white chocolate: A comparative study. J Food Eng 169:165–171. https://doi.org/10.1016/j.jfoodeng.2015.08.011
Vásquez C, Henríquez G, Lópe JV, Penott-Chang EK, Sandoval AJ, Müller AJ (2019) The effect of composition on the rheological behavior of commercial chocolates. Lwt-Food Sci Technol 111:744–750. https://doi.org/10.1016/j.lwt.2019.05.101
Ghazani SM, Marangoni AG Molecular origins of polymorphism in cocoa butter. Food Sci Technol 12:567–590, doi: https://doi.org/10.1146/annurev-food-070620-022551
Gonçalves EV, Lannes SCS (2010) Chocolate rheology. Food. Sci Technol 30:845–851. https://doi.org/10.1590/S0101-20612010000400002
Sandoval JA (2022) Large amplitude oscillatory shear (LAOS) behavior of chocolates of different compositions. J Rheol 66:859–879. https://doi.org/10.1122/8.0000425
Torres-Moreno M, Tarrega A, Costell E, Blanch C (2012) Dark chocolate acceptability: Influence of cocoa origin and processing conditions. J Sci Food Agric 92:404–411. https://doi.org/10.1002/jsfa.4592
Bordiga M, Locatelli M, Travaglia F, Coïsson JD, Arlorio MG, M, (2015) Evaluation of the effect of processing on cocoa polyphenols: Antiradical activity, anthocyanins and procyanidins profiling from raw beans to chocolate. Int J Food Sci Technol 50:840–848. https://doi.org/10.1111/ijfs.12760
Di Mattia CD, Sacchetti G, Mastrocola D, Serafini M (2017) From cocoa to chocolate: The impact of processing on in vitro antioxidant activity and the effects of chocolate on antioxidant markers in vivo. Front Immunol 8:1–7. https://doi.org/10.3389/fimmu.2017.01207
Le Rèvèrand BJD, Fryer PJ, Bakalis S (2009) Modelling crystallization and melting kinetics of cocoa butter in chocolate and application to confectionery manufacturing. Soft Matter 5:4. https://doi.org/10.1039/b809446b
Ladd-Parada M (2018) Fast field cycling NMR relaxometry studies of molten and cooled cocoa butter. Mol Phys 117(2):2018. https://doi.org/10.1080/00268976.2018.1508784
Kiumarsi M, Majchrzak D, Jäger H, Song J, Lieleg O, Shahbazi M (2021) Comparative study of instrumental properties and sensory profiling of low-calorie chocolate containing hydrophobically modified inulin. Part II: Proton mobility, topological, tribological and dynamic sensory properties. Food Hydrocoll 110:106144 https://doi.org/10.1016/j.foodhyd.2020.106144
Salimon J, Omar TA, Salih N (2014) Comparison of two derivatization methods for the analysis of fatty acids and trans fatty acids in bakery products using gas chromatography. Sci World J 25:906407. https://doi.org/10.1155/2014/906407
Afoakwa EO, Paterson A, Fowler M, Vieira J (2009) Fat bloom development and structure-appearance relationships during storage of under-tempered dark chocolates. J Food Eng 91:571–581. https://doi.org/10.1016/j.jfoodeng.2008.10.01
Do TA, Hargreaves JM, Wolf B, Hort, J Mitchell JR (2007) Impact of particle size distribution on rheological and textural properties of chocolate models with reduced fat content. J Food Sci 72:E541–52. https://doi.org/10.1111/j.1750-3841.2007.00572
Conte P (2021) Applications of fast field cycling NMR relaxometry. Annu Rep NMR Spectrosc 104:141–188. https://doi.org/10.1016/bs.arnmr.2021.05.001
Morozova-Roche LA, Jones JA, Noppe W, Dobson CM (1999) Independent nucleation and heterogeneous assembly of structure during folding of equine lysozyme. J Mol Biol 289:1055–1073. https://doi.org/10.1006/jmbi.1999.2741
Landi G, Spinelli GV, Zama F, Conte MDC (2023) An automatic L1-based regularization method for the analysis of FFC dispersion profiles with quadrupolar peaks. Appl Math Comput 444:1–16. https://doi.org/10.1016/j.amc.2022.127809
Hebbar KB, Ramesh SV, Ghosh DK, Beegum PPS, Pandiselvam R, Manikantan MR, Mathew AC (2022) Coconut sugar- a potential storehouse of nutritive metabolites, novel bio-products and Prospects. Sugar Tech 24:841–856. https://doi.org/10.1007/s12355-021-01101-3
Saraiva A, Carrascosa C, Ramos F, Raheem D, Lopes M, Raposo, (2023) A Coconut sugar: chemical analysis and nutritional profile; health impacts; safety and quality control; food industry applications. Int J Environ Res Public Health 20:4. https://doi.org/10.3390/ijerph20043671
Ghazani SM, Marangoni AG (2021) Molecular origins of polymorphism in cocoa butter. Annu Rev Food Sci Technol 12:567–659. https://doi.org/10.1146/annurev-food-070620-022551
Delbaere C, Van de Walle D, Depypere F, Gellynck X, Dewettinck K (2016) Relationship between chocolate microstructure, oil migration, and fat bloom in filled chocolates. Eur J Lipid Sci Technol 118:1800–1826. https://doi.org/10.1002/ejlt.201600164
Svanberg L, Ahrné L, Lorén N, Windhab E (2011) Effect of sugar, cocoa particles and lecithin on cocoa butter crystallization in seeded and non-seeded chocolate model systems. J Food Eng 104:70–80. https://doi.org/10.1016/j.jfoodeng.2010.09.023
Bourne M (2002) Texture, Viscosity and Food. Food Sci Technol, Concept and Measurement 2nd Edition https://doi.org/10.1016/B978-012119062-0/50001-2.
Szczesniak AS (2002) Texture is a sensory property. Food Qual Prefer 13:215–225. https://doi.org/10.1016/S0950-3293(01)00039-8
Cardarelli HR, Aragon-Alegro LC, Alegro JH, Castro IA, Saad SM (2008) Effect of inulin and Lactobacillus paracasei on sensory and instrumental texture properties of functional chocolate mousse. J Sci Food Agric 88:1318–1324
Machálková L, Hřivna L, Nedomová Š, Jůzl M (2015) The effect of storage temperature on the quality and formation of blooming defects in chocolate confectionery. Potr 9:39–47. https://doi.org/10.5219/425
Bikos D, Samaras G, Cann P, Masen M, Hardalupas Y, Hartmann C, Vieirac J,. Charalambides MN (2021) Effect of micro-aeration on the mechanical behaviour of chocolates and implications for oral processing. Food Funct 12:4864
Taşoyan İC, Yolaçaner ET, Öztop MH (2023) Physical characterization of chocolates prepared with various soybean and milk powders. J Texture Stud 54:34–346
Lim PY, Wong KY, Thoo YY, Siow LF (2021) Effect of inulin, fructo-oligosaccharide, trehalose or maltodextrin (M10 and M30) on the physicochemical and sensory properties of dark compound chocolate. LWT Food Sci Technol 149:111964. https://doi.org/10.1016/j.lwt.2021.111964
Afoakwa EO, Paterson A, Fowler M (2007) Factors influencing rheological and textural qualities in chocolate - a review. Trends Food Sci Technol 18:290–298. https://doi.org/10.1016/j.tifs.2007.02.002
Nebesny E, Żyżelewicz D (2005) Effect of lecithin concentration on properties of sucrose-free chocolate masses sweetened with isomalt. Eur Food Res Technol 220:131–135. https://doi.org/10.1007/s00217-004-1009-z
Abbasi S, Farzanmher AH (2009) Optimization of the formulation of prebiotic milk chocolate based on rheological properties. Food Technol Biotechnol 47:396–403. https://doi.org/10.1007/s13197-020-04536
Mujumdar A, Beris AN, Metzner AB (2002) Transient phenomena in thixotropic systems. J Non-Newton Fluid Mech 102:157–178. https://doi.org/10.1016/S0377-0257(01)00176-8
Piacenza E, Chillura Martino DF, Cinquanta L, Conte P, Lo Meo P (2022) Differentiation among dairy products by combination of fast field cycling NMR relaxometry data and chemometrics. Magn Reason Chem 60:369–385. https://doi.org/10.1002/mrc.5226
Somawiharja Y, Purnomo H, Wonohadidjojo D., Kartikawati M, Suniati F (2018). Indigenous technology of tapping, collecting and processing of coconut (Cocos Nucifera) sap and its quality in Blitar Regency, East Java, Indonesia J Food Sci 2:398, doi: https://doi.org/10.26656/fr.2017.2(4).075
Pocan P, Grunin L, Oztop MH (2022) Effect of different syrup types on turkish delight (Lokum): a TD-NMR relaxometry. ACS Food Sci Technol 2:1819–1831
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Torregrossa, F., Cinquanta, L., Albanese, D. et al. Vegan and sugar-substituted chocolates: assessing physicochemical characteristics by NMR relaxometry, rheology, and DSC. Eur Food Res Technol 250, 1219–1228 (2024). https://doi.org/10.1007/s00217-023-04457-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04457-w