Abstract
This study focuses on analyzing the impact of various fining agents on apple cider and evaluating their effects on its physical–chemical and sensory properties. Despite its common cloudy state, some consumers prefer clear apple cider. Within this study, two variations of apple cider were analyzed: original cider and cider with added barrique extract. Eight different fining agents were applied to these samples, specifically bentonite, gelatin, polyvinylpolypyrrolidone, egg white protein, activated carbon, pea protein, isinglass, and silica gel. The results revealed that the use of fining agents significantly impacted the antioxidant content in apple cider. The most substantial reduction in antioxidants was observed in samples with added activated carbon, resulting in a 75% decrease to values of 0.11–0.26 mmol TE/L. This decrease in antioxidants correlated with a decline in total polyphenols. Sensory analysis demonstrated statistically significant differences among the various apple cider samples. Bentonite and egg white protein had minimal adverse effects on sensory properties, while activated carbon had the most pronounced negative impact on the aroma and taste of the cider. This study provides valuable insights into the use of fining agents in winemaking technology and their effects on the quality of apple cider, considering consumer preferences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cider is a beverage produced by fermenting apple juice, with an alcohol content between 0.95 and 6.71 g/L (low-alcohol cider may have less than 0.95 g/L) obtained though partial or complete fermentation of juice (whether fresh or reconstituted), with or without the addition of sugar, water or flavoring [1, 2]. Global cider production is steadily growing, with the UK being the world leader in cider consumption [3]. According to 2018 statistics, more than 1 million tons of apples were processed worldwide solely within the cider industry [4].Apple ciders have distinct chemical compositions and organoleptic characteristics [5], which depend on the technological processes of production [2]. One of the critical technological operations is clarification [6], i.e., after pressing, apple cider numerous solid sludge particles need to be removal [7]. The most commonly employed clarification methods include sedimentation, centrifugation, microfiltration, enzymatic clarification, and clarification using clarifying agents [8]. In the technology of fruit wines, clarifying agents are the most frequently used materials. Clarifiers work based on either positive (albumen/gelatin) or negative (bentonite/silica gel) electric charge [9]. Bentonite is the most widely used clarifying agent in the production of alcoholic beverages [10]. It effectively removes yeast sludge, tannins, and other protein-based substances from wine [11]. Polyvinylpolypyrrolidone (PVPP) and activated carbon are also employed to remove natural polyphenols found in fresh fruit juice [12]. Gelatin is mainly used in the clarification of juices that are not intended for further processing. It effectively removes cloudiness that could arise during the storage of finished products [13]. In recent years, also plant-based fining agents have become increasingly common. Proteins extracted from plants (cereals, grape seeds, potatoes or legumes) and non-protein substances (polysaccharides obtained from seaweed) show significant potential [14].
Despite the fact that ciders can be cloudy, some consumers prefer clear alcoholic beverages, especially in regions where grape wine consumption is more prevalent. In countries where cider production does not have a long history, cider-specific fining agents are usually not readily available. Therefore, the potential of traditional winemaking fining agents to reduce turbidity in cider was investigated. While the influence of clarifying agents on the chemical composition of wines and fruit wines has been documented in many publications, describing the effects of bentonite, gelatin, PVPP, egg white, as well as casein [15,16,17], these publications primarily describe the effect of fining agents applied to cider before fermentation and do not address the application of the agents and post-fermentation effects on finished products [13, 18]. The aim of this study was to assess the suitability of selected fining agents for cider production and to provide recommendations for the most appropriate agent based on the conducted analytical and sensory analyses. The most efficient fining agent should significantly reduce turbidity and minimally affect the antioxidant content, polyphenols, and sensory expression of cider.
Materials and methods
Apple cider
The apple cider used in this experiment was made from a blend of 'Melodie' and 'Idared' apple cultivars, with 'Melodie' accounting for 60% and 'Idared' for 40% of the total volume. To enhance juice stability prior to fermentation, 40 mg/L of sulfur dioxide in the form of powdered potassium disulphite was added to freshly pressed apple juice, which had soluble solids at 12.3°Bx, titratable acids at 6.4 g/L, and a pH of 3.4. The apple juice underwent fermentation in a stainless steel container for 21 days at a temperature of 18 °C. After fermentation, the apple cider was separated from the yeast sludge and transferred to another stainless steel container where it aged for 6 months at a temperature of 8 °C before being used for sample preparation. A temperature controller ITC-308-Wifi (Inkbird, USA) monitored the cider's temperature, measuring it every 12 h, with temperature fluctuations within a range of ± 2 °C. The final apple cider contained 3.71 g/L of alcohol, titratable acids at 5.8 g/L, and had pH of 3.4.
Samples preparation
Two different variants were prepared (variant A and variant B). Variant A included cider with a fining agent; variant B included cider with the fining agent and 250 µl/L of a barrique extract. The barrique extract (Vinoferm OAK-a-VIN, Brouwland, Belgium) was added to the samples just before clarification. The purpose of adding the barrique extract to apple cider was to imitate the conditions of aging in a wooden oak barrel. For clarification, eight different fining agents were used—bentonite, gelatin, polyvinylpolypyrrolidone (PVPP), activated carbon, pea protein, isinglass, egg white, and silica gel. The maximum quantity and ½ of the maximum quantity of fining agents were dosed according to the specifications outlined in Table 1. All variants were prepared in 1-l containers with five replicates.
Chemical standards and fining agents
Folin–Ciocalteu reagent, 2,4,6-tris-(2-pyridyl)-s-triazine (TPTZ), and 2,2 -diphenyl-1-picrylhydrazyl were obtained from Sigma-Aldrich (St. Louis, MO, USA). Polyphenol standards were as follows: tyrosol, caffeic acid, p-coumaric acid, chlorogenic acid, ferulic acid, (+)-catechin, (−)-epicatechin, all supplied by Glentham Life Sciences Ltd. (Corsham, United Kingdom). Fining agents for this experiment, namely bentonite, gelatin, polyvinylpolypyrrolidone, activated carbon, pea protein, isinglass, egg white, and silica gel were purchased from Lamothe-abiet (Canéjan, France). Pectolytic enzyme Panzym F2 was purchased from Eaton (Bloomfield, NJ, USA). Acetic acid (99%, Lachema, Czech Republic) and methanol (suitable for HPLC, ≥ 99.9%, Sigma Aldrich, St. Louis, MO, USA), were used for HPLC measurements.
Antioxidant capacity
The antioxidant capacity of the researched samples was determined using modified FRAP and DPPH methods, as applied by Tomasina et al. [19] and Sethi et al. [20].
FRAP method The samples were mixed with ethanol in a concentration of 75.74 g/L (in a 1:1 ratio) and then diluted with distilled water (in a 1:4 ratio). 100 µL of each sample was pipetted, along with 400 µL of distilled water, were pipetted into an Eppendorf tube and shaken using an orbital shaker (IKA MS 3). The following solutions were prepared: FeCl3.6H2O (0.081 g dissolved in distilled water), TPTZ (2,4,6-tris(2-pyridyl)-s-triazine) with hydrochloric acid (0.078 g of TPTZ in a 25 ml flask with 0.08825 ml of 35% HCl) and a buffer (0.075 g of sodium acetate and 4 ml of acetic acid in a 250 ml flask). The reaction mixture was prepared by mixing these solutions at a ratio of 1:1:10 (in the order mentioned above). The Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) solution was used as a standard (0.0125 g/100 ml). 2000 µL of the reaction mixture and 25 µL of the diluted sample were pipetted into a cuvette. The cuvette was shaken for 10 s using the IKA MS 3 orbital shaker. 10 min after the start of the reaction, absorbance was measured in a 10 mm cuvette on a spectrophotometer (SPECORD 50 PLUS, Analytik Jena GmbH, Jena, Germany) at a wavelength of 593 nm against a blank sample (a reaction mixture with water). The results were specified as mmol Trolox/L (mmol TE/L).
DPPH method The samples were mixed with ethanol in concentration 75.74 g/L (in a 1:1 ratio) and then diluted with distilled water (in a 1:4 ratio). 100 µL of each sample, along with 400 µL of distilled water, were pipetted into an Eppendorf tube and shaken using an orbital shaker (IKA MS 3, IKA-Werke GmbH, Staufen im Breisgau, Germany). A solution of DPPH in methanol with a concentration of 0.1 mmol/L was prepared in a 250 ml volumetric flask. 100 µL of the diluted sample and 2000 µL of the DPPH solution were pipetted into a 10 mm cuvette. Cuvettes with samples were thoroughly shaken for 20 s using an orbital shaker (IKA MS 3). After the cuvettes were placed in the dark for 30 min, absorbance was measured on a spectrophotometer (SPECORD 50 PLUS, Analytik Jena GmbH, Jena, Germany) at a wavelength of 515 nm. A blank sample was used containing 100 µL of pure methanol. The results were specified as mmol Trolox/L (mmol TE/L).
Total polyphenols
The total polyphenol content was determined using the Folin–Ciocalteu reagent, with modifications compared to the method described by Vrhovsek et al. [21]. This approach relies on the spectrophotometric measurement of the color products resulting from the reaction of the hydroxyl groups in phenolic compounds using the Folin–Ciocalteu reagent. The procedure involved adding 1 ml of the sample to a 50 ml volumetric flask, followed by the addition of approximately 20 ml of demineralized water and 1 ml of the Folin–Ciocalteu reagent. After shaking the flask and waiting for 3 min, 5 ml of Na2CO3 (conc. 200 g/L) was added to the mixture, followed by another round of shaking and dilution with water. After allowing the mixture to stand for 30 min, its color was measured in 10 mm cuvettes at a wavelength of 700 nm, with the spectrophotometer (Specord 50 Plus) being used for this purpose. Total polyphenols were quantified using a calibration curve with gallic acid as the standard. The total polyphenol content was expressed in milligrams of gallic acid per liter of apple cider (mg GA/L).
Individual phenolic compounds
Individual phenolic compounds were identified through high-pressure liquid chromatography (HPLC). The HPLC analysis followed the method outlined by Picinelli Lobo et al. [22] with the modifications made for the selected column. The HPLC instrument (Agilent 1260 Infinity II, Germany) was equipped with pumps including an isocratic and a binary pump, an auto sampler, a thermostat, and diode array and fluorescence detectors (DAD and FLD). Data analysis was managed using Agilent OpenLAB CDS 2.x software. The separation of polyphenolic compounds occurred on a Kinetex 5 µm C18 100A column, 150 × 2.1 mm, at a temperature of 25 °C (Phenomenex). The mobile phase flow rate was 0.4 ml/min. The elution phases consisted of a 2% aqueous acetic acid (mobile phase A) and 100% methanol (mobile phase B). The chosen elution gradient exhibited linearity, transitioning from 0 to 45% mobile phase B over 55 min. From minute 55 to minute 75, the gradient was held at 45% mobile phase B. At minute 75–76, it returned to 0% mobile phase B. A post-time of 3 min was employed. The injection volume was 5 µl. Quantification was achieved through external standards and validated by adding a standard to the sample. Detection of phenolic compounds was carried out at specific wavelengths: 313.4 nm for gallic acid, chlorogenic acid, coumaric acid, and ferulic acid; 280.4 nm for tyrosol, catechin, syringic acid, and epicatechin; and 255 nm for caffeic acid. The R2, LOD, LOQ values for individual analytes are provided in Table 2.
Color parameters
To measure the spatial coordinates of the CIELAB color space, including lightness (L*) and color-opponent dimensions (a* and b*), a colorimeter (Lovibond RT850i-X-Rite, Tintometer Ltd., Greenwich, London) was employed. The cider samples were placed in plastic cuvettes and measured with an optical path length of 10 mm. The Oncolour Premium software application (Tintometer Ltd., Greenwich, London) was utilized for evaluation [23].
Turbidity
Turbidity was determined using a turbidimeter TN-100, (Thermo Fisher Scientific, Waltham, Massachusetts, USA). This device operates based on the nephelometric principle of turbidity measurement and conforms to ISO 7027 and DIN 27027 standards. Prior to measuring the first sample, the instrument underwent calibration using basic standards (800 NTU, 100 NTU, 20 NTU, and 0.02 NTU). The recorded values are expressed in NTU (Nephelometric Turbidity Units).
Sensory assessment
A sensory evaluation of the apple cider samples involved 13 assessors selected in accordance with ISO 8586. The testing environment met the criteria outlined in ISO 8589. The preparation, labeling, and presentation of samples to the assessors followed ISO 6658 guidelines. The samples were served in transparent 50 ml sample glasses at a temperature of 12 ± 2 °C. Differences in the intensity of aroma and taste were assessed using a graphical 100-point scale (ISO 4121). Assessors used a 100 mm line segment, equivalent to 100 points, to indicate the value of the tested parameter being tested (0 = very weak; 100 = very strong).
Statistics
All the calculations were carried out using STATISTICA 14 (StatSoft, USA). Analysis of variance was performed using the ANOVA procedure, with p < 0.05 considered statistically significant. In addition, the Tukey HSD test was employed to demonstrate statistically significant differences.
Results and discussion
Antioxidant capacity
Ljevat et al. [24] focused in their study on fruit wines and highlighted a direct connection between the content of phenolic substances and the value of antioxidant capacity. In the analyzed samples, there was either significant or not significant decrease in the values of the antioxidant capacity of individual samples in all variants (Figs. 1 and 2).
In the FRAP method, the control sample exhibited the highest antioxidant capacity, with a value of 1.31 mmol TE/L for variant A (without barrique extract) and 1.47 mmol TE/L for variant B (with the addition of 250 µl/L of a barrique extract). The most substantial decrease in antioxidant capacity in the FRAP method was observed in cider samples clarified with activated carbon (AC 500/AC 1000), where the values ranged from 0.23 to 0.80 mmol TE/L. The DPPH method also indicated that the control samples also had the highest antioxidant capacity, with values ranging from 0.75 to 1.12 mmol/L. The findings align with the research conducted by Kowalczyk et al. [25], Los et al. [26], and Budak et al. [27]. The samples with isinglass (IGS 30, IGS 60) and egg white (EW 50 and EW 100) experienced a 2–8% decrease in the antioxidant capacity for both variants A and B. The low presence of antioxidants in the samples treated with PVPP and activated carbon was used as clarifiers which can be attributed to the binding of phenolic substances with antioxidant properties by these clarifiers. Satora et al. [28] delved into the changes in the phenolic content of substances in apple wines, noting that phenolic substances degrade during processing, potentially leading to a reduction in antioxidant capacity. However, they reported that cider had twice the antioxidant capacity of apple juice, likely due to the lower solubility of certain phenolic substances in water, which are better dissolved in alcohol. The results of both the FRAP and DPPH methods were highly correlated (R = 0.93), suggesting the consistency of the findings between the two methods. Interestingly, despite the expectation that the addition of barrique extract would enhance antioxidant capacity, as shown by the FRAP method in the control sample, the DPPH method did not reveal a significant difference. Analyzing the presented graphs allows for the interpretation of the varying abilities of individual fining agents to reduce the levels of substances that scavenge free radicals, as opposed to substances capable of reducing trivalent iron compounds to divalent forms.
Total polyphenols
Fining agents had a significant impact on the total content of polyphenolic compounds. The values of total polyphenols showed either a highly significant decrease or no significant change (Fig. 3). The content of total polyphenols in ciders ranged from 290 to 1023 mg/L. The highest concentration of polyphenolic compounds was found in the control samples, with 893 mg/L for variant A, and 1023 mg/L for variant B. These results indicate that the barrel extract significantly influences the content of phenolic substances. In samples containing the barrel extract, the content of phenolic substances was 15 ± 2.6% higher than in samples without it, translating to 130 mg more in variant B than in variant A. The smallest reduction in phenolic compounds was observed in samples clarified with bentonite (BEN 500/BEN 1000) and gelatin (GEL 150/GEL 300). On the other hand, samples clarified with isinglass (IGS 30/IGS 60) had the lowest phenolic compound content. Based on Tukey's HSD test, statistically significant differences were identified between control samples and all other samples containing fining agents. These findings align with Picinelli Lobo et al. [22] who reported polyphenols concentrations ranging from 447 to 1180 mg/L in Austrian apple cider. In a publication by Alonso-Salces et al. [29], phenolic substances were measured in Basque ciders, finding values ranging from 24 to 331 mg/L. In a study by [30], polyphenol concentrations in ciders from Latvian apple varieties ranged from 794 to 3400 mg/L. Kowalczyk et al. [25] investigated the total content of phenolic substances in apple juice from Polish apple varieties, with concentrations ranging from 210 to 300 mg/L.
Individual phenolic compounds
In the analyzed cider samples (variants A/B), we identified six polyphenols and one phenylethanoid. Tyrosol, classified as a phenylethanoid, predominated in all cider samples, followed by chlorogenic acid, caffeic acid, coumaric acid, ferulic acid, epicatechin, and catechin for both variants (Tables 3 and 4). Tyrosol, as the principal polyphenol, exhibited a range of 13.84 to 25.20 mg/L. Madrera et al. [31] reported that tyrosol was among the predominant constituents in Asturian natural cider, with levels ranging from 10.09 to 38.16 mg/L. In addition, our samples displayed significant concentrations of chlorogenic acid (2.20 to 16.78 mg/L) and caffeic acid (4.12 to 8.96 mg/L).. The content of phenolic acids may be strongly influenced by the plant species, variety, and physiological stage [32]. In contrast, Picinelli Lobo et al. [22] documented that chlorogenic acid was the predominant constituent in cider apple juice, ranging from 1.1 to 32.2 mg/L, considerably higher than our findings. Epicatechin and catechin, two flavanols within flavanol group exhibited noteworthy content, ranging from 0.83 to 7.58 mg/L for epicatechin and from 2.53 to 10.82 mg/L for catechin. Epicatechin content in cider samples displayed variability. Madrera et al. [31] reported that epicatechin values ranged from 0.11 to 3.03 mg/L. Comparatively, values of epicatechin in Asturian ciders ranged from 1.4 to 22.5 mg/L in a publication [22]. Epicatechin values that are comparable to our results are reported in a study by Riekstina-Dolge et al. [30], with epicatechin content ranging 0.77 to 8.63 mg/L. Regarding the individual phenolic compounds, differences were observed across all samples. Typically, the highest values were found in the control samples. The presence of fining agents led to subsequent decrease, a significant average reduction 85% (± 5%) in individual phenolic compounds (except tyrosol). This reduction influenced the sample color and sensory attributes, as demonstrated in the stage of sensory evaluation phase. In the study [33], they observed the impact of aging apple brandy for 15 months in wooden barrels (Quercus alba). They noted an increase in caffeic acid from 0.64 to 1.21 mg/L, and ferulic acid from 0.30 to 0.43 mg/L. Comparing the results of this study, it can be seen that the addition of oak extract led to an increase in caffeic acid by 1 mg/L, but there was no significant change in the concentration of ferulic acid.
Turbidity and color parameters
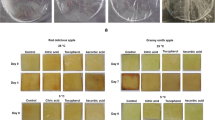
The highest turbidity values were measured in the control samples, ranging from 25 to 32.5 NTU. For variant B, the turbidity values in the samples containing gelatin (GEL 150/GEL 300) were higher than in the control samples for variant A. It was also generally true that samples in which a higher dose of a fining agent was applied had a higher NTU value (Fig. 4). The lowest turbidity values were determined in samples containing PVPP (PVPP 400/PVPP 800), activated carbon (AC 500/AC 1000), and bentonite (BEN 500/BEN 1000). To improve efficiency, a combination of gelatin and silica gel was used in one sample. Gelatin, as a fining agent, carries a positive charge, and it often happens that during fining, it does not precipitate well in the beverage and settles poorly. In winemaking practice, a combination of silica gel and gelatin is commonly used to enhance the clarifying capacity of gelatin. From the obtained results, it is evident that this effect was not demonstrated in the case of cider. Particularly in variant A, the turbidity of sample with gelatin was reduced more significantly than in the sample with combination of gelatin with silica gel. Turbidity is usually highest in fresh apple juice and it is reduced many times during technological processing. Oszmiański and Wojdyło [34] reported turbidity values in fresh apple juice after processing to be 3055 NTU and, after 6 months of storage, 2877 NTU. They also mentioned that after the application of enzymes, turbidity values ranged from 2133 to 2707 NTU. Benitez and Lozano [13] investigated the effect of bentonite and gelatin on the degree of turbidity in apple juice in their study. They reported that at bentonite concentrations of > 3.2 g/L, the apple juice was practically clarified when the concentration of gelatin was greater than 0.2 g/L. The addition of barrique extract caused cloudiness in the samples, with the presumed reason being the presence of barrique tannins in an alcohol–water mixture. When introduced to low-alcohol cider, some tannins may precipitate. In the case of the sample with gelatin, cloudiness was observed, and it sedimented poorly, resulting in higher turbidity values for these samples.
Parameter a* and parameter b*
Parameter a* represents the green–red color scale. A positive value indicates an increased red tint in the sample, while a negative value suggests a greenish tint. In summary, there were no significant differences in the samples regarding parameter a* values. Parameter b* represents a yellow–blue color scale. As the positive value increases, the proportion of the yellow tint grows. All cider samples with fining agents (for both variants, i.e., A and B) displayed lower values of the yellow tint as shown in Table 5 compared to the control sample, which had the highest value of yellow tint value. In the samples with PVPP and activated carbon, the value of the yellow tint value was so low that this change was noticeable to the naked eye. Oszmiański and Wojdyło [34] observed a wider range of color parameter b* in apple juice made from the 'Idared' variety and treated with bentonite, gelatin, and silica gel. The value of parameter a* ranged from − 1.66 to −0.29, while parameter b* ranged from 9.74 to 23.66.
Sensory assessment
Figures 5 and 6 illustrate changes in the taste and aroma intensity resulting from the addition of the fining agents. Variant B exhibited a fuller taste and positive aroma due to the inclusion of a barrique extract in the samples. Variant B experienced a smaller loss of sensorially significant components compared to variant A. A decrease in the sensorially significant substances occurred in all samples, which is reflected in the evaluation of fullness of taste and positive aroma. For both variants, samples containing pea protein and activated carbon were identified to feature the most significant negative changes in the fullness of taste. Samples with activated carbon, completely lost their taste character; for aroma, ethanol was predominant as the content of other aromatic components was rapidly reduced due to the effect of fining agents. Samples containing bentonite and isinglass received the most positive rating. Although it was lower than for the control sample, the assessors still considered the samples to be above average from the sensory aspect. The Tukey's HSD test showed statistically significant differences between control samples and other samples containing fining agents. Significant differences between individual variants were also noted; for example, in samples containing bentonite, there was a statistically significant difference in taste fullness between variant A and variant B in the BEN 1000 sample.
Conclusion
Ciders can be produced with a cloudy appearance, although some consumers prefer clear alcoholic beverages, particularly in regions where wine is more prevalent. Clarifying agents are primarily employed in winemaking technology, which is why this study investigates the utilization of clarifiers designed for grape wines and their suitability in cider production. The antioxidant capacity, total polyphenols, chemical composition, and the intensity of smell and taste in ciders are significantly affected by the presence of added fining agents. In sensory evaluation, there was a loss of aromatic and flavor compounds that enhance the sensory perception of smell and taste in cider. In certain samples, a notable decrease in color intensity was observed, which sensory assessors also identified as a negative impact. From a chemical perspective, a reduction in antioxidant substances was confirmed through both FRAP and DPPH methods. The most substantial decrease was evident in samples treated with activated carbon, characterized by its extensive adsorbent surface, thereby confirming initial assumptions. A greater reduction in antioxidant capacity was also noted for PVPP, known for its ability to bind to phenolics. Conversely, fining agents such as bentonite, pea protein, and isinglass contributed to the reduction in antioxidant capacity. The content of polyphenolic compounds decreased in all examined samples treated with fining agents. In the samples treated with activated carbon, there was a 75% decrease (± 4%) in antioxidant capacity and an 80% decrease (± 5%) in phenolic compounds, resulting in a shift in color from dark yellow to nearly transparent. The results’ findings suggest that the most suitable fining agents for cider clarification are bentonite and egg white, which had the least adverse impact on the tested cider samples.
Data availability
The authors verify that the data substantiating the conclusions of this study can be found within the article. Additional data can be obtained upon request from the corresponding author.
References
AICV. (2018). The European Cider & Fruit Wine Associaton. European Cider Trends. https://aicv.org. Brusels, Belgium: AICV. Retrieved 2023–03–10, from https://aicv.org/files/attachments/.55/CiderTrends2018.pdf
AICV. (2022). The European Cider & Fruit Wine Associaton. European Cider Trends. https://aicv.org. Brusels, Belgium: AICV. Retrieved 2023–03–10, from https://aicv.org/files/attachments/.504/AICV_Cider_Trends_2022.pdf
Alonso-Salces RM, Guyot S, Herrero C, Berrueta LA, Drilleau J-F, Gallo B, Vicente (2005) Chemometric classi-fication of Basque and French ciders based on their total polyphenol contents and CIELab parameters. Food Chem 91(1):91–98. https://doi.org/10.1016/j.foodchem.2004.05.049
Awe S (2018) Effect of clarifying agents (gelatin and kaolin) on fruit wine production. Int J Agric Innov Res 6(4):130–132
Benitez E, Lozano J (2007) Effect of gelatin on apple juice turbidity. Lat Am Appl Res 4(37):261–266
Braga A, Cosme F, Ricardo-da-Silva JM, Laureano O (2016) Gelatine, casein and potassium caseinate as distinct wine fining agents: different effects on colour, phenolic compounds and sensory characteristics. OENO One 41(4):203–214. https://doi.org/10.20870/oeno-one.2007.41.4.836
Budak NH, Ozçelik F, Güzel-Seydim ZB (2015) Antioxidant activity and phenolic content of apple cider. Turkish J Agric Food Sci Technol 3(6):356–360. https://doi.org/10.24925/turjaf.v3i6.356-360.265
Calugar PC, Coldea TE, Salanță LC, Pop CR, Pasqualone A, Burja-Udrea C, Mudura E (2021) An overview of the factors influencing apple cider sensory and microbial quality from raw materials to emerging processing technologies. Processes. https://doi.org/10.3390/pr9030502
Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK (2004) Clarification of fruit juice with chitosan. Process Biochem 39(12):2229–2232. https://doi.org/10.1016/j.procbio.2003.11.024
Duenas M, Irastorza A, Fernandez C, Bilbao A, Campo GD, Munduate A (1997) Influence of apple juice treatments on the cider making process. J Inst Brewing 103(4):251–255. https://doi.org/10.1002/j.2050-0416.1997.tb00953.x
Fukumoto L, Delaquis P, Girard B (1998) Microfiltration and ultrafiltration ceramic membranes for apple juice clarification. J Food Sci 63(5):845–850. https://doi.org/10.1111/j.1365-2621.1998.tb17912.x
Kemp B, Marangon M, Curioni A, Waters E, Marchal R (2022) New directions in stabilization, clarification, and fining. Manag Wine Qual. https://doi.org/10.1016/B978-0-08-102065-4.00002-X
Kowalczyk A, Ruszkiewicz M, Biskup I (2015) Total phenolic content and antioxidant capacity of polish apple ciders. Indian J Pharm Sci 77(5):637–637. https://doi.org/10.4103/0250-474X.169024
Lambri M, Dordoni R, Silva A, De Faveri DM (2012) Comparing the impact of bentonite addition for both must clarification and wine fining on the chemical profile of wine from Chambave Muscat grapes. Int J Food Sci Technol 47(1):1–12. https://doi.org/10.1111/j.1365-2621.2011.02800.x
Le Quéré J-M, Husson F, Renard CM, Primault J (2006) French cider characterization by sensory, technological and chemical evaluations. LWT Food Sci Technol 39(9):1033–1044. https://doi.org/10.1016/j.lwt.2006.02.018
Ljevar A, Ćurko N, Tomašević M, Radošević K, Gaurina Srček V, Kovačević Ganić K (2016) Phenolic composition, antioxidant capacity and in vitro cytotoxicity assessment of fruit wines. Food Technol Biotechnol 54(2):145–155. https://doi.org/10.17113/ftb.54.02.16.4208
Los PR, Braga CM, Carvalho JR, Simões DR, Nogueira A (2017) Application of sensory analyses in the development of a new apple cider. Revista Brasileira de Tecnologia Agroindustrial 11(1):2150–2168. https://doi.org/10.3895/rbta.v11n1.3301
Macdougall D (2010) Colour measurement of food: principles and practice. Colour Meas. https://doi.org/10.1533/9780857090195.2.312
Macheix J-J, Sapis J-C, Fleuriet A, Lee CY (1991) Phenolic compounds and polyphenoloxidase in relation to browning in gra-pes and wines. Crit Rev Food Sci Nutr 30(4):441–486. https://doi.org/10.1080/10408399109527552
Madrera RR, Lobo AP, Valles BS (2006) Phenolic profile of Asturian (Spain) natural cider. J Agric Food Chem 54(1):120–124. https://doi.org/10.1021/jf051717e
Mangas J, Rodríguez R, Moreno J, Suárez B, Blanco D (1997) Furanic and phenolic composition of cider brandy. A chemometric study. J Agric Food Chem 45(10):4076–4079. https://doi.org/10.1021/jf9701300
Mierczynska-Vasilev A, Smith P (2015) Current state of knowledge and challenges in wine clarification. Aust J Grape Wine Res 21:615–626. https://doi.org/10.1111/ajgw.12198
Muhlack R, Nordestgaard S, Waters E, Oneill B, Lim A, Colby C (2006) In-line dosing for bentonite fining of wine or juice: contact time, clarification, product recovery and sensory effects. Austr J Grape Wine Res 12(3):221–234. https://doi.org/10.1111/j.1755-0238.2006.tb00062.x
Oszmiański J, Wojdyło A (2007) Effects of various clarification treatments on phenolic compounds and color of apple juice. Eur Food Res Technol 224(6):755–762. https://doi.org/10.1007/s00217-006-0370-5
Picinelli Lobo A, García YD, Sánchez JM, Madrera RR, Valles BS (2009) Phenolic and antioxidant composition of cider. J Food Compos Anal 22(7–8):644–648. https://doi.org/10.1016/j.jfca.2009.03.008
Riekstina-Dolge R, Kruma Z, Dimins F, Straumite E, Karklina D (2014) Phenolic Composition and sensory properties of ciders produced from Latvian apples. Proc Latvia Univ Agric 31(1):39–45. https://doi.org/10.2478/plua-2014-0005
Rosend J, Kaleda A, Kuldjärv R, Arju G, Nisamedtinov I (2020) The effect of apple juice concentration on cider fermentation and properties of the final product. Foods.
Satora P, Tarko T, Duda-Chodak A, Sroka P, Tuszyński T, Czepielik M (2009) Influence of prefermentative treatments and fermentation on the antioxidant and volatile profiles of apple wines. J Agric Food Chem 57(23):11209–11217. https://doi.org/10.1021/jf9025053
Sethi S, Joshi A, Arora B, Bhowmik A, Sharma RR, Kumar P (2020) Significance of FRAP, DPPH, and CUPRAC assays for antioxidant activity determination in apple fruit extracts. Eur Food Res Technol 246(3):591–598. https://doi.org/10.1007/s00217-020-03432-z
Sommer S, Sommer SJ, Gutierrez M (2022) Characterization of different bentonites and their properties as a protein-fining agent in wine. Beverages 8(2):31–31. https://doi.org/10.3390/beverages8020031
Stanković S, Jović S, Živković J (2004) Bentonite and gelatine impact on the young red wine coloured matte. Food Technol Biotechnol 42(3):183–188
Tomasina F, Carabio C, Celano L, Thomson L (2012) Analysis of two methods to evaluate antioxidants. Biochem Mol Biol Educ 40(4):266–270. https://doi.org/10.1002/bmb.20617
Vrhovsek U, Rigo A, Tonon D, Mattivi F (2004) Quantitation of polyphenols in different apple varieties. J Agric Food Chem 52(21):6532–6538. https://doi.org/10.1021/jf049317z
Youn K-S, Hong J-H, Bae D-H, Kim S-J, Kim S-D (2004) Effective clarifying process of reconstituted apple juice using membrane filtration with filter-aid pretreatment. J Membrane Sci 228(2):179–186. https://doi.org/10.1016/j.memsci.2003.10.006
Acknowledgements
Financial support for this research was provided by Project IGA-ZF/2021-SI2007 of Mendel University, Brno, Czech Republic. This work was instrumentally supported by project CZ.02.1.01/0.0/0.0/16_017/0002334. Research Infrastructure for Young Scientists is co-financed from Operational Program Research, Development and Education.
Funding
Open access publishing supported by the National Technical Library in Prague.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Ethical standard
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seriš, D., Balík, J., Híc, P. et al. Effect of fining agents on the chemical composition and sensory properties of apple cider. Eur Food Res Technol 250, 521–531 (2024). https://doi.org/10.1007/s00217-023-04395-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04395-7