Abstract
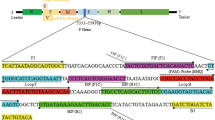
We established and validated an improved loop-mediated isothermal amplification (LAMP) method combined with a TaqMan probe (LAMp-Taqman method) for detection of Mycoplasma gallinis (MG). A test plasmid was constructed using a conserved sequence of the specific gene vlhA of MG, and the LAMP primer set was screened according to the conservative gene design. The loop primer modified by fluorescent/quenching dyes was used as the TaqMan probe to establish the LAMP-TaqMan detection method. The feasibility and application value of LAMP in detecting MG nucleic acids were evaluated by parallel testing using real-time fluorescence quantitative qPCR as a control. The study results showed that LAMP-TaqMan had good specificity in detecting MG nucleic acids. The optimal detection temperature was 63 ºC, and the linear correlation coefficient R2 of eight tenfold serial dilutions (2.607 × 106 copies/µL–2.607 × 10−1 copies/µL) was 0.995; the minimum detection limit was 2.607–1 copies/µL, and the detection rate of the minimum detection limit was 100%. The high-, medium-, and low-concentration test plasmids were tested for repeatability (the coefficients of variation (CVs) were 0.202%, 1.770%, and 3.771%, respectively). Parallel tests were conducted on 120 samples collected from pairs of chickens, the accuracy, sensitivity and specificity of TP-LAMP were 97.50%, 100.00% and 96.30%, respectively. The detection time is 1 h, in fact, test results can be obtained in 45 min. The LAMP-TaqMan isothermal nucleic acid detection method not only maintains the effect of the loop primer to accelerate amplification but also offers fast and simple detection, a low detection limit, and a low rate of false positives; it has a broad market application prospect in the accurate and rapid detection of poultry diseases.







Similar content being viewed by others
Data availability
The datasets generated during the current study are available from the corresponding author upon reasonable request.
References
Hakeem W, Farran MT, Shaib HA et al (2021) Does methionine enhance immunity in Mycoplasma gallispeticum F strain vaccinated broiler breeder pullets? J Appl Poult Res 30(3):100172
Javed MA, Salvatore Frasca J, Rood D et al (2005) Correlates of immune protection in chickens vaccinated with Mycoplasma gallisepticum strain GT5 following challenge with pathogenic M. gallisepticum strain rlow. Infect Immun 73(9):5410–5419
Liu M, Luo Y, Tao R et al (2009) Sensitive and rapid detection of genetic modified soybean (roundup ready) by loop-mediated isothermal amplification. Biosci Biotechnol Biochem 73(11):2365–2369
Kusum S, Reema B, Aman S et al (2014) Loop-mediated isothermal amplification for rapid diagnosis of tubercular uveitis. Jama Ophthalmol 132(132):777–778
Scheel CM, Zhou Y, Theodoro RC et al (2014) Development of a loop-mediated isothermal amplification method for detection of histoplasma capsulatum DNA in clinical samples. J Clin Microbiol 52(2):483–488
Hassan M, Vittal R, Raj JM et al (2022) Loop-mediated isothermal amplification (LAMP): a sensitive molecular tool for detection of Staphylococcus aureus in meat and dairy product. Braz J Microbiol 53(1):341–347
Hara-Kudo Y, Konishi N, Ohtsuka K et al (2008) Detection of verotoxigenic Escherichia coli O157 and O26 in food by plating methods and LAMP method: a collaborative study. Int J Food Microbiol 122(1–2):156–161
Pang B, Yao S, Xu K et al (2019) A novel visual-mixed-dye for LAMP and its application in the detection of foodborne pathogens. Anal Biochem 574:1–6
Zhu L, Xu Y, Cheng N et al (2017) A facile cascade signal amplification strategy using DNAzyme loop-mediated isothermal amplification for the ultrasensitive colorimetric detection of Salmonella. Sens Actuators, B Chem 242:880–888
Notomi T, Okayama H, Masubuchi H et al (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28(12):E63
Azizi M, Zaferani M, Cheong SH et al (2019) Pathogenic bacteria detection using RNA-based loop-mediated isothermal-amplification-assisted nucleic acid amplification via droplet microfluidics. Acs Sensors 4(4):841–848
Kokkinos PA, Ziros PG, Bellou M et al (2014) Loop-mediated isothermal amplification (LAMP) for the detection of salmonella in food. Food Anal Methods 7:512–526
Mori Y, Notomi T (2009) Loop-mediated isothermal amplification (LAMP): a rapid, accurate, and cost-effective diagnostic method for infectious diseases. J Infect Chemother 15(2):62–69
Dickinson M, Hodgetts J (2013) In-field diagnostics using loop-mediated isothermal amplification. Methods Mol Biol 938:291–300. https://doi.org/10.1007/978-1-62703-089-2_25
Patel JC, Lucchi NW, Srivastava P et al (2014) Field evaluation of a real-time fluorescence loop-mediated isothermal amplification assay, RealAmp, for the diagnosis of malaria in Thailand and India. J Infect Dis 8:1180–1187
Njiru ZK, Büscher P (2012) Loop-mediated isothermal amplification technology: towards point of care diagnostics. PLoS Negl Trop Dis 6(6):e1572
Fang X, Liu Y, Kong J et al (2010) Loop-mediated isothermal amplification integrated on microfluidic chips for point-of-care quantitative detection of pathogens. Anal Chem 82(7):3002
Safavieh M, Kanakasabapathy MK, Tarlan F et al (2016) Emerging loop-mediated isothermal amplification-based microchip and microdevice technologies for nucleic acid detection. ACS Biomater Sci Eng 2(2):278
Mori Y, Nagamine K, Tomita N et al (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Biophys Res Commun 289(1):150–154
Gray CM, Katamba A, Narang P et al (2016) Feasibility and operational performance of tuberculosis detection by loop-mediated isothermal amplification platform in decentralized settings: results from a multicenter study. J Clin Microbiol 54(8):1984–1991
Nagamine K, Hase T, Notomi T (2002) Accelerated reaction by loop-mediated isothermal amplification using loop primers. Mol Cell Probes 16(3):223–229
Parida M, Horioke K, Ishida H et al (2005) Rapid detection and differentiation of dengue virus serotypes by a real-time reverse transcription-loop-mediated isothermal amplification assay. J Clin Microbiol 43(6):2895–2903
Goto M, Honda E, Ogura A et al (2009) colorimetric detection of loop- mediated isothermal ampli cation reaction by using hydroxy naphthol blue. Biotechniques 46(3):167–172
Mahony J, Chong S et al (2013) Development of a sensitive loop-mediated isothermal amplification assay that provides specimen-to-result diagnosis of respiratory syncytial virus infection in 30 minutes. J Clin Microbiol 51(8):2696–2701
Imai M, Ai N, Minekawa H et al (2007) Rapid diagnosis of H5N1 avian influenza virus infection by newly developed influenza H5 hemagglutinin gene-specific loop-mediated isothermal amplification method. J Virol Methods 141(2):173–180
Zhang X, Xu G, Tang H et al (2017) Development of loop-mediated isothermal amplification (LAMP) assay for the rapid detection of Alternaria alternata. J AOAC Int 100(1):99–103
Wen H, Hao Z, Xu J et al (2017) Loop-mediated isothermal amplification method for the rapid detection of Ralstonia solanacearum phylotype I mulberry strains in China. Front Plant 8:76
Lucchi NW, Gaye M, Diallo MA et al (2016) Evaluation of the illumigene malaria LAMP: a robust molecular diagnostic tool for malaria parasites. Sci Rep 6:36808
Hirayama H, Kageyama S, Takahashi Y et al (2006) Rapid sexing of water buffalo (Bubalus bubalis) embryos using loop-mediated isothermal amplification. Theriogenology 66(5):1249–1256
Funding
The work described in this paper was fully supported by a grant from the High-level Science and Technology Innovation and Entrepreneurship Talent Project of Shijiazhuang (No. 05202001).
Author information
Authors and Affiliations
Contributions
YL developed the TaqMan-LAMP method and prepared the original draft; YZ and MW treated the samples; ZD, LY and LS edited the manuscript; and GS acquired funding. All the authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Zhang, Y., Wang, M. et al. Modified loop-mediated isothermal amplification method combined with a TaqMan probe for the detection of Mycoplasma gallisepticum. Eur Food Res Technol 249, 1469–1477 (2023). https://doi.org/10.1007/s00217-023-04226-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-023-04226-9