Abstract
In this study, the influence of iota carrageenan (IC) addition at different steps to the protein blends based on pea protein isolate (PPI) and wheat gluten (WG) as well as hydration mixing time and temperature of IC on the quality attributes of plant-based meat alternatives was studied. In more detail, IC was added before (B, in water with mixing times of 15 or 30 min and temperatures of 25 or 75 °C) or after (A, in powder form) the addition of PPI to the mixture with or without calcium chloride (Ca) in the formulation. The results showed that the addition of IC after PPI, especially combination with Ca resulted in the products with the most visible fibers, which can be considered as a quality improvement. IC addition to the formulations with or without Ca also increased the browning index, water holding capacity, tensile stress, and air bubble numbers compared to the PPI.WG formulation. However, no considerable difference in these parameters was found regarding the addition order of IC (before or after the addition of PPI). As the addition of IC after PPI hydration needs less energy for mixing, and, thus, less time for preparation, this order of addition can be recommended for improving the quality of plant-based meat alternatives containing IC. Therefore, hydration of IC in water, especially at high temperatures, is not necessary for the production of plant-based meat alternatives produced in the high-temperature shear cell (HTSC).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Julia Twigg, a sociologist, believes that “Meat is the most highly prized of food. It is the center around which a meal is arranged” [13]. Therefore, it is predictable that many people like to have meat in their diets. However, amongst others the increase of the world population to 9.1 billion by 2050 will lead to a doubling of the demand for meat if suitable plant-based meat alternatives are not found [8]. Plant-based meat alternatives can be defined as food products similar to meat produced from plant-based ingredients to mimic the appearance, texture, and nutritional value of real meat products [28]. Currently, a noteworthy growth of plant‐based meat alternatives to traditional animal proteins has been in the food market [34]. Thus, different plant-based meat alternatives can be found in the market to provide our diets with enough protein [35] which can help to mitigate consumer’s concerns about their health as well as the environmental and ethical aspects. The consumption of plant-based meat alternatives can decrease body weight, blood pressure, saturated fatty acids, and cholesterol resulting in a reduction in cancers, cardiovascular disease, stroke risk, diabetes, and mortality [2, 28]. Additionally, hormone usage for faster and higher meat production [35] and excessive antibiotics used to fight new infections produced by deadly pathogens are other health concerns related to meat consumption [9]. Ethically, the huge demand for meat in the near future also increases concerns about animal welfare. Moreover, it results in numerous environmental problems such as natural resource depletion [32], deforestation, pollution, damage to hydrogeological reserves, and loss of biodiversity [9] that negatively affect climate change [32].
It is often suggested that plant-based meat alternatives should have similar textural, sensorial, and nutritional properties at an affordable cost to convince many consumers [10]. Currently, soy protein isolate is mostly used to produce plant-based meat alternatives [6, 36]. However, soy is connected to GMO concerns and is allergenic. Furthermore, soy is mostly produced in South American countries, and, thus, its transportation to Europe could negatively affect the environment. Moreover, soil erosion and severe logging for soy cultivation harmfully impact the environment. These concerns explain a rising interest in pea as ingredient for meat alternatives. Pea can be cultivated in Europe, which solves the transport emission problem. In fact, regional production of products has different advantages in resource use and environment and climate protection [39]. In addition, pea protein isolate (PPI) has gained interest due to its low allergenicity, reasonable cost, its suitability for production in temperate climates, and acceptable nutritional value [20]. Although pea protein contains high levels of essential amino acids, especially lysine, it is low in sulfur-containing amino acids, especially methionine and cysteine [20, 24]. However, the combination of pea protein with cereal proteins such as wheat gluten (WG) can compensate for this problem as cereal proteins have high levels of sulfur amino acids. Thus, the mixture of PPI and WG has a balanced essential amino acid profile [20]. Thus, despite the fact that the development of plant-based meat alternatives containing PPI instead of soy protein isolate with appropriate textural and sensorial attributes is more challenging, PPI will probably be more easily accepted by consumers [31, 33].
Hydrocolloids are generally used in foods to obtain desired textural and sensorial properties [9] with their ability to retain water and form gels. Carrageenan is generally obtained from red seaweeds [27]. It is composed of D-galactose units in three primary forms (kappa, iota, and lambda) [47]. Those units differ with respect to the quantity and location of sulfate groups on the galactose chain. The water solubility increases with iota carrageenan (IC) which contains two sulfate groups per two galactose units [17]. Some studies found positive effects on the texture and firmness of plant-based meat alternatives and fiber formation in these products by adding IC to soy protein concentrate [25]. However, no research has been done yet on the effect of IC in plant-based meat alternatives based on PPI and WG in the high-temperature shear cell (HTSC). Furthermore, little attention has been paid to the appropriate moment of addition of IC into the formulation as well as to the temperature of the mixture at the moment of addition and the time allowed for hydration. IC is dissolves better and more quickly at higher temperatures [7]. It is not even clear whether hydration of IC in water is necessary to obtain good structuring, but it is reasonable that the hydration time and temperature will be of influence. A limitation of the extruder is that powder should simultaneously be mixed with water in the system and thus, the order of addition and degree of hydration cannot be tested. However, as the protein mixture is prepared outside HTSC, these parameters can be investigated. Additionally, IC constructs elastic gel networks and thermoreversible gels with calcium ions [1]. Therefore, salts will also influence the quality attributes of plant-based meat alternatives.
This study, therefore, aims to investigate the influence of IC on the structuring of plant-based meat alternatives in a HTSC process by measuring the textural and the properties related to sensorial profile of plant-based meat alternatives based on PPI and WG. The hypothesis we follow is that the formulation and the procedure of IC addition to the protein blends influence the morphology of plant-based meat alternatives. For instance, a higher water holding capacity and gel strength can be obtained with a blend of protein and IC [25, 42], and when IC should be hydrated, its hydration time and temperature will affect the structure of the processed materials obtained. Finally, the addition of Ca with IC in the product formulation improves the fibrous structure of plant-based meat alternatives and forms stronger gels [21, 42, 46].
Material and methods
Material
Pea protein isolate (PPI) (NUTRALYS® S85F) and vital wheat gluten (WG) (Viten®—VITAL WHEAT GLUTEN) were purchased from Roquette Frères S.A. (Lestrem, France). PPI and WG contain at least 83 and 77% protein (N × 6.25), respectively. PPI and WG also contain 92.6 and 92.0% dry matter content, respectively. Iota carrageenan (IC) (GENUVISCO® carrageenan CI-132) was obtained from CP KelcoApS (Lille Skensved, Denmark). CaCl2, Rhodamine B, and glutaraldehyde (25 v%) were obtained from Sigma-Aldrich (Sigma–Aldrich Co, St. Louis, Missouri, USA). Finally, ethanol 96% was obtained from VWR Chemicals BDH® (VWR International B.V., Amsterdam, Netherlands).
Methods
Preparation of different protein blends
Regarding the abbreviations of the product names in Table 1, the letters on the left side and before the comma show the ingredients containing pea protein isolate (PPI), wheat gluten (WG), iota carrageenan (IC), and calcium chloride (Ca) in the formulations. Moreover, the letters and numbers after the comma show the IC addition procedure (after (A) PPI addition and in powder form to the protein mixture or before (B) PPI addition to the water with IC hydration temperatures of 25 °C or 75 °C and IC hydration times of 15 min or 30 min). All formulations described here were based on 35 wt% ingredients, with equal weights used for PPI and WG. These procedures for preparing of different protein blends (100 g) are described in more detail in the following Sects. (“Preparation of protein blend (PPI.WG)”, “PPI.WG.Ca product”, “PPI.WG.IC,A product”, “PPI.WG.IC,B25°C-15min and PPI.WG.IC,B25°C-30min products”, “PPI.WG.IC,B75°C-15min and PPI.WG.IC,B75°C-30min products”, “PPI.WG.IC.Ca,B25°C-15min and PPI.WG.IC.Ca,B25°C-30min products”, “PPI.WG.IC.Ca,B75°C-15min and PPI.WG.IC.Ca,B75°C-30min products”, “PPI.WG.IC.Ca,A product”).
Preparation of protein blend (PPI.WG)
The control without IC was denominated as PPI.WG in Table 1 and contained pea protein isolate (PPI) and wheat gluten (WG). First, PPI (17.5 g) and demineralized water (65 g, room temperature) were mixed at room temperature by hand with a spatula for approximately 30 s in a beaker. Then, the beaker was covered with parafilm to prevent water evaporation and left for PPI hydration at room temperature for 30 min. Next, WG (17.5 g) was added to the mixture and mixed manually with the spatula. Finally, the blend was processed in the HTSC (Fig. 1).
PPI.WG.Ca product
To evaluate the effects of calcium chloride (Ca) in the product containing PPI and WG, Ca (0.4%) was added to the formulation. PPI (17.3 g) and Ca (0.4 g) were mixed together and added to a beaker with demineralized water (65 g). Then, all the mentioned ingredients were mixed manually with the spatula for approximately 30 s. Next, the beaker was covered with parafilm and left for hydration at room temperature. After 30 min, WG (17.3 g) was added to the hydrated mixture and was mixed with the spatula (Fig. 1).
PPI.WG.IC,A product
PPI.WG.IC,A contains PPI, WG, and IC with the addition of iota carrageenan (IC) after (A) PPI addition. Similar to standard procedure, PPI (16.5 g) and demineralized water (65 g) were mixed manually. After hydration for 30 min, WG (16.5 g) and IC (2 g) were mixed together and added to the mixture. Next, they were mixed by hand with the spatula (Fig. 1).
PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B25°C-30 min products
Both PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B25°C-30 min samples contain PPI, WG, and IC with the addition of IC before (B) PPI addition at room temperature (25 °C). First, IC (2 g) and demineralized water (65 g) were mixed at room temperature for 15 min and 30 min for PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B25°C-30 min samples, respectively with a stirrer (EUROSTAR 40 digital, IKA, Germany) and a four-flat-blade turbine impeller at 400 rpm. After mixing, the mixture was weighed to determine the amount of water that evaporated, and demineralized water was added to compensate for the loss. Then, PPI (16.5 g) was added to this mixture at room temperature and mixed manually with a spatula for approximately 30 s. After 30-min hydration at room temperature, WG (16.5 g) was added to the hydrated mixture and mixed with a spatula (Fig. 1).
PPI.WG.IC,B75°C-15 min and PPI.WG.IC,B75°C-30 min products
PPI.WG.IC,B75°C-15 min and PPI.WG.IC,B75°C-30 min samples have a similar formulation and preparation procedure as for PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B25°C-30 min samples in Sect. "PPI.WG.IC,B25°C-15min and PPI.WG.IC,B25°C-30min products". The only difference is that mixing IC and demineralized water took place in a water bath (HE-4 Heating Circulator, JULABO, Germany) at 75 °C for 15 min and 30 min for preparation of PPI.WG.IC,B75°C-15 min and PPI.WG.IC,B75°C-30 min, respectively.
PPI.WG.IC.Ca,B25°C-15 min and PPI.WG.IC.Ca,B25°C-30 min products
PPI.WG.IC.Ca,B25°C-15 min and PPI.WG.IC.Ca,B25°C-30 min samples contain PPI, WG, Ca, and IC with the addition of IC before (B) PPI hydration step at room temperature (25 °C) for 15 min and 30 min, respectively. First, IC (2 g) and demineralized water (65 g) were mixed at 25 °C for 15 min and 30 min, respectively, with the stirrer at 400 rpm. After mixing, the mixture was weighed, and demineralized water was added to compensate for possible water evaporation. Then, PPI (16.3 g) and Ca (0.4 g) were added simultaneously and mixed manually for approximately 30 s. After 30-min hydration at room temperature, WG (16.3 g) was added to the hydrated mixture and was mixed manually (Fig. 1).
PPI.WG.IC.Ca,B75°C-15 min and PPI.WG.IC.Ca,B75°C-30 min products
PPI.WG.IC.Ca,B75°C-15 min and PPI.WG.IC.Ca,B75°C-30 min samples have a similar formulation and preparation procedure in comparison to the procedure described for PPI.WG.IC.Ca,B25°C-15 min and PPI.WG.IC.Ca,B25°C-30 min samples in Sect. "PPI.WG.IC.Ca,B25°C-15min and PPI.WG.IC.Ca,B25°C-30min products" with the only difference being that mixing of IC and demineralized water took place at 75 °C in a water bath for 15 min and 30 min, respectively.
PPI.WG.IC.Ca,A product
PPI.WG.IC.Ca,A sample contains PPI, WG, Ca, and IC with the addition of IC after (A) hydration of PPI in the mixture. First, PPI (16.3 g) and Ca (0.4 g) were mixed together in the beaker with demineralized water (65 g) with a spatula for approximately 30 s. After 30-min hydration at room temperature, WG (16.3 g) and IC (2 g) were added to the protein blend and mixed manually.
High-temperature shear cell (HTSC) processing
Approximately 95 g of the described blends in Sects. "Preparation of different protein blends" and Table 1 were processed into a pre-heated (at 100 °C) HTSC designed at Wageningen University (The Netherlands). The shearing speed rotation for processing of all the formulations was 30 rpm for 15 min, and the processing temperature was at 100 °C. After 15 min of thermomechanical treatment, the device was cooled down to 25 °C for 10 min without any shearing (0 rpm). After cooling, the products were taken from the HTSC and placed in a resealable zipper bag at room temperature for at least 1 h before color and texture analyses and fiber observation. Then, the samples were stored at − 18 °C to continue with the subsequent analysis (SEM, CLSM, WHC, and XRT).
Visual inspection of fibrousness
Visualization of the macrostructure of the sheared samples was conducted by taking pictures inside an in-house white mini photo studio with three lights on the right, left, and upper sides. A picture of the complete products processed in the HTSC was taken inside a photo studio with standardized lighting. The macrostructure and fibrous structure of the products were visually evaluated by cutting and folding three pieces (Fig. 2) from products parallel to the shear-flow direction. Then, the cut pieces were collocated in a long pin for taking pictures via an iPhone 12, Apple.
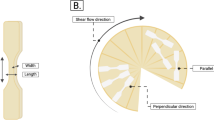
Red dash lines represent the pieces cut out of the processed product for visualization of fibers and the blue dash lines represent the sample folding line. Moreover, this figure shows the dog-bone mold and its dimensions (on the left side of this figure) to obtain the tensile bars and their positions in parallel and perpendicular directions to the shear flow
Color measurement
A colorimeter (Chroma Meters CR-400, KONICA MINOLTA, INC., Japan) was used to measure the L*, a*, and b* color data of samples. L* is associated with lightness (minimum value of 0) and darkness (maximum value of 100), a* with redness (positive values) and greenness (negative values), and b* with yellowness (positive values) and blueness (negative values). Browning index (BI) was calculated using L*, a*, and b* data by Eq. (1) [40]:
Tensile strength analysis
The mechanical characteristics of the products were evaluated with a Texture analyzer (TA.XTplusC, Stable Micro Systems, UK) and based on the procedure described by Taghian Dinani, van der Harst, et al. [39] and Schlangen et al. [29]. Specimens were cut out of the sample with a dog-bone mold, three in parallel and three in perpendicular direction to the shear flow (Fig. 2). The force (F in N) needed to break the tensile bars was recorded by Exponent Connect Software (Stable Micro Systems, UK). The tensile stresses (σ in Pa) in parallel and perpendicular directions were calculated using Eq. (2).
In Eq. (2), F(t) is the force [N] required to tear a tensile bar,\(h(0)\) corresponds to the initial length, \(h(t)\) is the length at time t, and \(A(0)\) (the initial contact surface area [m2]) is calculated by multiplying the width and thickness of the specimen. Moreover, the tensile strain \(\varepsilon\)[−] was determined for both parallel and perpendicular directions using Eq. (3):
Finally, Young’s modulus [Pa] was calculated from the slope of the linear part of the tensile stress versus the tensile strain curve.
Water holding capacity (WHC)
For the WHC test, the procedure described by Taghian Dinani, Broekema, et al. [36] was followed. Three specimens were taken out with a ring mold of approximately 16 mm in diameter per replication. These three circular pieces were weighed and placed in a beaker with 70 ml of demineralized water, and the beakers placed in a water bath (SW23, JULABO, Germany) at 50 °C for 16–17 h. After that, the hydrated samples were carefully taken out and placed on a tube rack; surface water was carefully and quickly removed with tissue paper. Finally, the hydrated samples were weighted, and WHC was calculated using Eq. 4:
In this equation \({W}_{a}\) and \({W}_{b}\) correspond to the weight of samples after and before hydration, respectively.
Confocal laser scanning microscopy (CLSM)
A similar procedure as described by Jia et al. [15] was followed to prepare CLSM images. First, frozen samples were cut in a right trapezoid shape (8 mm × 10 mm × 15 mm) parallel to the shear-flow direction. Then, the cut samples were glued with a CryoCompound (Immunologic a WellMed company, Duiven, The Netherlands) on a 35 mm cryostat chuck and collocated in a SLEF Cryostat MEV (SLEE, Mainz, Germany) to create slices with a smooth surface and with approximately 60 µm thickness at − 17 °C. The specimens were collocated on a microscope slide and stained with Rhodamine B (0.002% in MilliQ water). Consequently, the stained samples on the microscope slides were stored in a dark and humid box to maintain their moisture content constant at room temperature for at least 1 h. The samples were then confined by placing a coverslip. So-called spacers were used to create the appropriate distance between the microscope slide and the cover slip. Lastly, the visualization on a microstructure scale of the specimens was evaluated with an inverted Stellaris 5 DMi8 (Leica Microsystems CMS GmbH, Amsterdam, The Netherlands). White Light Lasers (WLL) provided the excitation of Rhodamine B at 543 nm, and a lens of HC PL APO CS2 20x/0.75 DRY was used for taking the images. These images were analyzed with LAS X (Leica Microsystems CMS GmbH, Amsterdam, The Netherlands).
Scanning electron microscopy (SEM)
The SEM method as described by Taghian Dinani et al. [39] was followed to obtain information on the microstructure of sheared samples. Frozen samples were cut in a rectangular shape with dimensions of 5 mm × 13 mm. Next, samples were collocated in a closed tube with 10 ml of 2.5% (v/v) glutaraldehyde while lightly shaking for 8 h using a Mini Rocker-Shaker (MR1, Riga, Latvia). The glutaraldehyde was then replaced by demineralized water. The samples were gently rotated overnight using the Mini Rocker-Shaker. Subsequently, the samples were immersed in a series of ethanol at 10, 30, 50, 70, 96, and 100% (v/v) for at least 1 h. Then, the samples were dried using a critical drying point (CPD 300, Leica, Vienna, Austria) and fractured manually perpendicular to the shear-flow direction. Consequently, the samples were fractured by hand parallel to the shear direction. Then, the fractured samples were mounted on the stubs via carbon cement glue and coated with 12 nm of tungsten (SCD 500, Leica, Vienna, Austria). Lastly, the surface of the samples was evaluated at magnifications of 250× (~ 300 μm) and 10,000× (~ 5 μm) by a field emission scanning electron microscope (Magellan 400, FEI, Eindhoven, the Netherlands) with a secondary electron detection of 13 pA and 2.00 kV.
X-ray microtomography (XRT)
To study the distribution of entrapped air, we followed the XRT procedure as described by Schreuders et al. [30]. Frozen sheared products were cut in a rectangular shape with dimensions of 9 mm × 20 mm parallel to the shear-flow direction. Then, the specimen was collocated in an Eppendorf tube to keep moisture content. Next, the sample was placed in an XRT (GE Phoenix v|tome|x m tomographer,General Electric Go., Wunstorf, Germany) with a spatial resolution of 6.0 µm using a distance between object and the X-ray source of 23 mm. An operating voltage of 75 kV, a current of 80 µA, and a power of 6.0 W were used. Images were taken using a GE DXR detector array with 2024 × 2024 pixels. Each specimen was pictured by taking 1501 images over 360° with a 0.24° step ratio and an exposure time of 150 ms when the first picture was omitted. Then, 3D structures were calculated via back projection and the software of Image Reconstruction GE software (Wunstorf, Germany). The 3D images were analyzed by the software of Avizo3D 2021.2 imaging (Thermo Fischer Scientific, Waltham, Massachusetts, USA). Finally, the overall amount of air entrapped in the structure (void fraction) was calculated by dividing the air volume [cm3] by the sample volume [cm3].
Statistics
All the experiments were analyzed with SPSS software (Version 28.0, IBM Corporation, USA) by a general linear model procedure (GLM) which performs regression analysis and analysis of variance (ANOVA) for the dependent variables with a homogeneity test. A significance level of 95% (p ≤ 0.05) was considered for all dependent variables. Consequently, if there was a significant effect, Duncan’s post hoc was performed to evaluate the differences among the means of the dependent variables. All the tests except the XRT test were performed at least three times and all the results were shown as the mean ± the standard deviation (SD) in this study. XRT experiment was performed in duplicate for each sample.
Results and discussion
Macrostructure
Figure 3 shows the pictures of PPI.WG (containing PPI and WG) and PPI.WG.Ca (containing PPI, WG, and Ca) products. Products containing PPI and WG with the addition of IC in the water before addition of PPI at 25 °C or 75 °C for 15 min or 30 min mixing time are shown as PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B25°C-30 min, PPI.WG.IC,B75°C-15 min, and PPI.WG.IC,B75°C-30 min, respectively. Products containing PPI and WG with the addition of both Ca and IC in water (before PPI) at 25 °C and 75 °C for 15 min or 30 min mixing time are shown as PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC.Ca,B75°C-15 min, and PPI.WG.IC.Ca,B75°C-30 min, respectively. The products containing PPI and WG with the addition of IC after PPI hydration with Ca (PPI.WG.IC.Ca,A) and without Ca (PPI.WG.IC,A) are also shown in this figure. More information about these formulations can be found in Sect. "Preparation of different protein blends" and Table 1. Figure 3 shows that all products have a similarly rounded form except PPI.WG.Ca, which was fragile, presenting some fractures and holes. Moreover, some differences in the color of these products can be seen in this figure. The products in the PPI.WG.IC.Ca group (PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC.Ca,B75°C-15 min, PPI.WG.IC.Ca,B75°C-30 min, and PPI.WG.IC.Ca,A) are slightly more brownish than the other materials. The color changes will be discussed in more detail in Sect. "Color".
The internal structure can be observed in the folded materials in Fig. 4. PPI.WG, selected as the control, has limited fiber formation. PPI.WG.Ca had a different structure in comparison with other products as it had a fragile and crumbly structure without any visible fibers making it difficult to fold without breaking. This product is not similar to the plant-based meat alternative containing 40 wt % dried ingredients including PPI (19.5 wt%), WG (19.5 wt%), and sodium chloride (1.0 wt%) processed at 120 °C with visible fibers that was reported by Schreuders et al. [30]. The differences could be because of the different formulations (less dried ingredients in our study (35 wt%) and using Ca instead of NaCl) or because of the different processing temperature (100 °C in our study) in the HTSC. Schreuders et al. [30] mentioned that a weak and fragile dough product without any fibers was obtained at 95 °C and small fibers were observed at 110 °C in the PPI-WG blend. Thus, the structure of PPI.WG.Ca processed at 100 °C in our study is in line with the structure of dough obtained at 95 °C and the structure with small fibers obtained at 110 °C reported by these researchers.
Visualization of the macrostructural fibers of products with different formulations and preparation procedures processed in the HTSC. In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively
Figure 4 shows that there were no significant macrostructural differences for the addition of IC without Ca after PPI addition (PPI.WG.IC,A) or before PPI addition at different mixing temperatures and times (PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B25°C-30 min, PPI.WG.IC,B75°C-15 min, PPI.WG.IC,B75°C-30 min). However, inside the samples containing Ca, the addition of IC after PPI (PPI.WG.IC.Ca,A) resulted in a more fibrous structure than the addition of IC before PPI (PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC.Ca,B75°C-15 min, and PPI.WG.IC.Ca,B75°C-30 min). In fact, PPI.WG.IC.Ca,A has the best fibrous structure in this figure. It could be suggested that adding IC after PPI in a PPI.WG.Ca mixture promotes protein phase alignment and fiber formation [12]. The results indicate that the hydration of PPI in a Ca solution is important for the PPI solubility and for structure formation in the PPI.WG.IC.Ca,A product. The initial absorption of Ca in the PPI phase could provide the opportunity for the WG to create the gluten network required for fibrous structure formation [3, 30, 36]. Finally, differences between the PPI.WG.IC.Ca,A sample containing both IC and Ca, compared to PPI.WG.IC,A sample containing IC without Ca could be attributed to the calcium ions, which were earlier found to be essential for forming anisotropic structures in case of caseinate [44, 45]. IC has an affinity to calcium ions, forming elastic gels [14]. Another possible explanation could be the effect of Ca on the viscosity of the protein blends. Krintiras et al. [16] mentioned that anisotropic structure formation is enhanced in highly viscous systems. Furthermore, the viscosity of the two phases affects the deformation of the dispersed phase [4, 5]. The existence of IC could be the reason that in the products of the PPI.WG.IC.Ca group ( PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC.Ca,B75°C-15 min, PPI.WG.IC.Ca,B75°C-30 min, and PPI.WG.IC.Ca,A), a more compact structure with more small and thin fibers was found in comparison with samples in PPI.WG.IC group (PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B25°C-30 min, PPI.WG.IC,B75°C-15 min, PPI.WG.IC,B75°C-30 min, and PPI.WG.IC,A).
Color
Color is an essential attribute of products that aim at resembling meat, and good control over the color may help the design of plant-based meat alternatives that are more attractive and acceptable to customers [18, 19]. Figure 5 shows that PPI.WG.IC.Ca,B25°C-30 min (42.11 ± 1.38) and PPI.WG.IC.Ca,B75°C-30 min (42.07 ± 1.28) have the highest browning index values, while PPI.WG.Ca (39.24 ± 2.07) has the lowest value. The browning index is generally associated with the Maillard reaction, which occurs during processing at high temperatures, where free carbonyl groups of reducing sugars and free amino acids of proteins are linked by a covalent bond during this reaction [38, 41]. The more galactose units, which are available in the backbone of IC hydrocolloid, the higher value for the browning index. However, all the formulations with IC hydrocolloid contain 2% IC. Therefore, variability in the number of galactose units is less probable. It is possible though that different numbers of carbonyl groups are available, which could be an effect of the IC solubilization, which is affected by the different addition procedures of IC, such as different mixing times and temperatures. The highest browning index values in Fig. 5 can be found for different formulations of the PPI.WG.IC.Ca group (PPI.WG.IC.Ca,A, PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC.Ca,B75°C-15 min, and PPI.WG.IC.Ca,B75°C-30 min) containing both IC and Ca. These observations suggest that Ca with IC are more reactive in the Maillard reaction. No statistically significant differences (p > 0.05) can be observed between PPI.WG.IC,A and PPI.WG.IC.Ca,A with their similar groups. As both treatments do not need any IC mixing in water, they could be recommended. However, PPI.WG.IC,A also has a simpler formulation, which does not include Ca in its preparation, and, thus, it is preferable in comparison to PPI.WG.IC.Ca,A.
Browning index ( −) values of products with different formulations and preparation procedures processed in the HTSC. In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively. Moreover, the first, second and third axis X lines from bottom to top show the formulations, hydration mixing temperatures (25 or 75 °C), and hydration mixing times (15 or 30 min), respectively. The black letters above data display statistically significant differences (p ≤ 0.05)
Tensile strength
Figure 6 summaries the tensile stresses of the different materials; Figure S1. A and B in the Appendix section show the tensile strains and Young’s modulus, respectively. In Fig. 6, the values for the parallel direction range from 12.25 ± 0.31 kPa for PPI.WG to 46.54 ± 11.07 kPa for PPI.WG.IC,B75°C-15 min. Although PPI.WG.IC,B75°C-15 min showed the highest tensile stress value parallel to the shear-flow direction, it is not really different (p > 0.05) from the other treatments, except for PPI.WG, PPI.WG.Ca, PPI.WG.IC.Ca,A, PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, and PPI.WG.IC.Ca,B75°C-15 min. The tensile stress value in the perpendicular direction for the PPI.WG.Ca sample could not be reliably measured, since this sample had a lot of cracks, and was too fragile (Fig. 3). The values for the shear stress in the perpendicular direction range from 4.43 ± 0.23 kPa for PPI.WG to 34.66 ± 6.51 kPa for PPI.WG.IC,B25°C-30 min. Although PPI.WG.IC,B25°C-30 min show the highest tensile stress perpendicular to the shear-flow direction, it was not significantly different (p > 0.05) compared to PPI.WG.IC,A, PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B75°C-30 min. The lowest value perpendicular to the shear flow is for PPI.WG which is significantly different (p ≤ 0.001) from the other treatments. PPI.WG showed the lowest tensile stress values in both parallel and perpendicular directions, but this was not significantly different (p > 0.05) from PPI.WG.Ca. Therefore, the addition of IC in both PPI.WG.IC and PPI.WG.IC.Ca groups results in increased tensile stresses in both parallel and perpendicular directions. Moreover, for almost all treatments except PPI.WG.Ca in Fig. 6, the tensile stress in the parallel direction to the shear flow is larger than those in the perpendicular direction, indicating at least some mechanical anisotropy [12].
Tensile stress (kPa) values of the products with different formulations and preparation procedures processed in the HTSC. In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively. Moreover, the first, second and third axis X lines from bottom to top show the formulations, hydration mixing temperatures (25 or 75 °C), and hydration mixing times (15 or 30 min), respectively. Dark red and black letters above and below data points display statistically significant differences in outcomes (p ≤ 0.001)
There are no significant differences (p > 0.05) within the treatments in the PPI.WG.IC group in the addition of IC before or after hydration, mixing time, or mixing temperature for parallel direction. Regarding the perpendicular direction in the PPI.WG.IC group, only PPI.WG.IC,B75°C-15 min is significantly lower than PPI.WG.IC,B25°C-30 min treatments in this group (p ≤ 0.001). Regarding all treatments in the PPI.WG.IC.Ca group (containing both IC with Ca), no significant differences (p > 0.05) in shear stress values in both parallel and perpendicular directions were found. Therefore, the hydration temperature and time for IC are not parameters that can be used to influence the tensile stress. Although it is believed that calcium improves protein–protein interactions ([44, 45], the PPI.WG.IC.Ca treatments with Ca gave lower tensile stress in both parallel and perpendicular directions than PPI.WG.IC treatments. A similar effect was shown in a study on meat, in which a weaker structure was found after addition of Ca [11]. Mcmahon et al. [22] found that a low calcium concentration led to weak bonds among proteins. On the other hand, Pan et al., [26] reported low WHC values and strengths with high concentrations of Ca in mixtures of carrageenan and porcine myosin. The gels became more compact and stronger upon an appropriate concentration of Ca addition. [48, 49]. observed that a low concentration of Ca in a mixture of soy and whey protein leads to fewer aggregates in the protein phase and weaker gels. The gelation process could be affected positively by the proper concentration of Ca that has effects on the cross-linking between molecules such as hydrogen bonds and hydrophobic interactions [48, 49].
Taghian Dinani, Broekema, et al., [36] found an increase in the tensile stress when IC is added to a 40 wt% PPI.WG mixture containing 1% Ca and processed in the HTSC (120 °C, 15 min, 30 rpm). Therefore, it could be suggested that adding different concentrations of Ca or using different processing conditions for the preparation of protein blends or for the processing of protein blends in the HTSC explains the reduction of the tensile stress for the treatments within the PPI.WG.IC.Ca group compared to our previous study. In summary, the treatments in the PPI.WG.IC group give higher tensile stress parallel to the shear-flow direction, while PPI.WG.IC,A results in similar tensile stresses for both parallel and perpendicular directions to the shear flow in this group.
Water holding capacity (WHC)
One of the most important attributes of the plant-based meat alternatives is the juiciness. The development of juiciness is often related to the WHC of plant-based meat alternatives [18]. Figure 7 depicts that WHC data range from − 1 ± 3.3% for PPI.WG.Ca to 124.3 ± 19.3% for PPI.WG.IC,B75°C-30 min. Although the WHC of PPI.WG.IC,B75°C-30 min treatment was higher than that with all other treatments (p ≤ 0.001), it was not significantly higher (p > 0.05) than those treatments within the PPI.WG.IC group. The WHC values of PPI.WG.IC group treatments were statistically higher (p ≤ 0.001) than WHC values of all other treatments except PPI.WG.IC.Ca,B25°C-30 min. The control, PPI.WG, was not significantly different (p > 0.05) from PPI.WG.Ca treatment. Generally speaking, hydrocolloids can be used as replacers for fat because of their high-water retention and propensity to form soft gels. They increase the viscosity and the plasticity of plant-based meat alternatives [23]. A higher WHC could result from the dry matter holding more water, or less dry matter getting lost from the sample into the water bath [37]. The PPI.WG.Ca exhibited high dry matter loss, and thus even resulted in negative WHC values for some replications.
Water holding capacity (%) values of products with different formulations and preparation procedures processed in the HTSC. In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively. Moreover, the first, second and third axis X lines from bottom to top show the formulations, hydration mixing temperatures (25 or 75 °C), and hydration mixing times (15 or 30 min), respectively. The black letters above data display statistically significant differences (p ≤ 0.001)
PPI.WG.IC yields statistically higher and different WHC (p ≤ 0.001) than PPI.WG, PPI.WG.Ca, and PPI.WG.IC.Ca treatments. J. Zhang et al. [48, 49] and Wang et al. [43] found that a high amount of Ca improves protein–protein interactions and cross-linking. Thus, a lower WHC could be expected for PPI.WG.Ca and PPI.WG.IC.Ca. Finally, insignificant differences (p > 0.05) are found between different mixing times (15 min or 30 min) and temperatures (25 °C and 75 °C) for similar treatments in each PPI.WG.IC and PPI.WG.IC.Ca group.
Microstructure
Confocal laser scanning microscopy (CLSM)
CLSM was performed for the different products described in Table 1 and Sect. "Preparation of different protein blends" at the scale of 100 µm in the shear-flow direction. In Fig. 8, PPI.WG (control) did not show considerable spatial differences in red color intensity. However, adding Ca and IC to the mixtures resulted in much more spatial variation in intensity, indicative of more heterogeneity than PPI.WG. For instance, PPI.WG.Ca contains large and dense agglomerates which are intensely colored. The Ca will result in more cross-linking in WG, and therefore increases the concentration in the WG rich domains. Moreover, as Ca absorbs water by itself, it decreases the availability of water to WG. It is clear from Fig. 8 that the addition of Ca leads to changes in microstructure and promotes the aggregation of spherical particles. For instance, PPI.WG.Ca contained more pseudo spherical structures with high-intensity red color than PPI.WG treatment [48, 49]. described that calcium ions promote the formation of small and dense particles, and they found that the addition of more Ca in a Mesona chinensis polysaccharide–whey protein isolate mixture resulted in the formation of large and dense agglomerates.
The addition of IC changed the product microstructures and increased the differences between high and low-intensity red colors in the pictures compared to PPI.WG. For instance, all PPI.WG.IC group treatments (PPI.WG.IC,A, PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B25°C-30 min, PPI.WG.IC,B75°C-15 min, and PPI.WG.IC,B75°C-30 min), as well as PPI.WG.IC.Ca group treatments (PPI.WG.IC.Ca,A, PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC.Ca,B75°C-15 min, and PPI.WG.IC.Ca,B75°C-30 min), show more or less spherical domains with different sizes with high-intensity red. However, the addition of IC after hydration (PPI.WG.IC,A) led to smaller aggregates with less heterogeneity than the products obtained with the treatments with similar ingredients of PPI, WG and IC but with the addition of IC before PPI hydration (PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B25°C-30 min, PPI.WG.IC,B75°C-15 min, and PPI.WG.IC,B75°C-30 min). The addition of IC before PPI hydration in PPI.WG.IC mixture results in significant absorption of water by IC. Again, the absorption of water by IC decreases the amount of water that is available for WG, therefore increasing the concentration in the WG domains.
The spatially different concentrations of PPI and WG causes differences in red color intensity. The lower fluorescence intensity corresponds to the PPI diluted phase and the higher red color intensity indicates the WG rich domains [30]. Less variation in color intensity was found with PPI.WG.IC group than with PPI.WG.IC.Ca. We suggest that the addition of Ca with IC in the formulations results in an IC network that absorbed more water. This will then reduce the water available to be absorbed by WG.
Comparison of PPI.WG.IC and PPI.WG.IC.Ca groups show that when IC is added to the mixture after PPI hydration, it competes with PPI for absorbing water, reducing the differences between PPI and WG. Thus, less differences in red color intensity with the addition of IC to the PPI.WG.IC,A and PPI.WG.IC.Ca,A are seen in comparison with PPI.WG.IC,B and PPI.WG.IC.Ca,B products, respectively. It is interesting also to notice in Fig. 8 that as an interconnected IC network can be produced with the addition of Ca, PPI.WG.IC.Ca group gives a more interconnected void (black) space which may be IC, air, or water without protein.
Mixing IC at 75 °C for 15 and 30 min in PPI.WG.IC.Ca group (PPI.WG.IC.Ca,B75°C-15 min and PPI.WG.IC.Ca,B75°C-30 min) or in PPI.WG.IC group (PPI.WG.IC,B75°C-15 min and PPI.WG.IC,B75°C-30 min) decreased the high-intensity red color intensity and increased the homogeneity in comparison to mixing at 25 °C for 15 min and 30 min in PPI.WG.IC.Ca (PPI.WG.IC.Ca,B25°C-15 min and PPI.WG.IC.Ca,B25°C-30 min) and PPI.WG.IC (PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B25°C-30 min), respectively. At 75 °C, IC absorbs considerably more water, and, thus, decreases the water absorption by PPI, increasing the homogeneity. In this condition, also the black regions are more in the samples with mixing IC at 75 °C for 15 and 30 min compared to the mentioned samples in PPI.WG.IC and PPI.WG.IC.Ca groups. Black regions could be because of domains with IC hydrocolloid only or elongated air bubbles. An explanation could be that IC absorbs more water and thus, its volume fraction increases and more black color regions in these samples can be observed.
Scanning electron microscopy (SEM)
Figure 9 shows SEM pictures of the described products (Table 1) at magnifications of 250× and 10,000×. At 250× magnification, PPI.WG showed a compact structure with some small globular particles (shown by yellow arrows). PPI.WG.IC products (PPI.WG.IC,A, PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B25°C-30 min, PPI.WG.IC,B75°C-15 min, and PPI.WG.IC,B75°C-30 min) containing IC showed more porous structures compared with PPI.WG. However, there is not a clear orientation. More openings and broken structures are observed in samples with 15 min mixing time of IC at both 25 °C and 75 °C temperatures (PPI.WG.IC,B25°C-15 min and PPI.WG.IC,B75°C-15 min) compared with 30 min mixing time of IC at both temperatures (PPI.WG.IC,B25°C-30 min and PPI.WG.IC,B75°C-30 min, respectively). PPI.WG.Ca contained more globules (yellow arrows) than in PPI.WG. The products in the PPI.WG.IC.Ca group shows similar globules, but more globules are observed by the addition of IC after PPI in PPI.WG.IC.Ca,A. These globules are elongated, but there is still no clear orientation to the shear-flow direction in this picture.
The SEM pictures of products with different formulations and preparation procedures processed in the HTSC In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively. Moreover, orange, blue, green, and pink boxes indicate cauliflower- like, sponge-like, spider web, and combined sponge and spider morphologies, respectively. The red and yellow arrows indicate string and glubules, respectively
A magnification of 10,000× reveals a sort of ‘cauliflower structure’ in PPI.WG (orange boxes) with very compact surface morphology. These structures change into an open sponge and spider web structure (pink box) with the addition of Ca in PPI.WG.Ca. On the other hand, all PPI.WG.IC treatments except PPI.WG.IC,B25°C-30 min (PPI.WG.IC,A, PPI.WG.IC,B25°C-15 min, PPI.WG.IC,B75°C-15 min and PPI.WG.IC,B75°C-30 min) presented a sponge-like structure (blue boxes), and show small spherical entities. This sponge-like structure could be attributed to a continuous phase with dispersed porosity. Grabowska et al., [12] mentioned that such a porous structure is caused by the evaporation of water when the products are prepared. Conversely, PPI.WG.IC,B25°C-30 min, PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B75°C-15 min and PPI.WG.IC.Ca,B75 °C-30 min showed an intermeshed porous structure, which could be defined as a spiderweb structure (green boxes). PPI.WG.Ca, PPI.WG.IC.Ca,A and PPI.WG.IC.Ca,B25°C-30 min showed a combined spider and sponge-like morphology (pink boxes). Therefore, in all treatments containing Ca, the spider web morphology can be observed to different extents. The combination of Ca and IC especially with the addition of IC before hydration (PPI.WG.IC.Ca,B25°C-15 min, PPI.WG.IC.Ca,B25°C-30 min, PPI.WG.IC,B75°C-15 min, and PPI.WG.IC,B75°C-30 min) results in more obvious spider web morphology.
When IC was mixed for a longer time in water (30 min) in PPI.WG.IC,B25 °C-30 min treatment, a denser spiderweb structure was obtained. PPI.WG.IC.Ca,B25°C-15 min showed again larger pores in a clear spider web structure, while with a 30 min mixing time of IC, a combined sponge and spider web morphology with a more compact structure was noticed in PPI.WG.IC.Ca,B25°C-30 min. Similarly, the spider web morphology is more pronounced in PPI.WG.IC.Ca,B75°C-15 min than in PPI.WG.IC.Ca,B75°C-30 min. Therefore, it seems that increasing the mixing time in the PPI.WG.IC.Ca products resulted in a more compact structure.
X-ray microtomography (XRT)
The XRT images in Fig. 10 show the bubble counts and overall air fractions in the processed products (Fig. 11A, B, respectively). A clear orientation and elongation of the air bubbles cannot be seen. The purple pictures show the external dimensions of the samples. The surfaces of PPI.WG and PPI.WG.Ca show a crumpled morphology, with no clear evidence of air bubbles. PPI.WG.IC and PPI.WG.IC.Ca groups only show small cavities on their surface, while PPI.WG.IC and PPI.WG.IC.Ca present a sponge-like external morphology, with PPI.WG.IC.Ca,B75 °C-30 min showing the most extensive cavities.
XRT images of solid part (in the purple rectangular shape) and air bubble distribution (in pink color) of the products with different formulations and preparation procedures processed in the HTSC. In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively
A Bubble numbers (−) and B air fraction (%) of the products with different formulations and preparation procedures processed in the HTSC. In this figure, PPI, WG, Ca, IC, A, and B correspond to pea protein isolate, wheat gluten, calcium chloride, iota carrageenan, after PPI addition, and before PPI addition, respectively. Moreover, the first, second and third axis X lines from bottom to top show the formulations, hydration mixing temperatures (25 or 75 °C), and hydration mixing times (15 or 30 min), respectively. No statistically significant differences were found in the outcomes of both parameters (p ˃ 0.05)
Figures 10 and 11A show that PPI.WG.Ca and PPI.WG are the densest products. Larger bubble numbers and a more uniform dispersion of air bubbles can be observed for all other samples containing IC in both PPI.WG.IC and PPI.WG.IC.Ca groups. It is in agreement with increasing air incorporation measured by XRT in the sheared calcium caseinate after addition of maltodextrin measured by XRT ([44, 45]. However, there are no clear differences between samples with different IC mixing times (15 and 30 min) and temperatures (25 and 75 °C). Furthermore, no clear visual distinction can be made for samples with the addition of IC before or after PPI hydration in both PPI.WG.IC and PPI.WG.IC.Ca groups. The air fraction in Fig. 11B ranges from 4.99 ± 0.83% for PPI.WG to 7.95 ± 2.03% for PPI.WG.IC,B75°C-15 min, without statistical differences (p ˃ 0.05) among the different products. Although the bubble numbers of PPI.WG and PPI.WG.Ca are considerably less than other materials (Fig. 11A, the difference in the total air fraction is much lower; indicating that these materials have on average much larger bubbles or cavities. (Fig. 11B).
Air bubbles have been found to be necessary for producing a more pronounced fibrous structure in case of SPI-based products [4, 5]. Mentioned that anisotropy appears when the deformation of phases occurs, and air bubbles are a separate phase, but Schreuders et al. [30] indicated that the hypothesis of air-enhancing anisotropic structure is not in line with the fiber formation in PPI.WG mixtures. In our study also the sample with the most pronounced fiber structure (PPI.WG.IC.Ca,A in Fig. 4) does not possess the highest bubble numbers and air fraction (Fig. 11), so we agree with Schreuders et al. [30].
Conclusions
We investigated the influence of the addition of iota carrageenan (IC), different preparation procedures and formulations on the morphology of plant-based meat alternatives based on pea protein isolate (PPI) and wheat gluten (WG) processed in the HTSC. Materials were made consisting of PPI and WG with or without CaCl2 (Ca) and with IC before PPI hydration (B), as well as after PPI hydration (A), and were assessed on their micro- and macrostructural properties such as color, WHC, tensile test, and air bubble counts and overall air fraction.
The browning index, WHC, tensile stress, and air bubble numbers increase with the addition of IC. Among all the products, PPI.WG.IC.Ca,A visually shows the best fiber formation. No significant influence was found by the addition order of IC or the hydration time and temperatures of IC. Therefore, adding IC after PPI hydration (in PPI.WG.IC,A and PPI.WG.IC.Ca,A) is the best option for improving the plant-based meat alternatives properties: these formulations, especially PPI.WG.IC,A, requires less preparation time, energy and ingredients for achieving a similar morphology. The prior hydration of IC is not necessary for plant-based meat alternatives preparation in the HTSC.
Data Availability
Data can be provided upon request from the corresponding author.
References
Blakemore WR (2016) Polysaccharide ingredients: carrageenan. Ref Module Food Sci. https://doi.org/10.1016/B978-0-08-100596-5.03251-0
Boukid F (2021) Plant-based meat analogues: from niche to mainstream. European food research and technology. Springer Science and Business Media Deutschland GmbH, Berlin, pp 297–308. https://doi.org/10.1007/s00217-020-03630-9
Cornet SH, Bühler JM, Gonçalves R, Bruins ME, Ruud G, van der Sman M, Jan A, der Goot V (2021) Apparent universality of leguminous proteins in swelling and fibre formation when mixed with gluten. Food Hydrocolloids 120(April):106788. https://doi.org/10.1016/j.foodhyd.2021.106788
Dekkers BL, Boom RM, Van Der Goot AJ (2018) Structuring processes for meat analogues. Trends Food Sci Technol 81:25–36. https://doi.org/10.1016/j.tifs.2018.08.011
Dekkers BL, Hamoen R, Boom RM, van der Goot AJ (2018) Understanding fiber formation in a concentrated soy protein isolate - pectin blend. J Food Eng 222:84–92. https://doi.org/10.1016/j.jfoodeng.2017.11.014
Dekkers BL, Nikiforidis C, van der Goot AJ (2016) Shear-induced fibrous structure formation from a pectin/SPI blend. Innov Food Sci Emerg Technol 36:193–200. https://doi.org/10.1016/j.ifset.2016.07.003
Douglas Goff H, Guo Q (2019) The role of hydrocolloids in the development of food structure. Food Chem Funct Anal 18:1–28
FAO (2009) How to Feed the World in 2050 https://www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf
Fiorentini M, Kinchla AJ, Nolden AA (2020) Role of sensory evaluation in consumer acceptance of plant-based meat analogs and meat extenders: a scoping review. Foods 9(9):1334
Foodvalley NL (2022) Breaking barriers to accelerate the transition. https://www.foodvalley.nl/
Gerelt B, Ikeuchi Y, Nishiumi T, Suzuki A (2002) Meat tenderization by calcium chloride after osmotic dehydration. Meat Sci 60:237–244
Grabowska KJ, Tekidou S, Boom RM, van der Goot AJ (2014) Shear structuring as a new method to make anisotropic structures from soy-gluten blends. Food Res Int 64:743–751. https://doi.org/10.1016/j.foodres.2014.08.010
Twigg J (1983) Vegetarianism and the meanings of meat. The sociology of food and eating. pp 18–30
Janaswamy S, Chandrasekaran R (2002) Effect of calcium ions on the organization of iota-carrageenan helices: an X-ray investigation. Carbohyd Res 337(6):523–535. https://doi.org/10.1016/S0008-6215(02)00017-4
Jia W, Curubeto N, Rodríguez-Alonso E, Keppler JK, Van Der AJ, van der Goot AJ, Van Der AJ (2021) Rapeseed protein concentrate as a potential ingredient for meat analogues. Innov Food Sci Emerg Technol 72:102758. https://doi.org/10.1016/j.ifset.2021.102758
Krintiras GA, Göbel J, Jan A, Goot VD, Stefanidis GD (2015) Production of structured soy-based meat analogues using simple shear and heat in a Couette Cell. J Food Eng 160:34–41. https://doi.org/10.1016/j.jfoodeng.2015.02.015
Krstonosic V, Jovicic-Bata J, Maravic N, Nikolic I, Dokic L (2021) Rheology, structure, and sensory perception of hydrocolloids. Food Struct Funct. https://doi.org/10.1016/B978-0-12-821453-4.00005-3
Kyriakopoulou K, Dekkers B, Van Der Goot AJ (2019) Plant-based meat analogues. Sustainable meat production and processing. Academic Press, pp 103–126. https://doi.org/10.1016/B978-0-12-814874-7.00006-7
Kyriakopoulou K, Keppler JK, van der Goot AJ (2021) Functionality of ingredients and additives in plant-based meat analogues. Foods. https://doi.org/10.3390/foods10030600Academic
Lam ACY, Can Karaca A, Tyler RT, Nickerson MT (2018) Pea protein isolates: Structure, extraction, and functionality. Food reviews international, vol 34. Taylor and Francis Inc, pp 126–147. https://doi.org/10.1080/87559129.2016.1242135
Manski JM, van der Goot AJ, Boom RM (2007) Advances in structure formation of anisotropic protein-rich foods through novel processing concepts. Trends Food Sci Technol 18(11):546–557. https://doi.org/10.1016/j.tifs.2007.05.002
Mcmahon DJ, Paulson B, Oberg CJ (2005) Influence of calcium, pH, and moisture on protein matrix structure and functionality in direct-acidified nonfat mozzarella cheese. J Dairy Sci 88(11):3754–3763. https://doi.org/10.3168/jds.S0022-0302(05)73061-7
Nanta P, Skolpap W, Kasemwong K (2021) Influence of hydrocolloids on the rheological and textural attributes of a gluten-free meat analog based on soy protein isolate. J Food Process Preserv 45(3):e15244. https://doi.org/10.1111/jfpp.15244
Owusu-Ansah YJ, Mc curdy SM (1991) Pea proteins: a review of chemistry, technology of production, and utilization. Food Rev Int 7(1):103–134. https://doi.org/10.1080/87559129109540903
Palanisamy M, Töpfl S, Aganovic K, Berger RG (2018) Influence of iota carrageenan addition on the properties of soya protein meat analogues. LWT Food Sci Technol 87:546–552. https://doi.org/10.1016/j.lwt.2017.09.029
Pan T, Guo H, Li Y, Song J, Ren F (2017) The effects of calcium chloride on the gel properties of porcine myosinek-carrageenan mixtures. Food Hydrocolloids 63:467–477. https://doi.org/10.1016/j.foodhyd.2016.09.026
Rhein-knudsen N, Ale MT, Meyer AS (2015) Seaweed hydrocolloid production: an update on enzyme assisted extraction and modification technologies. Mar Drugs 13(6):3340–3359. https://doi.org/10.3390/md13063340
Samard S, Ryu GH (2019) A comparison of physicochemical characteristics, texture, and structure of meat analogue and meats. J Sci Food Agric 99:2708–2715. https://doi.org/10.1002/jsfa.9438
Schlangen M, Ribberink MA, Taghian Dinani S, Sagis LMC, van der Goot AJ (2023) Mechanical and rheological effects of transglutaminase treatment on dense plant protein blends. Food Hydrocolloids 136:108261. https://doi.org/10.1016/j.foodhyd.2022.108261
Schreuders FKG, Dekkers BL, Bodnár I, Erni P, Boom RM, van der Goot AJ (2019) Comparing structuring potential of pea and soy protein with gluten for meat analogue preparation. J Food Eng 261(July):32–39. https://doi.org/10.1016/j.jfoodeng.2019.04.022
Schreuders FKG, Schlangen M, Bodnar I, Erni P, Boom RM, Van Der Goot AJ (2022) Structure formation and non-linear rheology of blends of plant proteins with pectin and cellulose. Food Hydrocolloids 1(124):107327. https://doi.org/10.1016/j.foodhyd.2021.107327
Scialabba NE (2022) Livestock and future food supply scenarios. Managing healthy livestock production and consumption. Academic Press, Rome
Shand PJ, Ya H, Pietrasik Z, Wanasundara PKJPD (2007) Physicochemical and textural properties of heat-induced pea protein isolate gels. Food Chem 102:1119–1130. https://doi.org/10.1016/j.foodchem.2006.06.060
Shi D, Nickerson MT (2022) Comparative evaluation of the functionality of faba bean protein isolates with major legume proteins in the market. Cereal Chem 99(6):1246–1260. https://doi.org/10.1002/cche.10589
Singh A, Sit N (2022) Meat analogues: types, methods of production and their effect on attributes of developed meat analogues. Food and bioprocess technology, vol 15. Springer, pp 2664–2682. https://doi.org/10.1007/s11947-022-02859-4
Taghian Dinani S, Broekema NL, Boom R, van der Goot AJ (2023) Investigation potential of hydrocolloids in meat analogue preparation. Food Hydrocolloids 135:108199. https://doi.org/10.1016/j.foodhyd.2022.108199
Taghian Dinani S, Hamdami N, Shahedi M, Keramat J (2014) Optimization of carboxymethyl cellulose and calcium chloride dip-coating on mushroom slices prior to hot air drying using response surface methodology. J Food Process Preserv 38(3):1269–1278. https://doi.org/10.1111/jfpp.12088
Taghian Dinani S, Hasić M, Auer M, Kulozik U (2020) Assessment of uniformity of microwave-based heating profiles generated by solid-state and magnetron systems using various shapes of test samples. Food Bioprod Process 124:121–130. https://doi.org/10.1016/j.fbp.2020.08.013
Taghian Dinani S, van der Harst JP, Boom R, van der Goot AJ (2023) Effect of l-cysteine and l-ascorbic acid addition on properties of meat analogues. Food Hydrocoll. https://doi.org/10.1016/j.foodhyd.2022.108059
Taghian Dinani S, Havet M (2015) Effect of voltage and air flow velocity of combined convective-electrohydrodynamic drying system on the physical properties of mushroom slices. Indust Crops Prod 70:417–426. https://doi.org/10.1016/j.indcrop.2015.03.047
Teodorowicz M, van Neerven J, Savelkoul H (2017) Food processing: the influence of the maillard reaction on immunogenicity and allergenicity of food proteins. Nutrients. https://doi.org/10.3390/nu9080835
Trius A, Sebranek JG, Lanier T (1996) Carrageenans and their use in meat products. Crit Rev Food Sci Nutr 36:69–85. https://doi.org/10.1080/10408399609527719
Wang Y, Zhou Y, Wang X, Li P, Xu B, Chen C (2020) Water holding capacity of sodium-reduced chicken breast myofibrillar protein gel as affected by combined CaCl2 and high- pressure processing. Int J Food Sci Technol 55:601–609. https://doi.org/10.1111/ijfs.14313
Wang Z, Dekkers BL, Boom R, van der Goot AJ (2019) Maltodextrin promotes calcium caseinate fibre formation through air inclusion. Food Hydrocolloids 95:143–151. https://doi.org/10.1016/j.foodhyd.2019.04.028
Wang Z, Tian B, Boom R, van der Goot AJ (2019) Air bubbles in calcium caseinate fibrous material enhances anisotropy. Food Hydrocolloids 87:497–505. https://doi.org/10.1016/j.foodhyd.2018.08.037
Yuliarti O, Kiat Kovis TJ, Yi NJ (2021) Structuring the meat analogue by using plant-based derived composites. J Food Eng 288:110138. https://doi.org/10.1016/j.jfoodeng.2020.110138
Zeece M (2020) Food additives. Introd Chem Food. https://doi.org/10.1016/B978-0-12-809434-1.00007-4
Zhang J, Jiang L, Yang J, Chen X, Shen M, Yu Q, Chen Y, Xie J (2022) Effect of calcium chloride on heat-induced Mesona chinensis polysaccharide-whey protein isolation gels: Gel properties and interactions. LWT 155:112907. https://doi.org/10.1016/j.lwt.2021.112907
Zhang X, Zhang S, Zhong M, Qi B, Li Y (2022) Soy and whey protein isolate mixture / calcium chloride thermally induced emulsion gels : Rheological properties and digestive characteristics. Food Chem 380:132212. https://doi.org/10.1016/j.foodchem.2022.132212
Acknowledgements
This study is part of the project PlantPromise, co-financed by Top Consortium for Knowledge and Innovation Agri & Food by the Dutch Ministry of Economic Affairs. The authors would like to thank Jarno Gieteling, Jos Sewalt, Maurice Strubel, Wouter de Groot, Martin de Wit, Jelmer Vroom, and MaaikeNieuwland for their valuable technical support.
Funding
This research work was partially funded by PlantPromise, co-financed by Top Consortium for Knowledge and Innovation Agri & Food by the Dutch Ministry of Economic Affairs.
Author information
Authors and Affiliations
Contributions
STD: Term, Conceptualization, Formal analysis, Validation, Visualization, Supervision, Writing—Review & Editing; MFCC: Investigation, Formal analysis, Validation, Visualization, Writing—Original Draft; RB: Supervision, Funding acquisition, Writing—Review & Editing; AJG: Term, Conceptualization, Supervision, Funding acquisition, Writing—Review & Editing.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that there is no conflict of interest.
Compliance with ethics Requirements
Not available.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Taghian Dinani, S., Charles Carrillo, M.F., Boom, R. et al. Quality improvement of plant-based meat alternatives by addition of iota carrageenan to pea protein–wheat gluten blend. Eur Food Res Technol 249, 1637–1654 (2023). https://doi.org/10.1007/s00217-023-04244-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04244-7