Abstract
Mixed fermentation is one of the methods used in sour beer production. The process requires initialization of the fermentation step by well-planned addition of brewing yeast and lactic acid bacteria to slightly hopped wort. The final product’s properties strictly depend on how the microorganisms are pitched and the initial wort composition. The experiment was performed to evaluate the impact of different initial conditions and pitching methods on the mixed fermentation process and the final product’s characteristics. With the aim of limitation of the number of experiments, the Box–Behnken design was applied. Three independent factors were considered while obtaining the response surface: initial extract, bitterness and order of pitching. The final product’s properties: ethanol and lactic acid concentration, appeared to depend strictly on initial conditions and pitching order. Several important observations have been made; for example, it appeared that the presence of LAB does not significantly impact the final ethanol concentration. Optimal conditions for obtaining the maximum or minimum of each quality were calculated using Matlab. Obtained results might improve the sour beer production process while shortening the duration and reducing the usage of ingredients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The presence of lactic acid bacteria (LAB) in beer has been an issue for ages, causing hard-to-avoid and irreversible beer spoilage. Traces of their spoiling activity could be traced even in beer bottles found in a shipwreck from the 1840s [12]. However, after being isolated in 1873 by Lister, it appeared that LAB can be used in many industry branches and improve the quality of products [19]. LAB are mostly applied in the dairy industry in the production of yoghurt, buttermilk, etc., while pharmaceutical and food industries make use of LAB's probiotic properties.
In the beer industry, LAB can be considered as spoilage microorganisms, whereas in some cases, the presence of LAB is required to obtain certain types of beer, such as sour beer, Belgian Lambic or Berliner Weisse.
It is estimated that LAB are responsible for approximately 70% of beer spoilage cases, mostly caused by Lactobacillus brevis and Pediococcus damnosus. Other strains—Lactobacillus plantarum, Lactobacillus lindneri, Lactobacillus paracasei and Lactobacillus buchneri—also appear in spoiled products [9, 21]. Due to the fact that it is extremely difficult to eliminate the presence of LAB, once contaminated installation might produce faulty products for many batches forward despite strict hygiene in the brewery. An uncontrolled presence of LAB in the brewery causes remarkable financial and material losses, thus thorough cleaning of the installation and high-quality materials play a crucial role.
LAB's metabolites can deeply affect the taste and aroma of beer thus spoilage of beer is difficult to overlook. The main metabolite, lactic acid, is responsible for the remarkable sourness of the beer. Other metabolites also leave their trace in the product’s properties. The presence of diacetyl gives the beer a butter-like aroma, while the so-called mousy aroma is caused by 2-acetyl tetrahydropyridine and 2-ethyl tetrahydropyridine. What’s more, LAB cells and heteropolysaccharides produced by them are the reason for the beer's high turbidity [11].
However, proper selection of LAB strain and intentional addition of the microorganisms can result in a product in which properties mentioned above (like remarkable sourness) are strongly desired—so-called sour beer. Production of beer characterized by sourness above average level has been conducted for ages, for example in the case of Belgian Lambic beer, where wild yeast strains are responsible for beer’s characteristic properties. In the case of wild yeast strains, LAB, and occasionally acetic acid bacteria (AAD), complex combinations of flavour compounds produced by them cannot be obtained in any other way—so obtaining high-quality sour beer cannot be artificially done by the simple addition of acids. Well-thought application of microorganisms can result in beers with a wide range of original flavour profiles, highly demanded on the market [20].
Numerous methods of beer acidification have been introduced in the breweries. For example, sour beer can be produced from special malt, previously exposed to LAB during malting. Here the most often used strain is Lactobacillus delbrueckii. This method helps to avoid further spoilage with unwanted microorganisms and lowers the wort’s pH, which has a positive impact on enzymes’ activity in the further brewing steps [14].
“Sour mashing” includes pitching the wort with LAB right after mashing. The wort is later left to ferment until the demanded pH is obtained and then it is heated up to 78°, filtered, and treated like normal wort in the following beer production steps. In this method, LAB are eliminated during wort boiling, so unwanted further spoilage is not possible [3].
Sour beer can also be obtained during “kettle souring”, where the wort is boiled without hops, cooled down and then pitched with LAB. The mixture is left to ferment for 24–48 h, boiled with the addition of hops, pitched with brewing yeast and left for further fermentation. Unfortunately, long fermentation taking place in the kettle excludes it from being used for other purposes, so unless other kettles are available at that time, the production capacity of the brewery becomes lower [8].
Another method of sour beer production is the so-called “mixed fermentation”. A mixture of yeast (conventional brewer’s yeast and/or non-conventional species) and lactic acid bacteria is added to slightly hopped wort. However, this method requires precise and thoughtful pitching of the microorganisms, as the composition and organoleptic properties of the beer strictly depend on the order of introducing the microbes into the wort [17].
The composition of the mixed culture is essential. Using fast-growing and/or fast-fermenting species like Lactobacillus spp. and Saccharomyces spp. drastically change the growth conditions in beer and affect the occurrence of other microorganisms [3]. Using Pediococcus spp. instead of Lactobacillus spp. cause the acidity to develop more gradually. This allows other species (for example Saccharomyces spp. and Brettanomyces spp.) to influence the flavour profile of the beer, making it more complex [15]. Moreover, these species may produce different aroma and flavour compounds if they are pitched as a single culture than in a culture combination [10].
The pitching rate plays a crucial role in the mixed fermentation process. It has been shown that adding the bacteria before inoculating yeast allows one to obtain appropriate lactic acid concentration and pH. Otherwise—when yeast is added before LAB—the pH of the beer tends to be higher, and the lactic acid content significantly lower. What’s more, LAB are highly sensitive to hop compounds, so strongly hopped wort might inhibit their activity. It has been shown that Lactobacillus brevis shows the highest resistance to hop compounds among other LAB [5].
The most challenging aspect of applying the mixed culture fermentation method is to control the optimal balance between the microorganisms involved in this process. The added cultures might not always behave in the same manner and it is not always simple to predict the outcome of the fermentation. To increase consistency, brewers can use dregs from previous, successful batches. However, it is recommended to pitch fresh yeast together with the dreg obtained from the previous batch because the bacteria in preserved dregs tend to grow faster than the yeasts [1].
This work aims to evaluate the mixed fermentation process with different pitching orders of S. cerevisiae and L. brevis, wort sugar content and hop dosage to achieve final products of various properties. Theoretical optimisation using the Box–Behnken design method was carried out to find the best way to achieve the extremal possible amounts of lactic acid and ethanol concentration in the beer.
Materials and methods
Application of Box–Behnken design (BBD)
Wort properties (initial extract, bitterness) and order of microorganisms pitching were chosen according to the Box–Behnken design (BBD) method, which allows to organize, conduct and interpret the results by performing a limited number of trials. The BBD method makes it possible to study the effect of various factors of the designs if, during the experiment considering one factor, the other factors are constant. In this case, three following independent variables were considered: initial extract (14, 10 and 6° Plato), bitterness (40, 30 and 10 IBU) and the order of pitching of the microorganisms (Table 1). Process optimization was performed using MATLAB® programme with Statistics and Machine Learning Toolbox.
For the calculations, the initial data shown in Table 2 were applied. The number after the letter “L” shows the day when L. brevis was pitched, and the number after the letter “S” shows the day when S. cerevisiae was pitched, e.g. L1:S3 means that L. brevis was pitched on the 1st day and S. cerevisiae on the 3rd day, etc. For the calculations in MATLAB®, the order of pitching was implied as shown in Table 3.
Wort preparation
7 L of wort with an extract value equal to 16.6° Plato was prepared using pilsner malt (Viking Malt, Poland). The solution was later diluted to obtain samples with 14, 10 and 6° Plato. 500 mL of hop extract was prepared by mixing 10 g of Marynka hop pellets (Browamator) with distilled water and boiling it for 1 h. Samples were prepared by diluting the previously obtained mixture. The bitterness of the initial mixture (in International Bitterness Units) was measured by adding 0.5 mL of 6 M HCl and 20 mL of isooctane to 10 mL of the sample. After centrifugation (3000 rpm, 3 min), the absorbance of the organic phase at 275 nm was measured [16]. Hopped worts of known extract values were distributed into previously sterilised 0.5-L Erlenmeyer flasks and the samples’ properties are as shown in Table 1.
Addition of microorganisms
Two microorganisms were used: Saccharomyces cerevisiae (SAFALE™ US-05 from Fermentis, OD550 = 0.554 in 10 × dilution) and Lactobacillus brevis (NCTC 13386/ATCC 8287, OD550 = 0.800 in 10 × dilution). L. Brevis was selected basing on literature research, as it was described to be the most resistant to beer-related environmental stress factors [6]. 5 mL of each inoculum was added to the wort as presented in Table 1. The 72 h difference between applications of each inoculum was applied based on the experiment which measured the impact of pitching sequence on sour beer production performed by A. Ciosek and others [5].
Fermentation process
The Erlenmeyer flasks containing the samples were covered with rubber stoppers with fermentation tubes filled with distilled water to apply anaerobic conditions, but at the same time to allow carbon dioxide to be removed from the flask. The samples were left to ferment at room temperature and the fermentation process was run for 14 days until the difference between extract values on the two following days did not exceed 0.2° Plato.
Physiochemical analysis
Samples were taken every day to monitor the progress of fermentation according to the extract and density values. Ethanol and lactic acid content were measured on the 14th day of the fermentation process.
The apparent extract was measured using an optical refractometer (Kerbl 1464). However, this method does not consider the presence of ethanol and lactic acid, with a refractive index equal to 1.36 and 1.43, respectively. Therefore, this method was applied only to monitor whether the fermentation is progressing or not.
The density of the samples is affected by the fermentation products, thus it was measured along with the extract to evaluate its progress. The density was measured using Anton Paar DMA-38 Density Meter.
Ethanol concentration in the samples was measured using a Shimadzu GC2014 gas chromatograph. The gas chromatography process was carried out under the following conditions:
-
Column: ZB-WAX plus, length = 30 m
-
Injector temperature: 140 °C
-
Detector temperature: 200 °C
-
Temperature program of the column: from 85 to 200° C, rate 25°/min, holding time of the sample in 200 °C: 1 min.
The retention time of ethanol was equal to approximately 2.79 min.
The lactic acid concentration was measured using a Shimadzu UV-1800 spectrophotometer. The presence and amount of lactic acid in the solution were determined, as it reacted with iron(II) chloride forming yellowish-green iron(IIII) lactate. The absorbance of the obtained solution was measured using a wavelength of 390 nm and is proportional to the concentration of lactic acid in the sample [2].
Organoleptic tests
Samples taken from each final product underwent organoleptic tests, which were performed by a group of 12 participants consisting of 6 males and 6 females aged 22–31. During the blind test, participants were asked to analyse the aroma and taste of each sample. Samples’ properties were rated between 1 and 10, where 1 means almost or completely unnoticeable and 10 stands for strong and dominant.
The following properties were analysed:
-
Fruity
-
Bitterness
-
Sweetness
-
Sourness
-
Sulphuric flavour
-
Refreshness
-
Yeasty flavour
For further purposes, the average value of test results for each sample for each trait was taken into consideration.
Results and discussion
Physiochemical measurements
The final results of ethanol concentration (ABV) in the samples (Table 4) show that in sample number 8 (initial extract = 14, IBU = 30, order of pitching: S. cerevisiae on 1st day, LAB on 3rd day), the value was the highest and equal to 6.49%. In general, samples starting with the highest initial extract and inoculated with yeast on the first day (either alone or with LAB) resulted in the highest alcohol contents. In the control sample, where only L. brevis was present (no. 14), trace amounts of ethanol were detected (approximately 0.16%). Comparing samples 9–15 and 16, which had initial extract equal to 10° Plato, similar ethanol concentrations can be observed. Therefore, it can be concluded that the presence of L. brevis did not have any significant impact on its value.
The final pH values of each sample (Table 4) were acidic. The sample with the lowest pH was number 3 (14° Plato, 10 IBU, both microorganisms pitched on the 1st day), with the value equal to 3.41. The highest pH was in sample number 2 (pH 4.55), with the initial extract equal to 6, 50 IBU and both microorganisms pitched on the 1st day. It appeared that a higher concentration of hop compounds results in less acidic beer.
Sour beers available on the market have a pH value between 3.0 and 3.9 [20]. Beer obtained by applying initial values like in samples number 1, 3, 7, 9, 10, 13, 14, and 15 would meet the commercial standards. These samples had low to moderate IBU and most of them (excluding number 10) were inoculated with L. brevis on the 1st day (alone or with yeast). According to the same criteria, samples with low initial extract and high IBU would not be considered as ‘sour’, as their acidity did not fit in the scale.
The concentration of lactic acid in each sample on the last day of fermentation is presented in Table 4. The highest value (10.25 g/L) was obtained in sample number 3, where the initial extract was the highest (14° Plato), and IBU had the lowest value (10). Simultaneously, its pH was the lowest. According to the literature, lactic acid concentration in commercially available beers should be in the range of 3–6 g/L, so in the case of samples 3 and 8 the content of lactic acid might be even too high to meet the commercial standards [4]. The lowest lactic acid concentration was measured in the sample number 2 and was equal to 0.81 g/L. In this case, the initial extract was low (6° Plato), and bitterness was relatively high (40 IBU), according to which it can be stated that such conditions negatively affect the efficiency of lactic acid fermentation. The presence of lactic acid was confirmed in both control samples, including trace amounts in sample number 17 with pure S. cerevisiae.
Organoleptic tests
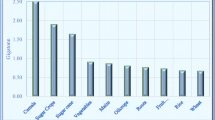
Organoleptic tests showed that all beers were perceived differently (Figs. 1, 2, 3). Each figure represents a group of samples with the same initial extract value (6, 10 and 14, respectively). In the case of the initial extract equal to 6° Plato (Fig. 1), the samples appeared to have a rather bland taste. Higher extract values (Figs. 2, 3) resulted in a more complex flavour. Most of the samples showed significant sourness. Samples with the lowest IBU (marked in green—sample numbers 1, 3, 9, 10) appeared to have the highest levels of perceptible sourness, which also responds to the fact that these samples were the most acidic. The most heightened sense of sourness was observed in sample number 3, containing lactic acid in the highest amount of 10.25 g/L. None of the samples appeared to be significantly refreshing or bitter. Sulphuric and yeasty flavours were not precipitated in any case. Surprisingly, higher IBU only slightly raised the feeling of bitterness.
The Box–Behnken design method results
The BBD method was applied to calculate initial conditions to obtain maximal and minimal ethanol and lactic acid concentrations in the final product. Calculated results are presented in Tables 5 and 6, and response surfaces generated by the program are shown in Figs. 4 and 5.
BBD results confirmed previously noticed correlation, according to which low IBU (10) and high initial extract (14) can lead to the highest concentration of lactic acid. However, the calculated result (9.5 g/L) is slightly lower than the highest result obtained during the experiment (sample 3, 10.25 g/L). This difference might suggest the imperfection of this method (Table 6). These values are relatively high compared to the range of lactic acid concentration in commercial products, which is between 3 and 6 g/L [4].
On the other hand, high bitterness (40 IBU) and similarly high extract (14° Plato) make ethanol the more favourable product of mixed fermentation, with its predicted concentration reaching up to 6.69% vol (Table 5).
Conclusions
Mixed fermentation is a promising method to produce new beer types. Our studies confirmed that L. brevis alone is less sufficient to efficiently produce lactic acid from the wort comparing to fermentation performed in co-culture. Low lactic acid yield could be caused by end-product inhibition observed previously in other LAB-involving research [18]. It has also been proven that the growth of Lactobacillus brevis is enhanced in co-culture with Saccharomyces cerevisiae, as the yeast cells only partially compete for the nitrogen sources with LAB, while synthesizing and excreting essential and stimulatory amino acids, which enhance the LAB cell yield [7]. Other experiments considering mixed fermentation including L. brevis and S. cerevisiae pitched simultaneously resulted in lower pH than in case when L. brevis was pitched alone [6].
The experiment showed that the order of pitching might inflict the final alcohol content of the beer. This observation may lead to the conclusion that beer with reduced ethanol content can be obtained during mixed fermentation. In some cases, co-fermentation using both microorganisms resulted in beer with ethanol content not exceeding 4%, with both satisfactory sourness and taste properties. Apart from adjusting the pitching order, other researches point out that while being pitched simultaneously, the proportion between LAB and yeast in the inoculum often plays a crucial role in the co-fermentation outcomes [13]. This adds another experimental factor to be considered in the further research.
Presented work also showed that hop compounds strongly affect LAB metabolism, which may be observed by decreased lactic acid content in beers produced with intensely hopped wort, which confirmed the results of previous studies [6].
The outcomes of mixed fermentation are highly dependent on the applied factors and to fully understand the process and easily predict the final product’s properties, another number of experiments needs to be performed.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
Bokulich NA, Bamforth CW (2017) Brewing microbiology: current research, omics and microbial ecology, brewing microbiology: current research, omics and microbial ecology. Caister Academic Press, Poole
Borshchevskaya LN, Gordeeva TL, Kalinina AN, Sineokii SP (2016) Spectrophotometric determination of lactic acid. J Anal Chem 71:755–758. https://doi.org/10.1134/S1061934816080037
Bossaert S, Crauwels S, Lievens B, De Rouck G (2019) The power of sour—a review: old traditions, new opportunities. Brew Sci. https://doi.org/10.23763/BrSc19-10bossaert
Ciosek A, Fulara K, Hrabia O, Satora P, Poreda A (2020) Chemical composition of sour beer resulting from supplementation the fermentation medium with magnesium and zinc ions. Biomolecules 10:1–14. https://doi.org/10.3390/biom10121599
Ciosek A, Rusiecka I, Poreda A (2020) Sour beer production: impact of pitching sequence of yeast and lactic acid bacteria. J Inst Brew 126:53–58. https://doi.org/10.1002/jib.590
Dysvik A, Leanti S, Rosa L, Liland KH, Myhrer KS, Østlie HM, Rouck GD, Rukke E, Westereng B, Arendt EK (2020) Co-fermentation involving Saccharomyces cerevisiae and Lactobacillus species tolerant to brewing-related stress factors for controlled and rapid production of sour beer. Front Microbiol 11:1–16. https://doi.org/10.3389/fmicb.2020.00279
Gobbetti M et al (1994) The sourdough microflora. Interactions between lactic acid bacteria and yeasts: metabolism of amino acids. World J Microbiol Biotechnol 10(3):275–279. https://doi.org/10.1007/BF00414862
Esslinger H (2009) Handbook of brewing: processes, technology, markets. Wiley, Wiley
Garofalo C, Osimani A, Milanović V, Taccari M, Aquilanti L, Clementi F (2015) The occurrence of beer spoilage lactic acid bacteria in craft beer production. J Food Sci 80:M2845–M2852. https://doi.org/10.1111/1750-3841.13112
Hoelzle RD, Virdis B, Batstone DJ (2014) Regulation mechanisms in mixed and pure culture microbial fermentation. Biotechnol Bioeng 111:2139–2154. https://doi.org/10.1002/bit.25321/abstract
König H, Unden G, Fröhlich J (2017) Biology of microorganisms on grapes, in must and in wine. Biol Microorg Grapes Must Wine. https://doi.org/10.1007/978-3-319-60021-5
Londesborough J, Dresel M, Gibson B, Juvonen R, Holopainen U, Mikkelson A, Seppänen-Laakso T, Viljanen K, Virtanen H, Wilpola A, Hofmann T, Wilhelmson A (2015) Analysis of beers from an 1840s’ shipwreck. J Agric Food Chem. https://doi.org/10.1021/jf5052943
Mahanta S, Pankaj PSS, Ranjan P, Gargi KM, Smita D (2022) Sour beer production in India using a coculture of Saccharomyces pastorianus and Lactobacillus plantarum: optimization, microbiological, and biochemical profiling. Braz J Microbiol. https://doi.org/10.1007/s42770-022-00691-8
Mallet J (2014) Malt A practical guide from field to brewhouse. Brewers Publications, Boulder
Martens H, Iserentant D, Verachtert H (1997) Microbiological aspects of a mixed yeast—bacterial fermentation in the production of a special Belgian acidic ale. J Inst Brew 103:85–91. https://doi.org/10.1002/j.2050-0416.1997.tb00939.x
MEBAK (2013) Wort, beer, beer-based beverages. MEBAK
Modzelewska A, Jackowski M (2022) Bakterie fermentacji mlekowej w produkcji piwa. Kierun Spożywczy 3:34–37
Peyer L (2017) Lactic acid bacteria fermentation of wort as a tool to add functionality in malting, brewing and novel beverages. PhD Thesis, University College Cork
Santer M (2009) Joseph Lister: first use of a bacterium as a “model organism” to illustrate the cause of infectious disease of humans. Notes Rec R Soc Lond 64:59–65. https://doi.org/10.1098/rsnr.2009.0029
Tonsmeire M (2014) American sour beer: innovative techniques for mixed fermentations. Brewers Publications, Boulder
Yansanjav A, Švec P, Sedláček I, Hollerová I, Němec M (2003) Ribotyping of lactobacilli isolated from spoiled beer. FEMS Microbiol Lett 229:141–144. https://doi.org/10.1016/S0378-1097(03)00817-6
Author information
Authors and Affiliations
Contributions
AM: methodology, formal analysis, conceptualisation, investigation, writing (original draft), visualisation. MJ: methodology, formal analysis, conceptualisation, investigation, writing (original draft). AT: supervision, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there are no conflicts of interest.
Compliance with ethics requirements
The authors declare this study was conducted in accordance with ethical guidelines and principles.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Modzelewska, A., Jackowski, M. & Trusek, A. Optimization of beer mixed fermentation using Saccharomyces cerevisiae and Lactobacillus brevis. Eur Food Res Technol 249, 3261–3269 (2023). https://doi.org/10.1007/s00217-023-04365-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04365-z