Abstract
Cranberry pomace considered as a by-product of fruit industry contains seeds which may be processed to highly nutritive oil. Conventional extraction methods may be, however, harmful to natural environment, thus alternative, ultrasound-assisted extraction method may be useful tool to reduce environmental impact. In the following study, sonication was applied to extract oil from cranberry seeds. The aim of the study was to determine the most efficient conditions of ultrasound-assisted extraction of oil and to investigate sonication influence on the properties of final product. Ultrasound amplitude and extraction time were independent variables; yield and maximum induction time of oils were responses. The most efficient conditions were amplitude of 95% and extraction time of 11.38 min. Model predicted extraction yield of 22.55 ± 0.36% (vs. actual 21.98 ± 0.08%) and induction time of 52.60 ± 0.95 min (vs. actual 61.95 ± 3.06 min). Detailed analyses of oil extracted in the most efficient conditions and the control sample were performed. Kinetic parameters of oil oxidation, fatty acid profile and distribution, melting characteristics studies were carried out. Sonication influenced activation energy of oxidation reaction, contribution of chosen fatty acids (oleic, α-linolenic and eicosenoic fatty acids) and distribution of oleic and α-linolenic fatty acids in sn-2 position of triacylglycerols. Slight changes in melting profile of oils were also recorded. Scanning electron microscopy of cranberry seeds revealed that ultrasound treatment resulted in pore enlargement and fat agglomeration damage. Additional studies of thermal properties of cranberry seeds: differential scanning calorimetry and modulated differential scanning calorimetry were performed, which confirmed that cranberry seeds may be a new source of oil with unique properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cranberry (Vaccinium macrocarpon) belongs to a family Ericaceae and genus Vaccinium. It is a berry fruit rich in vitamins, organic acids, sugar, fiber and mineral salts. Also, bioactive compounds, like polyphenols, terpenes and carotenoids are highly abundant in cranberry fruits [1]. According to Food and Agriculture Organization of the United Nations (FAO), in 2020 world production of cranberries exceeded 660,000 tons [2]. The fruits are industrially processed to obtain products such as: juice, jams, concentrates, jellies, powders. However, by-products including peel, seeds, stem cells, etc. are still a rich source of bioactive compounds. The recovery of valuable compounds which can bring financial and environmental benefits is also supported in circular economy statements [3]. Whole pomaces may be used in the preparation of value-added food enriched with fruit by-products. Mildner-Szkudlarz et al. [4] formulated muffins with raspberry and cranberry pomaces incorporation. Although, the textural properties of the muffins changed undesirably with increasing percentage of pomace, they were characterized by high bioactive phytocompounds contents. Also, Bajerska et al. [5] prepared muffins with sour cherry pomace, which turned out to be rich in polyphenols and dietary fiber.
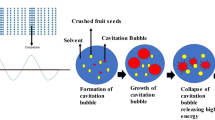
Valorization of fruit waste is also possible by extracting valuable compounds. However, conventional extraction methods may contribute to environment being degraded and are also cost-intensive due to high organic solvents consumption and high energy input needed. That is the reason why novel, unconventional extraction methods, which enable to reduce both solvent and energy usage, and additionally enhance the effectiveness and selectivity of the process are increasingly applied. One of those alternative extraction methods is ultrasound-assisted extraction (UAE). According to review by Chemat et al. [6] ultrasound-assisted extraction is based on several mechanisms including sonocapilary effect, sonoporation, detexturation and intense mixing. Those physical phenomena may be responsible for extraction enhancement when ultrasound is applied. Aside from that, acoustic cavitation bubbles collapse results in exceeded chemical activity of solvent and also improved mass transfer from solid to liquid phase. One of the most crucial advantages of ultrasound-assisted process of lipid extraction mentioned by Deng et al. [7] is time and energy saving, comparing to conventional extraction methods, which involve solvent heating. The comparable yields of oil may be achieved by UAE faster by decreasing effective extraction time. To specify economic issues some studies could be recalled. Jacotet-Navarro et al. [8] introduced laboratory-scale results comparing 30 min UAE with 5 h heat reflux conventional extraction of bioactive compounds from rosemary leaves. The ultrasound-assisted process consumed almost 37-fold less energy (in kWh/kg extract) comparing to the conventional extraction. Also, conventional extraction took 10 times longer to be comparably efficient to UAE. Gharibzahedi et al. [9] concluded similarly in case of oil extraction from lesser mealworm larvae, where UAE was almost 18.5-fold less electric energy consuming (in kWh/g oil) than conventional extraction described as two-hour process with stirring and increased temperature (57–80 °C).
The UAE was applied formerly by Górnaś et al. [10] as a method of fruit seed valorization in case of apples, red currant, gooseberries, grapes, pomegranates, quinces, sea buckthorns, watermelons and melons. Previous studies revealed that UAE significantly improved extraction yield with simultaneous decrease in solvent consumption and time of the process in case of flaxseed oil [11], soybean oil [12], rapeseed oil [13] or mango kernel oil [14]. The qualitative studies of UAE oils are, however, inconclusive. The mass transfer enhancement during sonication process may result in higher antioxidant release (especially lipophilic compounds) and thus improvement of oxidative stability of oil and that was observed in case of hemp seed oil [15], canola seed oil [16], grapeseed oil [17] or raspberry seed oil [18]. On the other hand, a few undesired effects of ultrasound application in oil extraction were reported. UAE of grapeseed oil resulted in elevated free radicals’ content and peroxide value [19], as well as decreased total polyphenol content [20]. Excessive ultrasound treatment, without constant temperature may result in decomposition of bioactive compounds naturally present in seeds. Moreover, within the temperature increase, higher free fatty acid concentration was observed in UAE pumpkin seed oil [21]. That indicates the triacylglycerol degradation to free fatty acids occurred during sonication due to hydrolysis or oxidation process. Aforementioned considerations force to study further the UAE effects on yield and quality of oil. To obtain reliable and the most efficient process conditions, applying response surface methodology (RSM) appears to be useful [22, 23]. In the following study, the extraction yield and oxidative stability as responses were determined by the objectives to obtain satisfying amount of oil characterized by maximum oxidative stability possible, since oxidative stability is an indicator of oil utility and shelf life. To assess the oxidative stability, accelerated oxidation study called pressure differential scanning calorimetry (PDSC) might be used [24]. RSM was successfully used to optimize oil UAE process of papaya seed oil [25], rice bran [26], apricot kernel oil [27], hemp seed oil [28], raspberry seed oil [18] or perilla oil [29]. Nevertheless, none of mentioned studies considered oxidative stability measured in PDSC study as a dependent variable of an experiment.
Apart from oxidative stability, thermal characteristics e.g., melting profile of oil may be assessed to define the quality and functionality of oil [30]. Differential scanning calorimetry (DSC) which records the heat flow associated with thermal transitions in the material in function of time or temperature is one of the methods to obtain melting characteristics of oil [31]. Fatty acid profile is another property of oil determining its nutritional value and utility. Moreover, detailed analysis of fatty acid distribution in sn-2 and sn-1,3 positions of triacylglycerols allows to assess the digestibility of fat [32]. Both features may be assessed in fatty acid methyl esters gas chromatography study.
The importance of sustainable acquisition of cranberry seed oil may be justified by the fact that oil obtained from cranberry seeds may be considered as rich in bioactive compounds. The polyphenol content in cranberry seed oil was investigated by Yu et al. [33] and it was reported at the level of 1.61 mg gallic acid equivalent (GAE) per gram of oil or at 13.68 µmol GAE/g reported by Luo et al. [34]. Other bioactive compounds were studied by Van Hoed et al. [35] total sterol content equal to 6.92 mg/g was reported with β-sitosterol as major sterol. From tocopherols and tocotrienols the most abundant one was γ − tocotrienol with the content of 1235 mg/kg oil. Comparing to other fruit sources, the cranberry seed oil has higher sterol content than gooseberry, pomegranate, apple and grape seed oils [10]. Sum of tocopherol and tocotrienol content in cranberry seed oil is higher than values reported for gooseberry, grape, watermelon and melon seed oils but not as high as for the apple, red currant, pomegranate or sea buckhorn seeds oils [36]. Taking those results into account it may be concluded that cranberry seed oil is a source of bioactive compounds with antioxidant properties.
The aim of the study was to determine the most efficient conditions of ultrasound-assisted extraction regarding cranberry seed oil yield and oxidation resistance assessed by pressure differential scanning calorimetry. Also, to identify the differences between oils extracted by applying ultrasound and oils extracted conventionally, qualitative assays such as: melting characteristics, fatty acid profile and fatty acid distribution in triacylglycerols were conducted. To analyze physical influence of ultrasound on the cranberry seed’s matrix, scanning electron microscopy study was carried out. To obtain a complete picture of thermal behavior of cranberry seeds, they were examined by differential scanning calorimetry and modulated differential scanning calorimetry studies. Overall purpose was to confirm if the UAE is applicable in valorization of cranberry fruit waste.
Materials and methods
Materials
Cranberry (Vaccinium macrocarpon) seeds purchased on the local market (Naturini, Poznań, Poland) were used in the following study. The water activity of whole seeds examined in Rotronic Hygrolab C1 (Rotronic AG, Switzerland) hygrometer at 25±0.3 ºC was lower than 0.2. Before extracting, samples of around 2 g of the seeds were milled in the laboratory IKA Tube Mill (IKA-Werke GmbH & Co. KG, Germany) applying 20,000 rpm in 60 s time.
Chemicals
The chemicals: n-hexane, hydrochloric acid, calcium chloride, diethyl ether, magnesium sulfate, acetic acid, methanol, potassium hydroxide and iodine were purchased in Chempur, Poland. Bile salts solution, TRIS and pancreatic lipase were purchased in Sigma-Aldrich, USA.
Thermal properties of cranberry seeds
Differential scanning calorimetry (DSC) of milled seeds was carried out in TA DSC Q200 calorimeter (TA Instruments, New Castle, DE, USA). The procedure was applied according to the method described by Ostrowska-Ligęza et al. [37] with slight modifications. The samples were cooled down to – 70 °C and then heated to the temperature of 200 °C with a heating rate equal to 5 °C/min. The peak temperatures (Tpeak) were determined based on the curves obtained as results of the studies.
Modulated differential scanning calorimetry (MDSC) was performed in the same apparatus to record the glass transition temperature of cranberry seeds, according to the method applied by Jakubczyk et al. [38].
Experimental design
The experimental plan was designed to assess the possible impact of extraction parameters on the yield and oxidative stability of obtained oils. Central composite design (CCD) plan with nine runs and three replications at central point, carried out in triplicate was applied. The two variable factors were ultrasound wave amplitude (X1) and sonication time (X2). The actual and coded values of ultrasound amplitude and sonication time are presented in Table 1. The 11 runs were performed in the triplicate and obtained results of responses were analyzed in the statistical software Design-Expert (v. 22.0.2, Stat-Ease Inc., Minneapolis, MN, USA). The model fit was carried out considering values of determination coefficient, lack of fit test, p-value and coefficient of variation. The threshold p-value was 0.05. Type of model was chosen by analyzing significance of model fit.
Ultrasound-assisted extraction
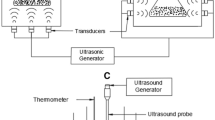
Obtained seed powder (2 g) was placed in 50 mL Falcon tubes. The extraction process was carried out using UP400S ultrasound processor supplied by Hielscher Ultrasonics (Tetlow, Germany), with adjustable wave amplitude level, the output power of 400 W and replaceable sonotrode, n-hexane was used in solid/liquid ratio at level 1:15. Solvent was added to the sample right before the ultrasound application. The tube with seed powder and n-hexane was placed in the ice bath and the sonotrode was dipped in the tube to the depth which disabled n-hexane spilling, additionally, tube was covered with the foil. The temperature of the process, controlled by immersion thermometer, did not exceed 45 ºC.
Conventional extraction
Conventional solid–liquid extraction was conducted to obtain reference, control sample. Two grams of ground cranberry seeds were placed in a Falcon tube with 30 mL of n-hexane and stirred for two hours [39].
Oil yield determination
After extraction, samples were centrifuged for 20 min applying 4000 rpm. Then, filtered and dried with anhydrous magnesium sulfate extracts were evaporated using Büchi R-215 Rotavapor vacuum rotary evaporator (Büchi Labortechnik AG, Flawil, Switzerland) at temperature of 40 °C and pressure at minimum level of 70 mbar. Samples of oil were then dried under nitrogen atmosphere to remove residual solvent. Oil yield was determined, gravimetrically, defined as the mass of extracted oil to the initial mass of powdered seeds using Eq. 1 [40]:
where: \(Y\)–oil yield (%); \({m}_{o}\)–mass of oil (g); \({m}_{s}\)–initial mass of powdered seeds (g).
Oxidative stability and kinetic parameters of oxidation reaction of cranberry seed oil
The oxidation reaction maximum induction time was determined to describe the oxidative stability of obtained cranberry seed oil samples. The procedure was described in previous studies [41, 42]. Measurements were conducted in a DSC Q20 TA Instrument (TA Instruments, New Castle, DE, USA). Briefly, aluminum pan containing 3–4 mg of oil and an empty pan that was used as a reference were placed in the measuring cell. The oxygen atmosphere at flow rate of 50 mL/min and initial pressure of 1400 kPa was provided. Experiment was carried out in isothermal conditions at 120 °C. The value of maximum induction time was obtained based on the maximum rate of oxidation recorded on the plot of heat flow in the function of time.
Additionally, oxidation processes kinetics of control oil sample (CR_USC) and sample of oil extracted using ultrasound in the most efficient conditions (CR_USO) were investigated [43]. The apparatus configuration and samples preparation were the same as for aforementioned analysis, however, experiments were carried out in isothermal conditions of 110, 115, 120, 125 and 130 °C. Parameters of oxidation reaction kinetics were calculated with the use of Ozawa-Flynn-Wall methodology [44, 45] and Arrhenius equation. Parameters of regression lines equations were calculated on the basis of the graph plotted between values of the logarithm of induction time versus reciprocal temperature, using following equations:
where: τ is an induction time in minutes, \(a\) and \(b\) are adjustable coefficients. Oil oxidation reaction is treated as first-order reaction, so activation energy value can be determined according to the Ozawa-Flynn-Wall methodology:
where: \({E}_{a}\) is activation energy, \(R\) is gas constant and \(a\) is a coefficient from Eq. (2). According to Arrhenius Eq. (4):
where: \(k\) is a reaction rate coefficient. Based on that activation energy, pre-exponential factors and reaction rate constants for all temperatures were calculated.
Gas chromatography
Percentage share of identified fatty acids, as well as fatty acid distribution in triacylglycerols of the control sample and sample obtained by applying chosen parameters of ultrasound-assisted extraction were determined in the GC method, using YL6100 GC gas chromatograph apparatus (Young Lin Bldg., Anyang, Hogye-dong, Korea) equipped with a flame ionization detector, as described by Bryś et al. [46]. To assess the fatty acid profile the samples of fat were methylated to fatty acid methyl esters (FAME), according to the PN-EN ISO:2001 method [47]. Detected FAMEs were identified by comparing their retention times with the standard FAMEs mixture. The area normalization procedure with calculation of each fatty acid percentage was applied to obtain fatty acid profile of oils. The analysis of fatty acids distribution in sn-2 and sn-1,3 positions of triacylglycerols was preceded by pancreatic lipase hydrolysis according to the method described by Pina-Rodriguez and Akoh [48].
Melting characteristics
Melting characteristics of control cranberry seed oil and oil extracted in chosen ultrasound conditions were obtained in a differential scanning calorimetry (DSC) study, applying the methodology described by Ostrowska-Ligęza et al. [49]. A DSC Q200 calorimeter (TA Instruments, New Castle, DE, USA) was used. Samples of fat were placed in aluminum, healed hermetically pans. The analysis started with heating the oils to 80 °C to promote melting crystals and erasing thermal memory of material. Then, samples were cooled to − 80 °C and heated again to 80 °C with a heating rate of 15 °C/min.
Scanning electron microscopy analysis
Microstructure of chosen seeds which underwent the conventional extraction and ultrasound-assisted extraction was analyzed using TM-3000 (HITACHI, Japan) at magnification of 2500x.
Statistical analysis
Statistical analysis of detailed oil’s studies (PDSC, DSC, GC) was carried out using Statistica (v. 13.3, StatSoft, Kraków, Poland) software. All the assays were performed in triplicate, one-way ANOVA and post-hoc Tukey’s test were performed. Differences were considered as significant at a p value of 0.05.
Results and discussion
DSC and MDSC studies of cranberry seeds
The results of cranberry seeds DSC were Tpeak of endotherm I − 34.71 ± 2.15 °C and Tpeak of endotherm II 155.32 ± 1.33 °C. The example of DSC curve is shown on the Fig. 1. It can be observed that the DSC curve is of similar course as in the case of previously studied blackberry and raspberry seeds [41]. The endothermic peak observed at around − 35 °C indicates low melting fat fraction presence in the material, which consists of mainly poly- and monounsaturated fatty acids. Second endothermic maximum, around 150 °C is observed due to carbohydrates residues in milled seeds [50]. The results are in agreement with fatty acid profile analysis described further.
MDSC study was carried out to define the glass transition temperature of the milled cranberry seeds. It is a helpful tool to determine the storage conditions of the food products as well as to describe its quality changes during storing. Mainly, food products may be considered as stable when stored under the glass transition temperature. Above the glass transition temperature, the solid state is transformed to supercooled melt highly dependent on temperature [51]. The results of the MDSC analysis, containing mean onset, midpoint and endpoint glass transition temperatures were: −14.55 ± 0.39 °C, −13.47 ± 0.06 °C and 12.50 ± 0.16 °C, respectively. According to the results, single glass transition was noted, as pictured in example, presented on Fig. 2.
Ultrasound-assisted extraction
Extraction yield
Generally, it can be observed that applying higher amplitude of ultrasounds in longer time results in higher extraction yield, detailed results are shown in Table 2. Additionally, graphs presenting observed vs. predicted by the model values of responses can be found in supplementary material (Fig. 1). Figure 3 shows surface plot of extraction time and amplitude of ultrasounds influence on the extraction yield. Enhanced mass transfer, as well as increased cavitation phenomena intensity leads to improved release of oil from the material due to solvent penetration of the matrix [52]. The results are in agreement with previous studies for raspberry seed oil [18] and hazelnut oil [53]. The yield of extraction was predicted significantly (p < 0.05) by applying quadratic model. That result was supported by high determination coefficient value (R2 < 0.90) and not significant p value result in lack of fit test (p > 0.05). Additionally, based on ANOVA results it can be concluded that extraction yield was significantly (p < 0.05) influenced by both ultrasound amplitude and extraction time. Multiple regressions coefficients were used to analyze the linear, quadratic and interaction terms of yield in response surface model. A second-order polynomial Eq. (5) was used to define the response of the yield the exact Eq. (6) allows to calculate the actual yield of ultrasound assisted cranberry seed oil extraction by using actual values of factors.
where Y is the response; β0 is the constant coefficient, β1, and β2 are the regression coefficients for the linear terms; β11 and β22 are the quadratic terms; β12 is the interaction terms; and X1 and X2 represent the coded values of independent variables.
Maximum induction time of oxidation
The observed mean results of τmax are shown in Table 2, the comparison of actual and predicted by the model values are shown in supplementary material (Fig. 1). Figure 4 shows the surface plot for the maximum induction time (τmax) response results. Linear model of prediction was chosen as significant (p < 0.05), as the ANOVA results revealed that only amplitude has significantly influenced τmax values. Quite high value of determination coefficient (R2 > 0.75) and insignificant result of lack of fit test indicate proper model fit (p > 0.05). The first-order polynomial Eq. (7) was utilized to define the response of τmax and exact τmax values may be calculated using Eq. (8).
where Y is the response; β0 is the constant coefficient, β1, and β2 are the regression coefficients for the linear terms.
Opposite findings were described by Samaram et al. [25] for the papaya seed oil. However different oil stability test was used but the results revealed that time was the only independent variable of the experiment (among extraction time, temperature, ultrasound power and solvent to sample ratio) that influenced the oil stability measured in peroxide and p-anisidine values tests. The increased stability of oil extracted applying higher amplitude of ultrasounds may be the result of antioxidant bioactive compounds release during the extraction process. As residual particles of pomace may be found on the seeds, which was confirmed in the following study in seeds DSC analysis, they may contain bioactive compounds commonly found in cranberry pomace, such as polyphenols [54]. Those compounds may act as natural anti-oxidative preservatives and improve resistance to oxidation of oils.
Optimization
The most efficient parameters of cranberry seed oil ultrasound-assisted extraction were calculated using RSM. Criteria for responses i.e., extraction yield and maximum induction time were set as ‘maximize’ and criteria for independent variables were set as ‘in range’ of 20–100 for amplitude and 2–12 for extraction time. 80 solutions were suggested by the software, with one selected as the most efficient, indicating amplitude of 95% and extraction time of 11.375 min to be the most preferable. The chosen solution predicted extraction yield of 22.55 ± 0.36% (vs. actual 21.98 ± 0.08%) and τmax of 52.60 ± 0.95 min (vs. actual 61.95 ± 3.06 min). As the optimized parameters of extraction were outside of the applied design space, biased observed value of τmax comparing to predicted by the model response was observed. However, it is in agreement with the criteria of maximizing the τmax response.
Kinetic parameters of oil oxidation
Based on the results of PDSC measurements conducted at five temperatures, kinetic parameters of oil oxidation reaction were calculated. Firstly, a graph of logarithm of maximum induction time in function of reciprocal temperature was plotted (supplementary material, Fig. 2). The R2 coefficients at a level above 0.95 meant that the data describing linear correlation could be used to further calculations.
The results of calculations are summarized in the Table 3. It was concluded that activation energy was significantly higher for oil extracted applying optimal chosen conditions of ultrasound extraction than activation energy of oil oxidation reaction for a control sample. However, activation energy values above 90 kJ/mol could be considered as high referring to the previous studies. Those results are similar as for linseed oil [55], soybean or sunflower oil [56]. Previous studies reported lowering activation energy of oxidation when ultrasound was applied [57]. However, both results are in agreement with studies by Torres et al. [58] which summarized that Ea of oil oxidation is in a range 41,842 to 104,605 kJ/mol.
Fatty acid profile and distribution in triacylglycerols
Results of cranberry seed oil’s fatty acid profile analysis are shown on Fig. 5. For both studied oils, linoleic acid (C18:2 n−6), followed by α-linolenic acid (C18:3 n−3) are fatty acids with the largest percentage share. It can be also concluded that cranberry seed oil was characterized by high contribution of polyunsaturated fatty acids in the studied samples. The method of extraction has significantly influenced the percentage share of oleic, α-linolenic and eicosenoic acids. Previous studies show that UAE did not affect the fatty acid composition of canola seed oil [16], grapeseed oil [17], papaya seed oil [59], flaxseed oil [11] or Moringa oleifera seed oil [60]. However, in case of pomegranate seed oil extracted using ultrasound, fatty acid profile was slightly influenced by the extraction method, especially considering SFA and PUFA [61]. Similar findings, assuming subtle changes in fatty acid composition were described for sunflower seed oil [62]. Opposite results were described for the UAE of raspberry seed oil by Teng et al. [18]. Also, Hosseini et al. [63] studied physicochemical properties of sesame, olive, sunflower and tallow olein oils treated with ultrasound. Sonication at 24 Hz at different amplitude values (25, 60 or 100%) was applied to extra virgin oils to analyze the effects of such treatment on the quality of fat. With increasing value of amplitude, a decrease in a content of linoleic fatty acid and α-linolenic fatty acid was observed. That may be connected to the higher cavitation caused by ultrasound resulting in oil degradation and as a consequence, lipid oxidation generated by free radicals formed in sonolysis process. However, overall conclusion is that UAE affects fatty acid profile to a small degree.
Fatty acid profile of cranberry seed oils obtained in different extraction methods, expressed as percentage share (%); CR_USC- control oil sample, CR_USO sample of oil extracted using ultrasound in the most efficient conditions; C16:0 palmitic acid, C18:0 stearic acid, C18:1 oleic acid, C18:2 linoleic fatty acid, C18:3 α-linolenic acid, C20:1- eicosenoic acid; different letters.a−b indicate significant differences at p < 0.05
In the fatty acid distribution study it was found out that the share of oleic and α-linolenic fatty acids in sn-2 position of TAG differed in studied oils (supplementary material, Fig. 3). Although, the TAG content was not determined in the following study, so it remains unknown is the yield of specific TAGs also dependent on extraction method applied. The polyunsaturated fatty acids present in cranberry seed oil were located mostly in internal position of TAG, only α-linolenic fatty acid occupied rather external sn-1,3 positions than sn-2. That kind of distribution is common for vegetable oils [64]. The saturated fatty acids occupied only outer sn-1,3 positions in TAGs. The structure of TAG determines its biochemical and physical properties. Enzymatic hydrolysis of fats in the digestive system of humans cause release of fatty acids from sn-1,3 positions. So, in case of vegetable oils, mostly saturated fatty acids are being liberated during digestion process. They may, however, bind free calcium ions and be removed with feces [65]. The decrease of α-linolenic acid percentage share in sn-2 position in cranberry seed oil extracted using ultrasound in the most efficient conditions may be connected to the degradation of unsaturated fatty acids due to free radical’s release during sonication process [66]. In other terms, a chain of chemical reactions induced particularly by hydroxyl and superoxide radicals and resulting in an autocatalytic process of fatty acid deterioration may occur during sonication [19] and it may affect the distribution of fatty acids in TAGs as well.
DSC study of oils
The melting curves of cranberry seed oils were characterized by two endothermic peaks, as presented in the example on the Fig. 6. Tpeak for endotherm I was significantly higher for control sample comparing to oil obtained in optimal conditions and was equal to − 35.59 ± 0.19 ºC in case of oil obtained in optimized conditions and − 33.59 ± 0.07 ºC in case of control sample, Tpeak values of endotherm II for cranberry seed oil extracted using sonication and control oil were measured at – 22.10 ± 0.08 ºC and – 20.74 ± 0.16 ºC, respectively, without any significant differences. The first peaks are connected with the presence of low-melting fractions of triacylglycerols containing mostly polyunsaturated fatty acids and second peaks indicate presence of medium-melting fractions rich in monounsaturated fatty acids [67]. The results of UAE influence on the melting characteristics of oils are lacking in the literature. Hosseini et al. [63] studied the effect of sonication treatment on extra virgin sesame, olive, sunflower and tallow olein oils’ melting profiles. Higher amplitude values were related to higher crystallization enthalpies which means, ultrasound- treated oils have less ordered crystal structure than untreated ones.
Scanning electron microscopy
Pictures of milled cranberry seeds SEM analysis taken at 2500 × magnification are shown in Fig. 7. The most significant difference visible in the pictures is the pores size and their permeability. Raw cranberry seeds (1) have visible pores, but they seem to be clogged. Seeds which underwent the UAE in optimized conditions (2) have more pores which are enlarged, comparing to the pores of seeds after conventional extraction process (3). However, both extractions seem to cause release of substance present inside the pores. It can be also noticed, how extraction breaks the oil agglomerations, which are visible only in the picture A. In the pictures B and C oil residuals are present as individual particles with their higher number in case of seeds after classical extraction which proves increased efficiency of UAE comparing to conventional method. Similar findings were described in case of SEM analysis of sunflower seeds [62] and flax seeds [11] after UAE.
Conclusions
Given results of cranberry seeds thermal analysis support the hypothesis that they are a promising material for obtaining oil. The extraction optimization results show that quadratic model dependance may be applied to evaluate the impact of ultrasound amplitude and extraction time on the yield and linear model may be applied to describe maximum induction time dependance on process conditions of ultrasound-assisted extraction. The most efficient parameters of extraction were amplitude of 95% and extraction time of 11.38 min and prediction of yield was 22.55 ± 0.36% and maximum induction time 52.60 ± 0.95 min. Detailed characteristics of oil obtained in the aforementioned conditions revealed its high oxidation reaction activation energy and dominance of linoleic fatty acid in the fatty acid percentage share. Additional analysis in scanning electron microscopy procedure confirmed ultrasound influence on the seed matrix which is visible as the increase in pore size and oil agglomeration breakdown. To summarize, ultrasound was successfully applied to obtain oil from cranberry seeds. The process was optimized within the specified parameters of ultrasound amplitude and extraction time to achieve highest possible yield and maximum induction time. Thus, it may be concluded that ultrasound-assisted extraction is applicable in valorization of cranberry fruit waste.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Oszmiański J, Kolniak-Ostek J, Lachowicz S, Gorzelany J, Matłok N (2017) Phytochemical compounds and antioxidant activity in different cultivars of cranberry (Vaccinium Macrocarpon L). J Food Sci 82:2569–2575. https://doi.org/10.1111/1750-3841.13924
(2019) FAOSTAT Crop database. http://www.fao.org/faostat/en/#data/QC. Accessed 6 May 2021
Patra A, Abdullah S, Pradhan RC (2022) Review on the extraction of bioactive compounds and characterization of fruit industry by-products. Bioresour Bioprocess 9:1–25. https://doi.org/10.1186/S40643-022-00498-3/TABLES/3
Mildner-Szkudlarz S, Bajerska J, Górnaś P, Segliņa D, Pilarska A, Jesionowski T (2016) Physical and bioactive properties of muffins enriched with raspberry and cranberry pomace powder: a promising application of fruit by-products rich in biocompounds. Plant Foods Hum Nutr 71:165–173. https://doi.org/10.1007/s11130-016-0539-4
Bajerska J, Mildner-Szkudlarz S, Górnaś P, Seglina D (2016) The effects of muffins enriched with sour cherry pomace on acceptability, glycemic response, satiety and energy intake: a randomized crossover trial. J Sci Food Agric 96:2486–2493. https://doi.org/10.1002/jsfa.7369
Chemat F, Rombaut N, Sicaire AG, Meullemiestre A, Fabiano-Tixier AS, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review Ultrason Sonochem 34:540–560. https://doi.org/10.1016/J.ULTSONCH.2016.06.035
Deng Y, Wang W, Zhao S, Yang X, Xu W, Guo M, Xu E, Ding T, Ye X, Liu D (2022) Ultrasound-assisted extraction of lipids as food components: mechanism, solvent, feedstock, quality evaluation and coupled technologies—a review. Trends Food Sci Technol 122:83–96. https://doi.org/10.1016/J.TIFS.2022.01.034
Jacotet-Navarro M, Rombaut N, Fabiano-Tixier AS, Danguien M, Bily A, Chemat F (2015) Ultrasound versus microwave as green processes for extraction of rosmarinic, carnosic and ursolic acids from rosemary. Ultrason Sonochem 27:102–109. https://doi.org/10.1016/J.ULTSONCH.2015.05.006
Gharibzahedi SMT, Altintas Z (2022) Ultrasound-assisted alcoholic extraction of lesser mealworm larvae oil: process optimization, physicochemical characteristics, and energy consumption. Antioxidants 11:1943. https://doi.org/10.3390/antiox11101943
Górnaś P, Rudzińska M (2016) Seeds recovered from industry by-products of nine fruit species with a high potential utility as a source of unconventional oil for biodiesel and cosmetic and pharmaceutical sectors. Ind Crops Prod 83:329–338. https://doi.org/10.1016/J.INDCROP.2016.01.021
Zhang ZS, Wang LJ, Li D, Jiao SS, Chen XD, Mao ZH (2008) Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol 62:192–198. https://doi.org/10.1016/J.SEPPUR.2008.01.014
Li H, Pordesimo L, Weiss J (2004) High intensity ultrasound-assisted extraction of oil from soybeans. Food Res Int 37:731–738. https://doi.org/10.1016/j.foodres.2004.02.016
Perrier A, Delsart C, Boussetta N, Grimi N, Citeau M, Vorobiev E (2017) Effect of ultrasound and green solvents addition on the oil extraction efficiency from rapeseed flakes. Ultrason Sonochem 39:58–65. https://doi.org/10.1016/J.ULTSONCH.2017.04.003
Mwaurah PW, Kumar S, Kumar N, Attkan AK, Panghal A, Singh VK (2021) Ultrasound and microwave-assisted solvent extraction of mango kernel oil and evaluation of physicochemical properties and fatty acid profile. J Food Process Preserv 45:e16090. https://doi.org/10.1111/JFPP.16090
Rezvankhah A, Emam-Djomeh Z, Safari M, Askari G, Salami M (2018) Investigation on the extraction yield, quality, and thermal properties of hempseed oil during ultrasound-assisted extraction: a comparative study. J Food Process Preserv 42:e13766. https://doi.org/10.1111/JFPP.13766
Jalili F, Jafari SM, Emam-Djomeh Z, Malekjani N, Farzaneh V (2018) Optimization of ultrasound-assisted extraction of oil from Canola seeds with the use of response surface methodology. Food Anal Methods 11:598–612. https://doi.org/10.1007/S12161-017-1030-Z/TABLES/6
Malićanin M, Rac V, Antić V, Antić M, Palade LM, Kefalas P, Rakić V (2014) Content of antioxidants, antioxidant capacity and oxidative stability of grape seed oil obtained by ultra sound assisted extraction. JAOCS 91:989–999. https://doi.org/10.1007/S11746-014-2441-2/FIGURES/3
Teng H, Chen L, Huang Q, Wang J, Lin Q, Liu M, Lee WY, Song H (2016) Ultrasonic-assisted extraction of raspberry seed oil and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. PLoS ONE 11:1–17. https://doi.org/10.1371/journal.pone.0153457
Böger BR, Salviato A, Valezi DF, Di Mauro E, Georgetti SR, Kurozawa LE (2018) Optimization of ultrasound-assisted extraction of grape-seed oil to enhance process yield and minimize free radical formation. J Sci Food Agric 98:5019–5026. https://doi.org/10.1002/JSFA.9036
Da Porto C, Porretto E, Decorti D (2013) Comparison of ultrasound-assisted extraction with conventional extraction methods of oil and polyphenols from grape (Vitis vinifera L.) seeds. Ultrason Sonochem 20:1076–1080. https://doi.org/10.1016/J.ULTSONCH.2012.12.002
Hernández-Santos B, Rodríguez-Miranda J, Herman-Lara E, Torruco-Uco JG, Carmona-García R, Juárez-Barrientos JM, Chávez-Zamudio R, Martínez-Sánchez CE (2016) Effect of oil extraction assisted by ultrasound on the physicochemical properties and fatty acid profile of pumpkin seed oil (Cucurbita pepo). Ultrason Sonochem 31:429–436. https://doi.org/10.1016/J.ULTSONCH.2016.01.029
Weremfo A, Abassah-Oppong S, Adulley F, Dabie K, Seidu-Larry S (2023) Response surface methodology as a tool to optimize the extraction of bioactive compounds from plant sources. J Sci Food Agric 103:26–36. https://doi.org/10.1002/JSFA.12121
Matys A, Dadan M, Witrowa-Rajchert D, Parniakov O, Wiktor A (2022) Response surface methodology as a tool for optimization of pulsed electric field pretreatment and microwave-convective drying of apple. Appl Sci 12:1–19. https://doi.org/10.3390/APP12073392
Ciemniewska-Żytkiewicz H, Ratusz K, Bryś J, Reder M, Koczoń P (2014) Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J Therm Anal Calorim 118:875–881. https://doi.org/10.1007/S10973-014-3861-9/TABLES/3
Samaram S, Mirhosseini H, Tan CP, Ghazali HM, Bordbar S, Serjouie A (2015) Optimisation of ultrasound-assisted extraction of oil from papaya seed by response surface methodology: oil recovery, radical scavenging antioxidant activity, and oxidation stability. Food Chem 172:7–17. https://doi.org/10.1016/J.FOODCHEM.2014.08.068
Rawson A, Vca K (2015) Ultrasound assisted extraction of oil from rice bran: a response surface methodology approach. J Food Process Technol 6:454. https://doi.org/10.4172/2157-7110.1000454
Gayas B, Kaur G, Gul K (2017) Ultrasound-assisted extraction of apricot kernel oil: effects on functional and rheological properties. J Food Process Eng 40:e12439. https://doi.org/10.1111/JFPE.12439
Esmaeilzadeh Kenari R, Dehghan B (2020) Optimization of ultrasound-assisted solvent extraction of hemp (Cannabis sativa L.) seed oil using RSM: evaluation of oxidative stability and physicochemical properties of oil. Food Sci Nutr 8:4976–4986. https://doi.org/10.1002/FSN3.1796
Li HZ, Zhang ZJ, Hou TY, Li XJ, Chen T (2015) Optimization of ultrasound-assisted hexane extraction of perilla oil using response surface methodology. Ind Crops Prod 76:18–24. https://doi.org/10.1016/J.INDCROP.2015.06.021
Fasina OO, Craig-Schmidt M, Colley Z, Hallman H (2008) Predicting melting characteristics of vegetable oils from fatty acid composition. LWT 41:1501–1505. https://doi.org/10.1016/J.LWT.2007.09.012
Gill P, Moghadam TT, Ranjbar B (2010) Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech 21:167
Dubois V, Breton S, Linder M, Fanni J, Parmentier M (2007) Fatty acid profiles of 80 vegetable oils with regard to their nutritional potential. Eur J Lipid Sci Technol 109:710–732. https://doi.org/10.1002/EJLT.200700040
Yu LL, Zhou KK, Parry J (2005) Antioxidant properties of cold-pressed black caraway, carrot, cranberry, and hemp seed oils. Food Chem 91:723–729. https://doi.org/10.1016/J.FOODCHEM.2004.06.044
Luo Y, Yuan F, Li Y, Wang J, Gao B, Yu L (2021) Triacylglycerol and fatty acid compositions of blackberry, red raspberry, black raspberry, blueberry and cranberry seed oils by ultra-performance convergence chromatography-quadrupole time-of-flight mass spectrometry. Foods 10:2530. https://doi.org/10.3390/foods10112530
Van Hoed V, De Clercq N, Echim C, Andjelkovic M, Leber E, Dewettinck K, VerhÉ R (2009) Berry seeds: a source of specialty oils with high content of bioactives and nutritional value. J Food Lipids 16:33–49. https://doi.org/10.1111/j.1745-4522.2009.01130.x
Górnaś P, Soliven A, Segliņa D (2015) Seed oils recovered from industrial fruit by-products are a rich source of tocopherols and tocotrienols: rapid separation of α/β/γ/δ homologues by RP-HPLC/FLD. Eur J Lipid Sci Technol 117:773–777. https://doi.org/10.1002/ejlt.201400566
Ostrowska-Ligęza E, Górska A, Wirkowska M, Koczoń P (2012) An assessment of various powdered baby formulas by conventional methods (DSC) or FT-IR spectroscopy. J Therm Anal Calorim 110:465–471. https://doi.org/10.1007/s10973-011-2158-5
Jakubczyk E, Ostrowska-Ligeza E, Gondek E (2010) Moisture sorption characteristics and glass transition temperature of apple puree powder. Int J Food Sci Technol 45:2515–2523. https://doi.org/10.1111/j.1365-2621.2010.02425.x
Dauber C, Carreras T, González L, Gámbaro A, Valdés A, Ibañez E, Vieitez I (2022) Characterization and incorporation of extracts from olive leaves obtained through maceration and supercritical extraction in Canola oil: oxidative stability evaluation. LWT 160:113274. https://doi.org/10.1016/J.LWT.2022.113274
Ni Q, Gao Q, Yu W, Liu X, Xu G, Zhang Y (2015) Supercritical carbon dioxide extraction of oils from two Torreya grandis varieties seeds and their physicochemical and antioxidant properties. LWT Food Sci Technol 60:1226–1234. https://doi.org/10.1016/J.LWT.2014.09.007
Piasecka I, Górska A, Ostrowska-Ligęza E, Kalisz S (2021) The study of thermal properties of blackberry, chokeberry and raspberry seeds and oils. Appl Sci 11:1–15. https://doi.org/10.3390/APP11167704
Wierzchowska K, Pakulska A, Derewiaka D, Piasecka I, Zieniuk B, Nowak D, Fabiszewska A (2022) Concept of batch and fed-batch cultures of Yarrowia lipolytica as a valuable source of sterols with simultaneous valorization of molasses and post-frying rapeseed oil. Appl Sci 12:12877. https://doi.org/10.3390/APP122412877
Wirkowska-Wojdyła M, Ostrowska-Ligęza E, Górska A, Bryś J (2022) Application of chromatographic and thermal methods to study fatty acids composition and positional distribution, oxidation kinetic parameters and melting profile as important factors characterizing amaranth and quinoa oils. Appl Sci 12:1–12. https://doi.org/10.3390/APP12042166
Dowdy DR (1987) Meaningful activation energies for complex systems—I. The application of the Ozawa-Flynn-Wall method to multiple reactions. J Therm Anal 32:137–147. https://doi.org/10.1007/BF01914556/METRICS
Popescu C (1996) Integral method to analyze the kinetics of heterogeneous reactions under non-isothermal conditions a variant on the Ozawa-Flynn-Wall method. Thermochim Acta 285:309–323. https://doi.org/10.1016/0040-6031(96)02916-4
Bryś J, Flores lnês FV, Górska A, Wirkowska-Wojdyła M, Ostrowska-Ligęza E, Bryś A, (2017) Use of GC and PDSC methods to characterize human milk fat substitutes obtained from lard and milk thistle oil mixtures. J Therm Anal Calorim 130:319–327. https://doi.org/10.1007/s10973-017-6452-8
(2001) Polish Norm: PN-EN ISO: 5509:2001: Oil and vegetable and animal fats. preparation of methyl ester of fatty acids. polish committee for standardization, Warsaw, Poland
Pina-Rodriguez AM, Akoh CC (2009) Enrichment of amaranth oil with ethyl palmitate at the sn-2 position by chemical and enzymatic synthesis. J Agric Food Chem 57:4657–4662. https://doi.org/10.1021/JF900242G
Ostrowska-Ligęza E, Dolatowska-Żebrowska K, Wirkowska-Wojdyła M, Bryś J, Górska A (2021) Comparison of thermal characteristics and fatty acids composition in raw and roasted cocoa beans from Peru (Criollo) and Ecuador (Forastero). Appl Sci 11:1–19. https://doi.org/10.3390/APP11062698
Falcão-Rodrigues MM, Moldão-Martins M, Beirão-da-Costa ML (2007) DSC as a tool to assess physiological evolution of apples preserved by edibles coatings. Food Chem 102:475–480. https://doi.org/10.1016/J.FOODCHEM.2006.05.016
Le Meste M, Champion D, Roudaut G, Blond G, Simatos D (2002) Glass transition and food technology: a critical appraisal. J Food Sci 67:2444–2458. https://doi.org/10.1111/J.1365-2621.2002.TB08758.X
Knorr D, Ade-Omowaye BIO, Heinz V (2002) Nutritional improvement of plant foods by non-thermal processing. Proc Nutr Soc 61:311–318. https://doi.org/10.1079/PNS2002162
Wong ST, Tan MC, Geow CH (2019) Optimization of ultrasound-assisted ethanol extraction of hazelnut oil. J Food Process Preserv 43:e14138. https://doi.org/10.1111/JFPP.14138
Roopchand DE, Krueger CG, Moskal K, Fridlender B, Lila MA, Raskin I (2013) Food-compatible method for the efficient extraction and stabilization of cranberry pomace polyphenols. Food Chem 141:3664–3669. https://doi.org/10.1016/J.FOODCHEM.2013.06.050
Symoniuk E, Ratusz K, Krygier K (2016) Comparison of the oxidative stability of linseed (Linum usitatissimum L.) oil by pressure differential scanning calorimetry and Rancimat measurements. J Food Sci Technol 53:3986–3995. https://doi.org/10.1007/s13197-016-2398-2
Farhoosh R, Niazmand R, Rezaei M, Sarabi M (2008) Kinetic parameter determination of vegetable oil oxidation under Rancimat test conditions. Eur J Lipid Sci Technol 110:587–592. https://doi.org/10.1002/EJLT.200800004
Pérez-Saucedo MR, Jiménez-Ruiz EI, Rodríguez-Carpena JG, Ragazzo-Sánchez JA, Ulloa JA, Ramírez-Ramírez JC, Gastón-Peña CR, Bautista-Rosales PU (2021) Properties of the avocado oil extracted using centrifugation and ultrasound-assisted methods. Food Sci Biotechnol 30:1051–1061. https://doi.org/10.1007/S10068-021-00940-W/TABLES/3
Torres A, Guerra M, Rosquete Y (2001) Estimación de la vida útil de una fórmula dietética en función de la disminución de lisina disponible. Food Sci Technol 21:129–133. https://doi.org/10.1590/S0101-20612001000200001
Samaram S, Mirhosseini H, Tan CP, Ghazali HM (2013) Ultrasound-assisted extraction (UAE) and solvent extraction of papaya seed oil: yield, fatty acid composition and triacylglycerol profile. Molecules 18:12474–12487. https://doi.org/10.3390/molecules181012474
Zhong J, Wang Y, Yang R, Liu X, Yang Q, Qin X (2018) The application of ultrasound and microwave to increase oil extraction from Moringa oleifera seeds. Ind Crops Prod 120:1–10. https://doi.org/10.1016/J.INDCROP.2018.04.028
Barizão ÉO, Boeing JS, Martins AC, Visentainer JV, Almeida VC (2015) Application of response surface methodology for the optimization of ultrasound-assisted extraction of pomegranate (Punica granatum L.) seed oil. Food Anal Methods 8:2392–2400. https://doi.org/10.1007/S12161-015-0135-5/FIGURES/4
Moradi N, Rahimi M, Moeini A, Parsamoghadam MA (2017) Impact of ultrasound on oil yield and content of functional food ingredients at the oil extraction from sunflower. Sep Sci Technol 53:261–276. https://doi.org/10.1080/01496395.2017.1384016
Hosseini S, Gharachorloo M, Tarzi BG, Ghavami M, Bakhoda H (2015) Effects of ultrasound amplitude on the physicochemical properties of some edible oils. J Am Oil Chem Soc 92:1717–1724. https://doi.org/10.1007/S11746-015-2733-1
Karupaiah T, Sundram K (2007) Effects of stereospecific positioning of fatty acids in triacylglycerol structures in native and randomized fats: a review of their nutritional implications. Nutr Metab (Lond) 4:1–17. https://doi.org/10.1186/1743-7075-4-16/TABLES/3
Bryś J, Wirkowska M (2010) Znaczenie struktury triacylogliceroli w projektowaniu lipidów strukturyzowanych. Postępy Techniki Przetwórstwa Spożywczego nr 2:86–89
Sivakumar M, Tang SY, Tan KW (2014) Cavitation technology—a greener processing technique for the generation of pharmaceutical nanoemulsions. Ultrason Sonochem 21:2069–2083. https://doi.org/10.1016/J.ULTSONCH.2014.03.025
Embaby HE, Miyakawa T, Hachimura S, Muramatsu T, Nara M, Tanokura M (2022) Crystallization and melting properties studied by DSC and FTIR spectroscopy of goldenberry (Physalis peruviana) oil. Food Chem 366:130645. https://doi.org/10.1016/J.FOODCHEM.2021.130645
Funding
Some research equipment was purchased as part of the “Food and Nutrition Centre—modernisation of the WULS campus to create a Food and Nutrition Research and Development Centre (CŻiŻ)” co-financed by the European Union from the European Regional Development Fund under the Regional Operational Programme of the Mazowieckie Voivodeship for 2014–2020 (Project No. RPMA.01.01.00-14-8276/17).
Author information
Authors and Affiliations
Contributions
Conceptualization of the study IP, AG, AW, methodology was prepared by IP, RB, EOL, AW and AG, formal analysis was performed by IP, statistical analysis was performed by IP and RB, manuscript was written by IP and reviewed by AG, figures were prepared by IP (Figs. 3, 4, 5 and Fig. 1 in supplementary material), EOL (Figs. 1, 2, 6 and Figs. 2, 3 in supplementary material) and AW (Fig. 7). Administration of the study and funding acquisition was performed by AG.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piasecka, I., Brzezińska, R., Ostrowska-Ligęza, E. et al. Ultrasound-assisted extraction of cranberry seed oil: food waste valorization approach. Eur Food Res Technol 249, 2763–2775 (2023). https://doi.org/10.1007/s00217-023-04326-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04326-6