Abstract
In order to evaluate the effect of the application of chitosan to red wines with different initial composition, four wines showing tannins/anthocyanins ratio (T/A) ranging from 0.15 to 2.44 were treated with this amino polysaccharide. As one of the main factors involved in red wine ageing is the oxidation, even a forced oxidation test was applied on all the samples. The addition of chitosan determined a decrease in total phenolic compounds mainly due to the adsorption of protein-reactive tannins which decreased from 10 to 50% of the initial value. The previous addition of chitosan determined a lower production of acetaldehyde after oxidation confirming the antioxidant activity of this amino polysaccharide. The production of acetaldehyde was lower in samples with a higher T/A ratio probably due to the involvement of acetaldehyde in reactions with flavanols and anthocyanins giving polymeric pigments. These results suggest a possible use of chitosan in red wine with a higher T/A ratio to decrease the content of tannins reactive towards proteins and, contemporary, to act as antioxidant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chitosan is a biological derivative that is obtained starting from the partial deacetylation of chitin (polysaccharide composed of units of N-acetyl-d glucosamine) in an alkaline environment [1]. It can be obtained from living organisms composed of exoskeleton such as insects, crustacean shells and cell walls of fungi and plants [2].
Nowadays, in the wine industry, chitosan has received considerable attention due to its antimicrobial activity against many microorganisms [3]. Chitosan resulted also active as growth inhibitor of yeasts [4, 5] and bacteria [6, 7]. Moreover, as reported by Chinnici et al. [8], chitosan shows a chelation activity towards metal in wine probably justifying its antioxidant activity [9]. The compendium of international methods of analysis of wines and musts of the international organisation of vine and wine [10] admitted its use as fining agent to reduce turbidity and prevent protein haze; nowadays, the limit of chitosan addition to wines ranges from 10 to 500 g/h L [11].
According to the different applications highlighted above, there has been an increase in its use in the wine industry but there are still few studies regarding the interaction of chitosan with the phenolic compounds present in red wines [12, 13].
Phenolics compounds are responsible for wine longevity [14, 15] and are fundamental to produce high-quality wines [16, 17]. Among phenolics compounds in red wines, the most important classes are anthocyanins and tannins. The anthocyanins influence the colour of wine [18, 19] while the tannins influence the main tactile sensations as astringency and taste sensation as bitterness [20].
Different studies [21, 22] reported that during the ageing of red wine the phenolic fractions play an important role in increasing wine shelf life. Therefore, the ratio between anthocyanins and tannins could affect the reactions of stabilisation and proper wine ageing [23, 24]. Recent studies highlighted the interactions that chitosan could have with phenolic compounds [25] mainly with tannins and polymeric pigments [13, 26] but the effect on red wines with different initial composition is not known.
The proposed study is aimed at evaluating the influence of chitosan on the changes on phenolic fractions and acetaldehyde in four red wines with three different T/A ratios before and after forced oxidation.
Materials and methods
Wines
The experimental plan was performed on a red wine produced in South Italy. Before preparation of the experimental samples, the wine was analysed to obtain the main base parameters reported in Table 1.
The wine was split in 4 × 2 experimental samples, and every experimental plot was treated to have four wines with different T/A ratio.
To obtain the different T/A ratios, we added different quantities of condensed tannins (VR Grape Laffort Oenologie, Bordeaux, France). The chemical characterisation of added tannins was reported by Harbertson et al. [27]. To get the different T/A ratios, the following quantities of condensed tannins were added: 600 mg/L to obtain the sample (WT0.5), 1200 mg/L to obtain the sample (WT1) and 2400 mg/L to obtain the sample (WT2). Thus, wines with four different T/A ratios were obtained (Table 2): W sample (control wine T/A Ratio 0.15), W 0.5 sample (T/A Ratio 0.48), W1 sample (T/A Ratio 1.24) and W2 sample (T/A Ratio 2.44). To evaluate the effect of chitosan for each experimental wine, 500 mg/L of chitosan (C) (Sigma-Aldrich CAS: 9012-764) was added. After the addition of chitosan further four wines with different T/A ratios added of chitosan were produced: W-C (T/A ratio 0.15 with chitosan), WT0.5C (T/A ratio 0.48 with chitosan), WT1-C (T/A ratio 1.24 with chitosan) and WT2-C (T/A ratio 2.44 with chitosan).
All samples were filtered (0.45 micron), stored at the temperature of 18° C and analysed after 7 days to evaluate the fining effect. All wines were successively treated to evaluate their wine oxidative response as described in the next paragraph.
Wine oxidation test
The evaluation of wine oxidative response was carried out as reported by Coppola et al. [28]. Experimental test was conducted at controlled temperature 18 °C and every sample was treated by adding 19 mg/L of H2O2 (equivalent to 18 mg/L O2) (30% Fluka, Sigma-Aldrich Chemie GmbH Steinheim, France).
The addition of hydrogen peroxide (o) triggered oxidation and the corresponding oxidated experimental samples were named as follows: “Wo” (control wine T/A Ratio 0.15 added with H2O2), “W Co” (control wine T/A Ratio 0.15 added with chitosan and H2O2), “WTo 0.5” (T/A ratio 0.48 added with H2O2), “WTo 1” (T/A ratio 1.24 added with H2O2), WTo 2 (T/A ratio 2.44 added with H2O2). The same procedure was applied to samples containing chitosan renamed as follows: “W Co” (control wine T/A Ratio 0.15 added with chitosan and H2O2), “WTo-C 0.5” (T/A ratio 0.48 added with chitosan and H2O2), “WTo-C 1” (T/A ratio 1.24 added with chitosan and H2O2) and “WTo-C 2” (T/A ratio 2.44 added with chitosan and H2O2). Treated samples after 15 days were centrifuged, filtered (0.45 micron) and then analysed. All the samples were prepared in duplicate.
High-performance liquid chromatography determination of acetaldehyde
The analysis of acetaldehyde was performed by HPLC analysis as described by Han, et al. [29]. The derivatisation of samples was carried out into glass vial as follows: sulphur dioxide solution (1120 mg/L) freshly prepared (20 µL) was added, the samples were then acidified with sulfuric acid (Carlo Erba reagent 96%) (25%) (20 µL). The derivatisation reagent (140 µL) 2,4-dinitrophenylhydrazine (Aldrich chemistry) (2 g/L) and an aliquot of wine sample (100 µL) were then added.
After mixing, the solution allowed to react for 15 min at 65 °C and then cooled at room temperature. The analyses were performed using a HPLC SHIMADZULC10 ADVP apparatus (Shimadzu Italy, Milan), consisting of a system controller SCL-10AVP, two pumps LC-10ADVP, a detector SPD-M 10 AVP, autosampler SIL-20HTA and a Waters Spherisorb column (250 × 4.6 mm, 4 μm particles diameter).
The chromatographic conditions were: sample injection volume, 50 µL; flow rate, 0.75 mL/min; column temperature, 35 °C; mobile phase solvents, (A) 0.5% formic acid (Sigma-Aldrich 95%) in water milli-Q (Sigma-Aldrich) and (B) acetonitrile (Sigma-Aldrich 99.9%). The following gradient elution protocol was used: 35% B to 60% B (t = 8 min), 60% B to 90% B (t = 13 min), 90% B to 95% B (t = 15 min, 2-min hold), 95% B to 35% B (t = 17 min, 4-min hold), total run time, 21 min. The calibration was performed using a derivatised acetaldehyde standard. All analyses were conducted through two experimental replicas and two analytical replicas.
High-performance liquid chromatography determination of anthocyanins and polymeric phenolics
Analyses of native anthocyanins and polymeric phenolics were performed by high-performance liquid chromatography and the apparatus used was the same reported above.
The separation of phenolic compound was carried out according to Waterhouse, et al. [30]. Samples were filtered through 0.45 micron, Durapore membrane filters (Millipore–Ireland), putted into glass vials and injected into the column (Agilent PLRP-S 100-Å reversed phase polystyrene divinyl benzene column 4.6 × 150 mm, 3 µm particle size protected with a guard cartridge with the same packing material PLRP-S, 5 × 3 mm). Detection was performed by monitoring the absorbance signals at 520 nm.
The chromatographic conditions were: sample injection volume, 20 µL; flow rate, 1 mL/min; column temperature, 35 °C; solvent A, 1.5% v/v ortho-phosphoric acid (EMP Chemicals, Gibbstown, NJ, USA) and solvent B, 80% acetonitrile (HPLC grade, Honeywell, Muskegon, MI, USA) and 20% of solvent A.
The gradient used was: zero-time conditions, B 6%; 73 min, B 31%; 78 min, B 62%, staying constant until 86 min; 90 min, B 6%. This zero-time solvent mixture was followed by a 15 min equilibrium period prior to injecting the next sample.
The calibration curve was performed by injecting increasing amount of malvidin-3-monoglucoside (Extrasynthese, Lyon, France) and the concentration was expressed as mg/L of malvidin-3 monoglucoside.
All analyses were conducted through two experimental replicas and two analytical replicas.
Spectrophotometric analyses
The chromatic characteristics and spectrophotometric measures were determined using a spectrophotometer (Jenway 7305 Spectrophotometer). The analysis of colour intensity (Abs 420 nm + Abs 520 nm + Abs 620 nm) and hue (Abs 420 nm/Abs 520 nm) were evaluated as reported by Glories [31]. Tannins reactive to BSA were determined by the Harbertson–Adams assay [32], while Vanillin reactive flavans (VRF) were determined as previously reported by Di Stefano and Guidoni [33].
31]. Tannins reactive to BSA were determined by the Harbertson–Adams assay [32], while Vanillin reactive flavans (VRF) were determined as previously reported by Di Stefano and Guidoni [33].
The evaluation of ∆E was made by the analysis of CIELAB parameters (L∗, a∗, b∗) using NomaSense Color P100 Equipped with a 0.5 cm cell, and the colour differences were calculated as the Euclidean distance between two points in the 3D space defined by L∗, a∗, and b∗, as reported by the Commission Internationale de L’Eclariage (CIE) [34].
All analyses were conducted through two experimental replicas and two analytical replicas.
Statistical analysis
Statistical analysis was performed using XLSTAT (software Addinsoft, 2017.1). The influence of treatments was assessment by the analysis of the variance (ANOVA) by using the Tukey’s method for the evaluation of significant differences (p < 0.05). All data are means of four values: two experimental replicas and two analytical replicas.
Results and discussion
The effect of chitosan on wines at different tannins/anthocyanins ratio
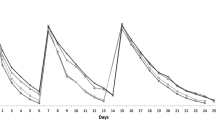
A comparison among wines at different T/A ratio before and after the treatment with chitosan is shown in Fig. 1. As expected, by increasing T/A ratio, wines exhibited an increase in concentrations of total phenol, vanillin reactive flavans and tannins reactive to BSA as previously reported [24]. The addition of chitosan determined slight decreases in total phenolics compound in samples W-C and WT2-C. Only for sample W was reported a significant influence on vanillin reactive flavans.
Chitosan effect on total phenols (A), vanillin reactive flavans (B), tannins reactive to BSA (C) and total anthocyanins (D). Different letters indicate a statistically significant difference among treated wines, according to Tukey’s (HSD) analysis (p < 0.05). The effect of T/A ratio is expressed with a capital letter on wines W, WT 0.5, WT 1, and WT 2 (A–C), and the effect of chitosan addition is expressed with a lowercase letter on wines W-C, WT 0.5-C, WT 1-C, and WT 2-C (a, b, c). The asterisk (*) indicates a statistical difference between each kind of wine before and after the treatment with chitosan. All data are expressed as means ± standard deviation
In contrast, and in agreement with previous study [12, 13], the addition of chitosan influenced the concentration of BSA-reactive tannins. The decrease was higher in sample W (− 50%) and lower in sample WT2 (− 10%). However, the maximum amount of adsorbed BSA-reactive tannins was detected in sample WT2 and was of 102.21 mg/L. As reported by Harbertson, et al. [35], BSA-reactive tannins do not involve all the tannins present in wine but only high molecular weight tannins. Authors showed that the parameter obtained by determining tannins precipitated by BSA is affected by the degree of polymerisation of tannins and it increases by increasing the degree of polymerisation (or size) from trimers (10%) to octamers (93%) [36]. Therefore, these data showed that chitosan showed a greater reactivity against high molecular weight tannins determined by the interaction with BSA. As shown in Fig. 1, the concentration of total anthocyanins was approximately 300 mg/L in all samples with slight lower content in the samples treated with chitosan.
A comparison among total monomeric anthocyanins and polymerics pigments is shown in Table 3. Native anthocyanins decrease proportionally with the decrease in the T/A ratio. Probably, a higher amount of oligomeric tannins promotes the reactions of formation of new pigments [37] involving free monomeric anthocyanins. Samples treated with chitosan showed a decrease in concentrations of total anthocyanins, anyhow, the involvement of native anthocyanins was negligible and differences between treated and untreated samples were observed only in sample W and WT1. Probably thus is due to the slight adsorption of these monomeric pigments on chitosan as previously reported by Ribeiro, et al. [38].
The reactivity of polymeric pigments towards chitosan was correlated with the change in the T/A ratio (Table 3). In the assessment of the chemical nature of these pigments, a large proportion was composed of oligomers (5 units and higher) as reported by Peng et al. [39]. Concerning the ability of chitosan to react with phenolic compounds in red wine, data confirm that chitosan strongly reacts with polymerised phenolic compounds.
As expected, the additions of oligomeric tannins influenced positively the colour intensity (Table 4). However, simultaneous slight increase of tonality was observed probably due on the effect of tannins on yellow tint [40].
In agreement with the literature [41], chitosan treatment showed a negative influence on colour intensity in each sample except for WT0.5. Because of chitosan determined a decrease of polymeric pigments in all samples (Table 2), it is likely that the decrease of the polymeric pigments could have influenced the colour intensity of the treated wines.
In addition, the assessment of ∆E (Table 5) was calculated before and after the treatment with chitosan. A variation of ∆E greater than two points for each wine added with tannins and treated with chitosan was detected. In agreement with Pérez-Magariño, et al. [42], it is clear that the fining treatment generated a colour variation visible by human eye for WT 0.5.
Effect of chitosan after an oxidative stress
In our experiment, the addition of chitosan did not affect the concentration of acetaldehyde in wines after the fining treatment. Even the initial T/A ratio slightly affects the acetaldehyde concentration (Table 6) showing statistical higher values only in samples treated with higher concentrations of condensed tannins (WT1), probably due to an increase in catechols that can oxidise to quinones affecting the Fenton reaction and acetaldehyde production [15, 43].
As expected, after the oxidative stress, the amount of acetaldehyde increased in according to Casellato et al. [44] and Singleton [45]. After oxidation, treated wines showed interesting differences in acetaldehyde (Table 6). The concentrations were lower in samples with a higher T/A ratio [24] and added with chitosan. Probably, the highest concentration of tannins facilitated the involvement of acetaldehyde in polymerisations reactions [46], while the positive effect of chitosan was due to the direct radical scavenging activity of this polymer [47]. A comparison among phenolic compounds was reported in Fig. 2, total phenols and vanillin reactive flavans were affected by fining but not by oxidation. Tannins reactive to BSA showed a significant increase probably due to the oxidative stress that enhanced the tannins polymerisations [35]. In contrast and as expected, a contemporary decrease of total anthocyanins occurred.
Oxidation effect on total phenols (A), vanillin reactive flavans(B), tannins reactive to BSA (C) and total anthocyanins (D). Different letters indicate a statistically significant difference among treated wines, according to Tukey’s (HSD) analysis (p < 0.05). The effect of T/A ratio is expressed with a capital letter on wines Wo, WTo 0.5, WTo 1, and WTo 2 (A–D), and the effect of chitosan addition is expressed with a lowercase letter on wines W-Co, WT 0.5-Co, WT 1-Co, and WT 2-Co (a, b, c, d). The asterisk (*) indicates a statistical difference between each kind of wine before and after the treatment with chitosan. The Greek symbol (α) is used if the value is significantly higher than the non-oxidised sample, and the symbol (β) is used if the value is significantly lower than the non-oxidised sample (Fig. 1). All data are expressed as means ± standard deviation
Chitosan addition negatively influenced the concentrations of phenolic compounds, in particular, the total anthocyanins and tannins reactive to BSA in agreement with literature [12, 36]. After oxidation, vanillin reactive flavans showed statistical differences only for sample WTo 2. Table 7 shows the concentrations of monomeric anthocyanins and pigmented polymers. As expected, the increase of T/A ratios caused a proportional increase of polymeric pigments; simultaneously, the sum of native anthocyanins drastically decreased, probably due to the involvement of monomeric pigments in polymerisation reactions [22]. These data confirmed the hypothesis previously reported about the involvement of acetaldehyde in the formation of new polymerised pigments. Even after oxidation, it is confirmed that the addition of chitosan resulted in a decrease in the compounds with the highest degree of polymerisation (BSA-reactive tannins and polymeric pigments) in all the experimental samples.
Differences observed in pigments affected colour intensity, hue and ∆E (Tables 8 and 9). Colour intensity was better preserved at lower T/A ratio, while the tonality slightly increased by increasing the T/A ratio. Chitosan additions before oxidation stress had a negative effect on the colour intensity and tonality likely due to the fact that the variations in anthocyanins and polymeric pigments influenced the chroma of wines. Analysis of ∆E in Table 9 confirms that the change in chromatic characteristics is well visible (∆E > 3) in samples at a higher T/A ratio and treated with chitosan.
Conclusion
The present study evaluated the effect of chitosan additions before and after oxidation on red wines characterised by four different levels of T/A ratio. Our results confirmed that the addition of chitosan influenced the concentration of phenolic compounds particularly reacting with high molecular weight tannins. Concerning the wine pigments, chitosan additions slightly affect monomeric anthocyanins, while strongly decrease the content of polymeric pigments.
Furthermore, our results confirmed that higher T/A ratios determined a greater concentration of stable colour pigments. Higher T/A ratio and chitosan addition also determine a lower production of oxidative reaction products, such as acetaldehyde after oxidation.
Data showed a great reactivity of chitosan towards compounds with a high degree of polymerisation; therefore, it is advisable to use it on wines with a high T/A ratio to avoid an excessive impoverishment of wines, especially for wines with a low concentration of phenolic compounds.
Data availability
All the data collected in this study are available upon request to the corresponding author.
References
Hayes M, Carney B, Slater J, Brück W (2008) Mining marine shellfish wastes for bioactive molecules: chitin and chitosan ndash; part A: extraction methods. Biotechnol J Healthc Nutr Technol 3(7):871–877
Rinaudo M (2006) Chitin and chitosan: Properties and applications. Prog Polym Sci 31(7):603–632
Shahidi F, Arachchi JKV, Jeon YJ (1999) Food applications of chitin and chitosans. Trends Food Sci Technol 10(2):37–51
Roller S, Covill N (1999) The antifungal properties of chitosan in laboratory media and apple juice. Int J Food Microbiol 47(1–2):67–77
Ferreira D, Moreira D, Costa EM, Silva S, Pintado MM, Couto JA (2013) The antimicrobial action of chitosan against the wine spoilage yeast Brettanomyces/Dekkera. J Chitin Chitosan Sci 1(3):240–245
Valera MJ, Sainz F, Mas A, Torija MJ (2017) Effect of chitosan and SO2 on viability of Acetobacter strains in wine. Int J Food Microbiol 246:1–4
Rhoades J, Roller S (2000) Antimicrobial actions of degraded and native chitosan against spoilage organisms in laboratory media and foods. Appl Environ Microbiol 66(1):80–86
Chinnici F, Natali N, Riponi C (2014) Efficacy of chitosan in inhibiting the oxidation of (+)-catechin in white wine model solutions. J Agric Food Chem 62(40):9868–9875
Marín AC, Culcasi M, Cassien M, Stocker P, Thétiot-Laurent S, Robillard B, Pietri S (2019) Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem 285:67–76
OIV (2009b) Resolution 368/2009, OENO 368/2009Codex Oenologique International. Paris: Organisation Internationale de la Vigne et du Vin
European Union (EU) (2011) Commission regulation (EU) 53/2011 of 21 January 2011. Of J Eur Union L19/1−L19/6
Spagna G, Pifferi PG, Rangoni C, Mattivi F, Nicolini G, Palmonari R (1996) The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res Int 29(3–4):241–248
Picariello L, Errichiello F, Coppola F, Rinaldi A, Moio L, Gambuti A (2021) Effect of chitosan on the removal of different types of tannins from red wines. Appl Sci 11(24):11743
Larrauri JA, Sánchez-Moreno C, Rupérez P, Saura-Calixto F (1999) Free radical scavenging capacity in the aging of selected red Spanish wines. J Agric Food Chem 47(4):1603–1606
Waterhouse AL, Laurie VF (2006) Oxidation of wine phenolics: A critical evaluation and hypotheses. Am J Enol Vitic 57(3):306–313
Chira K, Pacella N, Jourdes M, Teissedre PL (2011) Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem 126(4):1971–1977
Lukić I, Radeka S, Budić-Leto I, Bubola M, Vrhovsek U (2019) Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem 300:125251
Mazza G, Francis FJ (1995) Anthocyanins in grapes and grape products. Crit Rev Food Sci Nutr 35(4):341–371
Heredia FJ, Francia-Aricha EM, Rivas-Gonzalo JC, Vicario IM, Santos-Buelga C (1998) Chromatic characterization of anthocyanins from red grapes—I. pH effect. Food Chem 63(4):491–498
Arnold RA, Noble AC, Singleton VL (1980) Bitterness and astringency of phenolic fractions in wine. J Agric Food Chem 28(3):675–678
Morel-Salmi C, Souquet JM, Bes M, Cheynier V (2006) Effect of flash release treatment on phenolic extraction and wine composition. J Agric Food Chem 54(12):4270–4276
Atanasova V, Fulcrand H, Cheynier V, Moutounet M (2002) Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal Chim Acta 458(1):15–27
Singleton VL, Trousdale EK (1992) Anthocyanin-tannin interactions explaining differences in polymeric phenols between white and red wines. Am J Enol Vitic 43(1):63–70
Picariello L, Gambuti A, Picariello B, Moio L (2017) Evolution of pigments, tannins and acetaldehyde during forced oxidation of red wine: Effect of tannins addition. LWT 77:370–375
Dıblan S, Özkan M (2021) Effects of various clarification treatments on anthocyanins, color, phenolics and antioxidant activity of red grape juice. Food Chem 352:129321
Castro Marin A, Chinnici F (2020) Physico-chemical features of sangiovese wine as affected by a post-fermentative treatment with chitosan. Appl Sci 10(19):6877
Harbertson JF, Parpinello GP, Heymann H, Downey MO (2012) Impact of exogenous tannin additions on wine chemistry and wine sensory character. Food Chem 131(3):999–1008
Coppola F, Picariello L, Forino M, Moio L, Gambuti A (2021) Comparison of three accelerated oxidation tests applied to red wines with different chemical composition. Molecules 26(4):815
Han G, Wang H, Webb MR, Waterhouse AL (2015) A rapid, one step preparation for measuring selected free plus SO2-bound wine carbonyls by HPLC-DAD/MS. Talanta 134:596–602
Waterhouse AL, Price SF, McCord JD (1999) [11] Reversed-phase high-performance liquid chromatography methods for analysis of wine polyphenols. In: Methods in enzymology, vol 299. Academic Press, New York, pp 113–121
Glories Y (1984) La couleur des vins rouges. Conn Vigne Vin 18(4):253–271
Harbertson JF, Picciotto EA, Adams DO (2003) Measurement of polymeric pigments in grape berry extract sand wines using a protein precipitation assay combined with bisulfite bleaching. Am J Enol Vitic 54(4):301–306
Di Stefano R, Guidoni S (1989) La determinazione dei polifenoli totali nei mostie nei vini. Vignevini 16(12):47e52
CIE Publication 15 (2004) Colorimetry, 3rd edn. Commission Internationale de l’Eclairage (ISBN 3-901-906-33-9)
Harbertson JF, Kilmister RL, Kelm MA, Downey MO (2014) Impact of condensed tannin size as individual and mixed polymers on bovine serum albumin precipitation. Food Chem 160:16–21
Picariello L, Rinaldi A, Blaiotta G, Moio L, Pirozzi P, Gambuti A (2020) Effectiveness of chitosan as an alternative to sulfites in red wine production. Eur Food Res Technol 246(9):1795–1804
Remy S, Fulcrand H, Labarbe B, Cheynier V, Moutounet M (2000) First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J Sci Food Agric 80:745–751
Filipe-Ribeiro L, Cosme F, Nunes FM (2018) Data on changes in red wine phenolic compounds and headspace aroma compounds after treatment of red wines with chitosans with different structures. Data Brief 17:1201–1217
Peng Z, Hayasaka Y, Iland PG, Sefton M, Høj P, Waters EJ (2001) Quantitative analysis of polymeric procyanidins (tannins) from grape (Vitis vinifera) seeds by reverse phase high-performance liquid chromatography. J Agric Food Chem 49(1):26–31
Bautista-Ortín AB, Martínez-Cutillas A, Ros-García JM, López-Roca JM, Gómez-Plaza E (2005) Improving colour extraction and stability in red wines: the use of maceration enzymes and enological tannins. Int J Food Sci Technol 40(8):867–878
Quintela S, Villarán MC, De Armentia IL, Elejalde E (2012) Ochratoxin A removal from red wine by several oenological fining agents: bentonite, egg albumin, allergen-free adsorbents, chitin and chitosan. Food Addit Contam Part A 29(7):1168–1174
Pérez-Magariño S, González-Sanjosé ML (2003) Application of absorbance values used in wineries for estimating CIELAB parameters in red wines. Food Chem 81(2):301–306
Danilewicz JC (2003) Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: central role of iron and copper. Am J Enol Vitic 54(2):73–85
Casellato U, Tamburini S, Vigato PA, Vidali M, Fenton DE (1984) Binuclear oxovanadium (IV) complexes as catalyst for the oxygenation of the catechols. Inorg Chim Acta 84(1):101–104
Singleton VL (1987) Oxygen with phenols and related reactions in musts, wines, and model systems: observations and practical implications. Am J Enol Vitic 38(1):69–77
Gambuti A, Picariello L, Rinaldi A, Moio L (2018) Evolution of Sangiovese wines with varied tannin and anthocyanin ratios during oxidative aging. Front Chem 6:63
Park PJ, Je JY, Kim SK (2004) Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr Polym 55(1):17–22
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no known competing financial interests or conflict of interests that could have influenced the work reported in this paper.
Compliance with ethics requirements
This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Picariello, L., Errichiello, F., Coppola, F. et al. Effect of chitosan addition on acetaldehyde and polymeric pigments production after oxidation of red wines with different tannin/anthocyanins ratio. Eur Food Res Technol 249, 2447–2455 (2023). https://doi.org/10.1007/s00217-023-04292-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04292-z