Abstract
The possibility of using unmalted millet with the help of the enzymes for the production of gluten-free beer was investigated. The enzymes under different conditions were examined to completely saccharify the wort. The optimal conditions for enzymes activity were 85 °C 60 min for amylosubtilin and 60 °C 60 min for glucavamarin and β-glucanase. Since the gluten-free beverage has no colour, roasted buckwheat and boiled coffee as colourants were used. Sample with 30% roasted buckwheat showed good results in colouring the drink and had the best overall impression. Gluten-negative results were obtained for beer samples and all ingredients separately. Nevertheless, further improvements in brewing methods of gluten-free beer are needed. Almost all samples tasted more like cider than beer, without foam and with low pH values (3.5–3.8). The production of gluten-free beer provides an opportunity to attract new customers with gluten intolerance. Imperfect competition is a great advantage for manufacturers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nowadays, celiac disease is one of the most common immune-mediated diseases, diagnosed in 1% of the European and American population [1, 2]. Symptoms of this disease can include abdominal pain, diarrhoea, nausea, fatigue, weight loss, mouth ulcers, bloating, and anaemia [3]. These symptoms can be caused by products that contain gluten, such as wheat, barley, rye and oats [4]. The gluten proteins of wheat belong to two of the Osborne fractions, the gliadins and the alcohol-insoluble glutenins [5]. Gliadins contain large amounts of proline and glutamine residues in the form of repeated sequence motifs, which makes them resistant to human intestinal proteases and preserves their antigenic nature [6]. The immunogenicity of non-degradable gliadin peptides is also enhanced by human tissue transglutaminase (TG2)-mediated deamidation [7].
Such popular gluten-containing grains as barley and wheat are used to produce beer, making it impossible for people with coeliac disease to consume this drink. One gram of malt contains 1.6 mg of barley hordein (gluten), and one pint of beer contains 1.5 mg of hordein [8]. There is some research on gluten-free beer with rice and none with millet or unmalted grains. Mayer et al. [9] and Ceccaroni et al. [10] made a gluten-free beer with 100% malted rice. There is a possibility to produce gluten-free beer with barley by enzymatic degradation of gluten using prolyl endopeptidase from Aspergillus niger [4]. Prolyl endopeptidase was not available on our market. To brew gluten-free beer, unmalted proso millet (Panicum miliaiceum) from Ukraine was used with added enzymes (amylosubtilin, glucavamarin, and β-glucanase). Roasted buckwheat and boiled coffee were used as the colourant for gluten-free beer. Unmalted grains do not contain enzymes, so exogenous enzymes have to be added. The beer produced with enzymes reduces the use of water, raw materials and natural gas by 7%, 14% and 78%, respectively. The largest exergy losses result from the use of natural gas for kilning and from starch loss during barley germination. This makes enzyme-assisted brewing more exergy-efficient than brewing with malted barley [11].
Millet is an environmentally friendly crop as it consumes more carbon dioxide, thereby releasing more oxygen, and has a low water requirement [12]. Thanks to a low glycaemic index, proso millet reduces the risk of type 2 diabetes [13]. Millet is rich in calcium, fibre, polyphenols, and protein [14]. Millet contains 65–75% complex carbohydrates, with amylose ranging from 1.50 to 25.93% (w/w db) and amylopectin ranging from 17.06 to 46.26% (w/w db), 5.6–12% protein, 2–5% fat, 15–20% crude fibre and 2.5–3.5% minerals [13, 15]. Lipoxygenases account for 40.1–47.4% of the total fatty acids in various millet species [16]. The main polyphenols are phenolic acids and tannins, while flavonoids are present in small amounts; they act as antioxidants and play a major role in immune system defence [14, 17]. The absence of gluten makes millet suitable for the production of gluten-free beer.
The aim of this work was to achieve complete saccharification in the mashing step. An additional goal was to use colourants to colour the beer. The production of gluten-free beer provides an opportunity to attract new customers with gluten intolerance. Imperfect competition is a great advantage for manufacturers.
Materials and methods
Enzymes
Amylosubtilin is a bacterial enzyme preparation containing α-amylase, which is used to dilute starch.
A-Amylase hydrolyses the internal α-1,4-glycosidic bonds of starch, resulting in a rapid decrease in the viscosity of pasteurised starch solutions. The products are soluble dextrins of low molecular weight and low content of mono- and disaccharides (glucose and maltose). Amylosubtilin is active at high temperatures (up to 95 °C) and high pH.
Glucavamarin is a bacterial enzyme preparation used to saccharify partially cleaved polymeric starch molecules, while glucoamylase sequentially hydrolyses –1,4- and –1,6-glycosidic bonds and cleaves from the non-reducing ends of starch molecules, dextrins, oligosaccharides and glucose residues that are the end product of hydrolysis.
Β-Glucanase is an enzymatic biocatalyst with a high degree of purification and enzymatic activity obtained from a high-quality microorganism by deep liquid fermentation and purification. This enzyme catalyses 1,3- and 1,4-glycosidic linkages of β-glucans and cleaves viscous polymer macromolecules into low-viscosity isomalt.
Β-Glucanase can reduce the viscosity of the wort and improve the quality of the final product.
Gluten-free beer preparation
Mashing: The wort was prepared in a 10-L laboratory brewery using the infusion method. Before brewing, the millet must be ground and gelatinized at 80–85 °C to break down the starch into fermentable sugars and dextrins. The malt was crushed in a two-roller mill with a 0.2 mm gap between the rollers. Millet was mixed with water in a 1:4 ratio (1.5 kg of millet and 5 L of water at 60 °C with the addition of amylosubtilin). Water was heated up to 80 °C (1 °C /min) and wort was stirred from time to time. After exposure, the wort to amylosubtilin and 5 L of warm water were added, the temperature was lowered to 60 °C and citric acid was used to bring the wort to a pH of 5, after which glucavamarin and β-glucanase were added. Activity of the enzymes was checked by the iodine test to indicate the breakdown of starch into sugar. In the first step of the experiment, various combinations of enzymes under different temperatures and time conditions were investigated. Results can be seen in the Results section.
Lautering and boiling: The mash was separated through the sieve into liquid wort and residual grain. Hop pellets (Fuggle, 5.6% α-acids) were used at the beginning of the boil (9 g, 60 min) and after the boil (10 g, 20 min) to achieve 17.5 International Bitter Units (IBU). Boiling was conducted at 100 °C for 60 min. After hot trub removal, the wort was cooled to 20 °C.
Addition of colourants before fermentation: boiled ground coffee (10 g coffee per 150 mL water, boil for 10 s)—added to the fermenter after yeast pitching: 2 ± 0.01 g L−1, 4 ± 0.01 g L−1; millet was partially substituted with roasted buckwheat during mashing: 20%, 30%.
Final samples with colourants and the optimal amount of enzymes (85 °C 60 min for 8 g amylosubtilin and 60 °C 60 min for 20 g glucavamarin and 12 g β-glucanase) were prepared (Table 1).
Fermentation: For the fermentation, which took place in plastic food fermenters, the top-fermenting yeast Safale S-04 was used (Fermentis, Marcqen-Barœul Cedex, France). Attenuation: 72–75%. Yeast Cell Count: 69,000,000,000 (69 billion) per 11.5 g sachet. This is a yeast with a high fermentation power, which has the ability to form a compact sediment at the end of the fermentation. The recommended ideal fermentation temperature for this type of yeast is 15–20 °C. Pitching of the yeast 107 cells/mL in the wort, that had been cooled to 20 °C. For the different samples, the fermentation lasted on average 7 days, it was stopped when the alcohol content reached about 6°Bx. After the fermentation, the beers were not pasteurised or filtered. Maturation took place in bottles with added glucose (3 ± 0.01 g L−1) for a fortnight.
Gluten analyses
Gluten analyses were carried out for beer and all ingredients separately. Gluten analyses were performed using the Imutest Gluten-In-Food Test Kit, which is sensitive enough to confirm that gluten levels in a variety of foods and products are comply with International Codex Standard 118:1979 (2015) and contain less than 20 mg.kg−1 of gluten in accordance with Regulation (EU) 1169/2011 and 609/2013 for ‘gluten-free’ foods. These qualitative tests use highly specific Mendez R5 monoclonal antibodies to detect gluten in food. They detect mainly alpha, gamma and omega gliadins, secalins and hordeins. The Gluten-in-Food test does not react to ingredients that do not contain gluten: corn, oats, rice, and soy.
Performance of the test
The 0.5 cc measuring spoon was filled with the sample and placed in the extraction tube, which was recapped and shaken for 2 min, which helped to dissolve the gluten from the sample. The tube was left for 10–15 min and then shaken again for 2 min. The sample then stood for 20 min. Using a pipette, the clear yellow extract was taken by tightly squeezing the bulb and releasing it until the solution overfilled the measuring tube and flowed into the lower bulb. Then the pipette bulb was squeezed into the tube with the diluted extract and mixed carefully. The diluted extract was put into the test unit and was allowed to absorb. After absorption, the colour reagent was shaken and poured into the test unit where it was absorbed for about 5 min. A pink test spot on the left side of the test area, marked T regardless of intensity, indicates that gluten was detected. The darker the test spot, the more gluten is detected in the sample extract tested. A pink control spot of medium intensity should appear on the right-hand side of the test area marked C. This indicates that the Gluten-in-Food test is valid, and the test has been performed correctly and all reagents are functional.
Sensory evaluation of the beer
The sensory properties of beer were evaluated according to the ČSN ISO 6658 by ten panellists (f = 4, m = 6, age = 21–52 years). The panellists are regular consumers of beer. The beer panel was conducted in a tasting room at 18 °C. Before tasting, the beer samples were cooled to 7 °C. The amount of beverage to be evaluated was 150 mL. The beer was poured into colourless, clear glasses. The panellists filled in the sensory evaluation form. The blank sheet had the following items: aroma, off-flavour, taste (fullness and saturation), bitterness and overall impression. The rating ranged from 1 to 5. A score of 1 meant that the attribute was absent whereas a score of 5 indicated that the attribute was extremely strong. The overall impression rating ranged from 1 to 5, with 1 being excellent and 5 being very bad. Blind-tasting was conducted on all the experimental samples. The results were expressed as the mean of the two technological replicates.
Colour measurements
Spectrophotometer GRANUM 721 (ATO, Diamond Bar, CA 91765 USA) was used to measure the colour of the beer. The largest allowable error of the transmittance (T): ± 0.5%. The beer was poured into a 1 cm thick cuvette, which was previously rinsed several times with the same beer sample. The measurement length is 450 nm for the Standard Reference Method (SRM) [18] and The European Brewery Convention (EBC) [19]:
SRM = 12.7 × D × A450
EBC = 25 × D × A450, where D is the dilution factor (D = 1 for undiluted samples, D = 2 for 1:1 dilution, etc.), and A450 = light absorbance at 450 nm.
Foam height and stability
The constant method was used. Foam height (F) is calculated according to liquid height (L) and total height (T) (in duplicate) after elapsed times (t) in the range 0–5 min. The rate of foam collapse calculated according to ln(F) = a + bt. In turn, foam half-life (min) was determined as ln(0.5)/b.
Alcohol content
Alcohol content was analysed on the device for the automatic analysis of beer FermentoFlash (Funke Gerber, Berlin, Germany). During brewing was used portable digital refractometer PAL-3 (Atago Corp., Tokyo, Japan).
All the statistical analyses and the related graphics were performed in XLSTAT (Lumivero, Denver, United States). Variables were auto-scaled (mean-centring + scaling by standard deviation).
Results and discussion
The iodine test is supposed to indicate the breakdown of starch into sugar by the absence of colouring after mixing with wort. Samples without starch did not colour and remained yellow (Table 2). Samples with starch present in the post-mash wort had turned black (Table 2). Complete saccharification of the wort was achieved due to the optimised conditions for the enzyme activity (85 °C 60 min for 8 g amylosubtilin and 60 °C 60 min for 20 g glucavamarin and 12 g β-glucanase). The limitation of the study is the type of used enzymes, which could be found only in some countries of Eastern Europe, which reduces the possibility of reproducing this part of investigation. The optimal temperature and boiling time may vary for different enzymes. But this does not diminish the importance of research on unmalted grains for the production of gluten-free beer.
Colour measurements
Samples (2, 3, 4) with 30% roasted buckwheat and boiled coffee (2 g L−1 and 4 g L−1) showed good results in colouring the drink (Fig. 1), respectively, SRM 2, SRM 3, and SRM 4. Sample 1 with 20% roasted buckwheat was pale (SRM 1). Mayer et al. [9] brewed malted rice beer and obtained pale yellow colour for all beer samples (EBC 5.4, 5.5, 6.9 converted to SRM 2.7–3.5). We achieved the same colouring results only by roasting buckwheat, which was used to replace 30% of unmalted millet, eliminating the malting step, which saves time and resources. The reasons roasted buckwheat was used are a darker colour and a special taste. Ceccaroni et al. [10] made caramelised rice with the help of malting and obtained a colour of 25 EBC-U (12.7 SRM), which is the typical colour of ales, while our “ale” had typical colour of lager and bottom-fermented beers. Millet does not have husks, thereby the haze was present in every sample. It would be appropriate to use rice husks as a filter layer during lautering as Mayer et al. [9] did in their study.
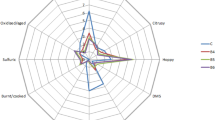
Sensory evaluation
Almost all samples tasted like cider, they had a fruity aroma, and were sour (Fig. 2). In addition, some of them had the yeasty taste. High-lipid content led to increased yeast growth, which is the cause of the yeasty taste. Lipids do not affect beer quality if their content is less than 1.5% [20]. In addition, the protein content in the wort influences the fermentation process [21]. The finished beverage in the bottle had turbidity, which means that the proteins were not broken-down during mashing and affected the sensory parameters of the beer, such as taste and aroma. The overall quality of the beer is affected by the amount of protein; both less and too much protein is unfavourable [21].
The cider-like taste was influenced by yeast metabolism, especially in Safale-04 yeasts, which optimal fermentation temperature is 18–20 °C. As experiment was provided in summer the temperature of fermentation was higher, respectively 23–25 °C. Higher fermentation temperatures stimulate the formation of acetaldehyde, which produces the green apple flavour found in our beverage. Therefore, changing the temperature could be an effective way to get rid of acetaldehyde [22]. In future investigations, it is needed to perform a primary fermentation at a lower temperature with bottom-fermented yeast.
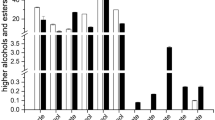
The body of every sample of the beer was thin/watery without carbonation and bitterness. Ceccaroni et al. [10] made caramelised and dark rice beer and obtained a malty profile and an amber beer. The body score was not particularly high, despite the presence of special rice malts [10]. Mayer et al. [9] reported that the malted rice beer had the relatively flat character. Flavour attributes were in a low range [9]. Gluten-free grains had flat body and do not have much carbonation. The weak hop flavour and foam stability influenced by the fat content of the millet (2–5%) [13, 23]. The presence of lipoxygenase, which accounts for 40.1–47.4% of the total fatty acids of millet, leads to the foam and flavour instability of beverages [16].
Foam height and stability
All samples had almost no foam, as it can be seen in Table 3. They were small or large bubbles that quickly disappeared. High amount of lipoxygenase in millet and the absence of protease were affected foam stability. Proteins, which are not cleaved by proteases, affect the formation and stability of beer foam, as it is formed by the interaction of medium molecular weight proteins and carbon dioxide [24]. During malting, malt proteins are broken down by proteases into smaller peptides that contribute to foam formation [25]. In the case of millet beverages, the proteins were not broken down, so that no foam formed. Mayer et al. [9] reported that rice malt beers had coarse foam, which rapidly collapsed. Compared results lead to the conclusion that using of gluten-free grains for beer production requires the application of foaming agents.
pH measurements
All beer samples (Table 4) were below the optimal pH for most beers, but sour ales can have a pH as low as 3.0. There are some sour beer styles such as Belgian Lambic, which are exposed to wild yeasts and bacteria that produce the flavour of cider. Belgian Lambic is a cloudy, non-carbonated, tangy sour drink similar to ours. Mayer et al. [9] reported pH values of 4.21–4.24 for malted rice beer, which is the optimal pH for most beer styles. Afterwards, Ceccaroni et al. [10] made caramelised and dark rice beer with the pH values of 3.9 ± 0.1, which is a little higher value than our 20% and 30% buckwheat samples had, respectively, 3.75 ± 0.07 and 3.8 ± 0.06. Coffee lowered down pH of the beer, which is seen in sample 1 (2 g L−1) and sample 2 (4 g L−1), respectively, 3.5 ± 0.03 and 3.3 ± 0.08.
All ingredients and beer samples did not contain gluten, unlike the barley beer that was bought from a shop for comparison. The alcohol contents of the gluten-free beers were in an estimated range 2.8 ± 0.2% ABW. All beverage samples were sour, without foam, with cider taste and flavour. Sample 2 with 30% of roasted buckwheat showed the best taste and coloration results. While previous research has focussed on beer production with malted gluten-free grains, these results demonstrate that unmalted gluten-free grains can also be used with addition of enzymes to fully saccharify the wort. This new product can be presented as more comparable to cider than beer. As this work aims to produce gluten-free beer, fermentation conditions and filtering methods must be optimised in further research. The production of gluten-free beer provides an opportunity to attract new customers with gluten intolerance. Imperfect competition is a great advantage for manufacturers.
Data availability
All data and materials support their published claims and comply with field standards.
Code availability
Not applicable.
References
Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S (2003) Prevalence of celiac disease in at-risk and not-at-risk groups in the United States. Arch Intern Med. https://doi.org/10.1001/archinte.163.3.286
Mustalahti K, Catassi C, Reunanen A, Fabiani E, Heier M, McMillan S, Murray L, Metzger MH, Gasparin M, Bravi E, Mäki M (2010) The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann Med. https://doi.org/10.3109/07853890.2010.505931
Lowth M (2018) Coeliac disease: Clinical features, diagnosis and management. Practice Nurse 44(8):36–40
Wieser H, Koehler P, Konitzer K (2014) Celiac disease and gluten multidisciplinary challenges and opportunities. Academic Press Elsevier
Juhász A, Békés F, Wrigley CW (2015) Wheat proteins. Appl Food Protein Chem. https://doi.org/10.1002/9781118860588.ch11
Shan L, Qiao SW, Arentz-Hansen H, Molberg Y, Gray GM, Sollid LM, Khosla C (2005) Identification and analysis of multivalent proteolytically resistant peptides from gluten: implications for celiac sprue. J Proteome Res. https://doi.org/10.1021/pr050173t
Vader LW, de Ru A, van der Wal Y, Kooy YM, Benckhuijsen W, Mearin ML, Drijfhout JW, van Veelen P, Koning F (2002) Specificity of tissue transglutaminase explains cereal toxicity in celiac disease. J Exp Med. https://doi.org/10.1084/jem.20012028
Ellis HJ, Freedman AR, Ciclitira PJ (1990) Detection and estimation of the barley prolamin content of beer and malt to assess their suitability for patients with coeliac disease. Clin Chim Acta. https://doi.org/10.1016/0009-8981(90)90082-4
Mayer H, Ceccaroni D, Marconi O, Sileoni V, Perretti G, Fantozzi P (2016) Development of an all rice malt beer: a gluten free alternative. Food Sci Technol. https://doi.org/10.1016/j.lwt.2015.11.037
Ceccaroni D, Sileoni V, Marconi O, De Francesco G, Lee EG, Perretti G (2019) Specialty rice malt optimization and improvement of rice malt beer aspect and aroma. LWT. https://doi.org/10.1016/j.lwt.2018.09.060
van Donkelaar LH, Mostert J, Zisopoulos FK, Boom RM, van der Goot AJ (2016) The use of enzymes for beer brewing: thermodynamic comparison on resource use. Energy. https://doi.org/10.1016/j.energy.2016.09.011
Kumar A, Tomer V, Kaur A, Kumar V, Gupta K (2018) Millets: a solution to agrarian and nutritional challenges. Agric Food Secur. https://doi.org/10.1186/s40066-018-0183-3
Ramashia SE, Mashau M, Onipe O (2021) Millets cereal grains: nutritional composition and utilisation in Sub-Saharan Africa. Cereal Grains. https://doi.org/10.5772/intechopen.97272
Devi PB, Vijayabharathi R, Sathyabama S, Malleshi NG, Priyadarisini VB (2011) Health benefits of finger millet (Eleusine coracana L.) polyphenols and dietary fiber: a review. J Food Sci Technol. https://doi.org/10.1007/s13197-011-0584-9
Shen R, Ma Y, Jiang L, Dong J, Zhu Y, Ren G (2018) Chemical composition, antioxidant, and antiproliferative activities of nine Chinese proso millet varieties. Food Hydrocoll. https://doi.org/10.1080/09540105.2018.1428283
Wennman A, Oliw EH, Karkehabadi S, Chen Y (2016) Crystal structure of manganese lipoxygenase of the rice blast fungus Magnaporthe oryzae. J Biol Chem. https://doi.org/10.1074/jbc.m115.707380
Chandrasekara A, Shahidi F (2010) Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J Agric Food Chem. https://doi.org/10.1021/jf100868b
The American Society of Brewing Chemists (ASBC). Standard Reference Method (SRM). (1950). https://www.asbcnet.org/. Accessed 20 Dec 2022
European Brewery Convention (EBC) (2021) https://europeanbreweryconvention.eu. Accessed 20 Dec 2022
Resurrection AP, Juliano BO, Tanaka Y (1979) Nutrient content and distribution in milling fractions of rice grain. J Sci Food Agric. https://doi.org/10.1002/jsfa.2740300506
Tan YW, Li M, Devkota L, Attenborough E, Dhital S (2021) Mashing performance as a function of malt particle size in beer production. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2021.2018673
Petkova N, Jonkova G (2010) Effect of some technological factors on the content of acetaldehyde in beer. Sci Study Res: Chem Chem Eng 46:1
Bamforth CW (2008) Beer: a quality perspective. Academic Press
Marconi O, Sileoni V, Ceccaroni D, Perretti G (2017) The use of rice in brewing. In: Li JQ (ed) Advances in international rice research. South China Agricultural University. https://doi.org/10.5772/66450
Steiner E, Gastl M, Becker T (2011) Protein changes during malting and brewing with focus on haze and foam formation: a review. Eur Food Res Technol. https://doi.org/10.1007/s00217-010-1412-6
Funding
Open access publishing supported by the National Technical Library in Prague. Mendel University in Brno, Czech Republic provided the financial support. National University of Life and Environmental Sciences of Ukraine provided the laboratory.
Author information
Authors and Affiliations
Contributions
Conceptualisation: AD, OO, YK, LB-P; methodology: AD, YK; formal analysis and investigation: AD, YK; writing—original draft preparation: AD; writing—review and editing: YK, OO; funding acquisition: AD; resources: LB-P, OO, YK; supervision: YK, OO, LB-P.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
The authors declare this study was conducted in accordance with ethical guidelines and principles. The study was explained to the consumers prior to sensory evaluation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dymchenko, A., Kirilenko, Y., Ochkolyas, O. et al. Gluten-free beer with unmalted millet. Eur Food Res Technol 250, 999–1005 (2024). https://doi.org/10.1007/s00217-023-04405-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04405-8