Abstract
The aim of the paper was to determine the potential of using grape pulp, marc and must in the beer production process. Samples were fermented using non-Saccharomyces yeasts (Dekkera bruxellensis 3429, Metschnikowia pulcherrima MG970690), while Saccharomyces cerevisiae Safale US-05 was used as a control. Grape marc was obtained by pressing grape must. The grape marc, must and pulp were pasteurized and, together with wort, volumetrically introduced into fermentation flasks for fermentation. Mass changes taking place during the process were analyzed. Real extract, alcohol content, free amino nitrogen (FAN) content, titratable acidity, pH, color, organic acid profile and content of sugars were determined in obtained beers. The addition of grape marc, must and pulp increased the value of most of the tested parameters. It did not adversely affect the fermentation process. This offers the possibility of using grape marc, must and pulp in the brewing industry, even with the use of non-Saccharomyces yeast monocultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The modern beer market offers a variety of different types of beers and is subject to constant dynamic changes. Consumers are resigning from high-percentage alcohols in favor of consuming beer with a characteristic taste and aroma. Not only classic beers are popular, but also those containing unusual ingredients. Use of distinctive additions or unconventional yeasts in the beer production process improves taste and quality and enriches the nutritional value of the product. The increasing demand for non-classic beers causes constant competition among manufacturers to produce innovative products. One idea, which has arisen out of it is cooperation of beer and wine enthusiasts, is the production of beers with the addition of wine or grape must. The production is aimed at combining the advantages of both drinks. Such beer-wine hybrids can be an attractive option among new generation beers.
In recent years, the role of yeast strains in beer production has been increasingly acknowledged. They are responsible for metabolism of carbohydrates and production of ethyl alcohol, carbon dioxide, as well as higher alcohols, aldehydes, esters, carboxylic compounds and organic acids in the fermentation process. The aforementioned reaction products determine the quality and aroma-taste profile of obtained beers.
The wine industry in Poland is constantly developing. Favorable climate changes and increasing consumption of wine are constantly increasing its production. This contributes to the generation of a large amount of waste in the form of grape marc [1]. It is used to produce bioethanol, alcohol for cosmetic and pharmaceutical applications or as an animal feed additive. Grape marc includes grape peel, pulp damaged cells and seeds. Its addition can enrich beer with tannins, dietary fiber, polysaccharides, dyes and aroma and taste compounds [2]. Production of beers with such an addition offers a new possibility to use the waste. It can also contribute to the prevention of economic losses to the wine industry.
The aim of the paper was to determine potential of using grape pulp, marc and must in the beer production process. Samples were fermented using non-Saccharomyces yeasts (Dekkera bruxellensis 3429, Metschnikowia pulcherrima MG970690), while Saccharomyces cerevisiae Safale US-05 was used as a control. Unconventional strains were selected based on their natural occurrence in certain alcoholic fermentations and their prior use in winemaking. Grape marc was obtained by pressing grape must. Subsequently, the grape marc, must and pulp were pasteurized and, together with wort, volumetrically introduced into fermentation flasks for fermentation. Mass changes taking place during the process were analyzed. Real extract, alcohol content, free amino nitrogen (FAN) content, titratable acidity, pH, color, organic acid profile and content of sugars were determined in obtained beers.
Materials and methods
Materials
The yeast strains Saccharomyces cerevisiae Safale US-05, Dekkera bruxellensis 3429 and Metschnikowia pulcherrima MG970690 from the own collection of the Department of Fermentation Technology and Microbiology of the University of Agriculture in Kraków were used in the study. To make the Polish Pale Ale style wort the malts Strzegom Plizneński (Viking Malt), Abbey Malt (Weyermann) and Caramel Red (Viking Malt) as well as the hop pellets Iunga PL 2019 (10% alfa-acids) and Crystal US 2017 (3% alfa-acids) were used. The red grape variety Leon Millot from the Goja vineyard in Burów was used in the study.
Preparation of wort, grape marc, must and pulp
Wort was prepared by heating 35 L of water to the temperature of 66 °C and subsequently adding 10 kg of the Strzegom Pilzneński (Viking Malt) light malt, 0.5 kg of the Abbey Malt (Weyermann) caramel malt and 0.5 kg of red caramel malt (Viking Malt). Mash was kept at the temperature of 64 °C for 60 min. The temperature was then raised to 77 °C and the mash continued to be kept at such temperature for 1 min. An iodine test was performed to determine if all starch had been saccharified. To complete the mashing process, the wort was heated to the temperature of 78 °C and kept at it for 10 min. The mash was then transferred to a filter tank and left to develop a layer of spent grain. Subsequently the mash was filtered with liquor of 68 °C, yielding 80 L of wort. Then, the wort was boiled for an hour. 50 g of the Iunga PL 2019 (10% alfa-acids) hop pellets and 50 g of the Crystal US 2017 (3% alfa-acids) hop pellets were added in the beginning of boiling and 10 min prior to the end of it, respectively, to obtain an appropriate degree of hopping (approximately 30 IBU). After boiling, the wort was left to cool down (extract 11.4°P). Grape marc was obtained by pressing grape must and it was subsequently pasteurized (100 °C, 15 min) together with must and pulp.
Inoculation and fermentation
Pure yeast cultures were passaged in triplicate. In the first stage, the strains were grown on the Sabouraud agar (Biocorp, Poland) slants for 24 h. Then, the strains were transferred to 10 mL of Sabouraud Broth (Biocorp, Poland). After another 24 h dynamic propagation of the strains was conducted in 200 mL of Sabouraud Broth (Biocorp, Poland) on a water bath shaker (120 rpm, 20 °C) for 48 h. After the growth process, the dry yeast mass was determined on a moisture analyzer and an appropriate amount of yeast slurry was centrifuged (10 min, 4989 × g/min). Sediment obtained from centrifugation of the yeast slurry was washed with sterile water, centrifuged again under the same conditions and introduced to wort and wort with an addition of grape marc, must and pulp.
The basic raw material for fermentation was wort (extract 11.4°P, 30 IBU) and wort with the addition of grape marc, must and pulp. The samples of 0.3 L were fermented in 0.5 L glass flasks. The wort and appropriate volumes of grape marc, must and pulp were introduced into them (according to the variants below). The yeast slurry was introduced in an amount of 0.5 g d.w./L. The S. cerevisiae Safale US-05 yeast was used as a control. After carefully closing the flasks and attaching fermentation tubes filled with glycerin, the system was additionally sealed with parafilm. The fermentation process was conducted for 14 days at the temperature of 20 °C.
The fermentation was conducted using the yeast strains Saccharomyces cerevisiae Safale US-05, Dekkera bruxellensis 3429 and Metschnikowia pulcherrima MG970690 in the following variants (each sample in triplicate): wort; wort + 20% addition of grape marc/must/pulp; wort + 40% addition of grape marc/must/pulp.
Methods
Determination of fermentation dynamics
The fermentation rate was determined on the basis of a mass loss of samples weighted every 24 h with 0.01 g accuracy. Results from three independent repetitions were presented as a percentage loss of the fermentation media mass.
Determination of real extract and alcohol content
Alcohol concentration in final beer was determined using the pycnometric method. For this purpose, the sample after fermentation was distilled. The obtained distillate was filled up to 100 g with distilled water, its density was determined and the concentration of ethanol was read from the adequate tables (Analytica EBC Methods 9.2.1, Analytica EBC Methods 9.4), (Analytica EBC, European Brewery Convention, 1998).
Determination of titratable acidity
The potentiometric method was applied to determine titratable acidity, titrating a sample with 0.1 M NaOH solution to obtain pH = 8.
Determination of FAN content
Free amino nitrogen (FAN) was measured using ninhydrin-based methods with the use of the absorbance measurement at 570 nm (Beckman DU-650 UV–Vis) according to the method: 8.10 Free Amino Nitrogen in wort by Spectrophotometry (IM) (Analytica EBC, European Brewery Convention, 1998).
Determination of color
The color of the filtered samples was determined spectrophotometrically (Beckman DU-650 UV–Vis) at a wavelength of 430 nm (according to Analytica EBC Methods 8.5 and Analytica EBC Methods 9.6).
Determination of organic acids
Organic acids analysis was carried out on a Perkin–Elmer (USA) FLEXAR chromatograph equipped with a pump system, and a UV/Vis (monitored at 210 nm). Malic, tartaric, succinic, lactic, citric and acetic acids (Sigma-Aldrich) were determined using Rezex ROA-Organic Acid Aminex HPX-87H (300 mm, 18 cm × 7.8 mm). Samples were eluted isocratically at 40 °C with a mobile phase (0.005 mol/L H2SO4) at a flow rate of 0.4 mL/min.
Determination of content of sugars (HPLC)
Analyses were performed using a Shimadzu (Japan) NEXERA XR with an RF-20A refractometric detector. The separation was performed on a Asahipak NH2P-50 250 × 4.6 mm Shodex column (Showa Denko Europe, Germany), thermostated at 30 °C. The mobile phase was an aqueous solution of acetonitrile (70%), while the isocratic elution profile (0.8 mL/min) lasted for 20 min. For quantitative determination, standard curves were prepared for the respective sugars: fructose, glucose, sucrose, maltose and glycerol.
Statistical analysis
The results have been presented as the arithmetic mean of three repetitions, standard deviation included. Moreover, a repeated measures ANOVA and a Tukey`s (HSD) multiple range test at the significance level of α = 0.05 have been performed.
Results and discussion
Characteristics of wort and wort with the addition of grape marc, must and pulp
The tested wort had a pH of 5.89 (Table 1). The obtained result differs from literature data, according to which the value of this parameter should be at the level of 5.4–5.6 [3]. Introduction of grape marc and grape must into the wort caused a decrease in pH. However, the low results for the variants with additions did not inhibit the adaptation of yeasts to the environment, which is shown in the graphs of fermentation kinetics (Fig. 1).
The values of titratable acidity for the samples increased along with the addition of grape marc, must and pulp. The tested wort contained 107 mg/L of free amino nitrogen (Table 1), which is within the range of the recommended amount of FAN, i.e., 100–140 mg/L, for the proper growth and development of yeast [4]. The values of free amino nitrogen and color increased after addition of grape marc and grape pulp to wort (except for the samples with the addition of grape must) (Table 1). The darkening of the color of the samples with 20% and 40% addition of grape marc and pulp was caused by the introduction of dyes naturally occurring in grapes.
Fermentation dynamics
The yeast’s ability to utilize sugars largely determines the fermentation rate as well as the final quality of the beer produced. The efficiency of the process depends, inter alia, on the content of the substrates in wort needed for the proper growth of yeast. These include elements, such as free amino nitrogen, phosphorus, potassium and magnesium in an absorbable form. The pH and temperature of the process are also important [5].
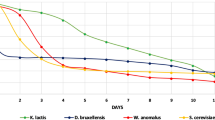
The data presented in Fig. 1a show the differences in the fermentation process of different yeast strains. The yeast strains S. cerevisiae Safale US-05 and D. bruxellensis 3429 were characterized by the shortest and similar adaptation time to the environmental conditions in the wort. In both cases there was a sharp decrease in the mass of the samples. Both strains probably utilized most of the sugars within 6 days after inoculation. However, in the case of the yeast strain D. bruxellensis 3429, there was another decrease in the mass on the ninth day of the fermentation process. In comparison with the first two yeast strains, M. pulcherrima MG970690 needed more time to adapt to the new environment and had a much slower fermentation rate. The fermentation process of the yeast strain was the most linear. This suggests that there was a gradual decrease in the mass caused by slower metabolism of sugars.
The data in Fig. 1b show that the fermentation process of the samples with the addition of grape marc and control samples (Fig. 1a) was similar. In the case of beers obtained with the use of D. bruxellensis 3429 and S. cerevisiae Safale US-05 there was a significant mass loss already on the second day after inoculation, which indicated the beginning of fermentation. Then, from the fourth day, the sample mass loss was slight for both strains. In the case of M. pulcherrima MG970690, however, the time of adaptation to the environment was longer, and the fermentation process started on the fourth day after inoculation. A period of turbulent fermentation and more pronounced decrease in the mass of the samples were observed for the M. pulcherrima MG970690 strain, which is different compared to the beers without the addition of grape marc (Fig. 1a). All strains showed a similar mass loss from the seventh day onward. Despite the increased addition of grape marc (40%), a similar fermentation rate was observed. The total mass loss was just slightly greater than in the variant with the addition of 20% of grape marc (Fig. 1b). According to studies by Blomqvist et al. [6], the strain D. bruxellensis, compared to S. cerevisiae, is characterized by a slower rate of growth and consumption of sugars, as well as greater or equal mass loss. The data presented in Fig. 1a, b confirm the information contained in the literature. The difference in fermentation rate between D. bruxellensis 3429 and S. cerevisiae Safale US-05 was slight. The aforementioned authors also noted in their research that in the case of using glucose as the carbon source the growth of yeast and alcohol production were faster than when using maltose. Both S. cerevisiae and D. bruxellensis have variable maltose fermentation abilities. The addition of grape marc introduced naturally occurring simple sugars to the samples. The increased amount of these compounds could contributed to the earlier occurrence of the fermentation process, which is shown in Fig. 1b. On the other hand, in all samples produced with the use of M. pulcherrima MG970690 a longer adaptation time and thus a delayed occurrence of mass loss was observed compared to the other two strains. The obtained results are consistent with literature data saying that M. pulcherrima is characterized by weak fermentation activity [7]. Yeasts not belonging to the genus Saccharomyces show a similar ability of fermentation and utilization of ingredients contained in wort as S. cerevisiae. However, these processes may take more time, especially for the strain M. pulcherrima. The addition of grape marc does not interfere with the fermentation process, and even contributes to the creation of conditions to which yeasts adapt faster.
In the samples with the addition of grape must (20%), the yeasts that adapted the fastest to the environment were D. bruxellensis 3429 which started fermenting already on the day of inoculation, causing a sharp decrease in the mass of the samples that continued until the fourth day (Fig. 1c). Then, the mass loss of the samples was minimal, up to 0.5%. For S. cerevisiae Safale US-05 a significant decrease in the mass occurred from the first to the fifth day, after which silent fermentation began. The species that adapted the least was M. pulcherrima MG970690. It started turbulent fermentation between the first and the second day after inoculation and used up available sugars relatively quickly (Fig. 1c). Fermentations of the samples with a 40% addition of grape must obtained a higher degree of attenuation than the samples with a 20% addition of must (Fig. 1c), which may be related to the greater availability of fermentable sugars derived from grape must. The increased percentage content of grape must thus caused a slight extension of the duration of turbulent fermentation for M. pulcherrima MG970690. The strain adapted to the environment the least. Only after the second day it started fermenting. The fermentation process in this case was rather mild than turbulent and also the longest among the tested yeasts. The yeast M. pulcherrima MG970690 can be used in the fermentation of non-alcoholic/low alcohol or fruit beers, e.g., based on must. In the studies of Meneghin et al. [8] it was found that S. cerevisiae fermented faster than D. bruxellensis, which was confirmed by the obtained results (Fig. 1a).
Figure 1d shows the fermentation process of the samples with 20% and 40% grape pulp addition. As in other variants (Fig. 1a–c), it was observed that the species that showed the fastest adaptation to the environment was D. bruxellensis 3429 (Fig. 1d). These yeasts started a turbulent fermentation already on the first day and it continued until the fifth day. Then, the process slowed down and the sample mass fluctuations were slight. The slowest fermentation process was recorded for M. pulcherrima MG970690 (Fig. 1d).
Despite the different adaptation times to the environment, all the tested yeasts fermented satisfactorily. This shows that both S. cerevisiae Safale US-05 as well as non-Saccharomyces strains are suitable for the fermentation of beer to the lesser or greater extent.
Alcohol and real extract content
Alcohol content
The most important stage in the production of alcoholic beverages is fermentation. During this process complex biochemical reactions take place that produce alcohol, CO2, as well as other metabolic by-products. The most important compound among alcohols is ethanol. It affects the texture of beer, improves its viscosity and also makes the foam more stable. In addition, ethanol is important in creating the taste of the beverage and is a precursor to some esters [9]. The effectiveness of yeast strains in performing alcoholic fermentation depends on their ability to use the sugars present in wort [5].
Depending on the alcohol content, beers are classified as non-alcoholic (up to 0.5% vol.), low alcohol (0.5–1.5% vol.), light (up to 3.5% vol.), regular (3.5–5% vol.) and strong (over 5% vol.) [10]. The attenuated wort exhibited an alcohol content of 4.11–4.55% vol. (Table 2), thus corresponding to the parameters of regular beer. The values obtained and showed in Table 2 indicate a relatively high alcohol content in the analyzed beers. The addition of grape marc, pulp and must contributed to the increase of this parameter. It was probably caused by the introduction of more sugars that the yeast could utilize during the fermentation process. The increase in alcohol content depended on the amount of added raw materials and ranged from 1% to 2.5% (v/v) (Table 2). The amount of alcohol produced in beers by S. cerevisiae Safale US-05, D. bruxellensis 3429 and M. pulcherrima MG970690 strains was comparable (Table 2). The obtained results are consistent with literature data. During fermentation processes, the strain D. bruxellensis were capable of producing the same or even slightly higher alcohol concentrations as S. cerevisiae [6]. It was also found that during fermentation using monocultures M. pulcherrima there was usually up to 4.5% (v/v) of ethanol produced [11], which was confirmed by our tests (control sample, Table 2).
Real extract content
Real extract consists of all substances soluble in water. These mainly include carbohydrates, nitrogen compounds, minerals, and glycerol. After fermentation, the extract content in beer lowers, because some of the sugars are processed by the yeast [3].
The data in Table 2 show that the yeasts S. cerevisiae Safale US-05 produced lower amount of extract in beers, compared to the non-Saccharomyces strains. The only exception were the samples with 40% addition of grape marc. In their case beers obtained with the yeasts D. bruxellensis 3429 exhibited lower extract content (Table 2). Due to the presence of ß-D-glucosidase, D. bruxellensis has the ability to metabolize complex sugars [12]. However, the results obtained for D. bruxellensis 3429 do not give extract values low enough for proving high enzymatic activity and increased utilization of carbohydrates. The results obtained indicate that the non-Saccharomyces yeasts exhibit similar utilization abilities for sugars contained in wort and delivered with grape marc. The values of this parameter did not exceed 60 g/L (Table 2) for any of the obtained samples. The addition of grape marc, pulp and must contributed to an increase in the alcohol content and a decrease in the real extract content in the tested samples. The obtained values confirm the changes in the mass of the samples presented in Fig. 1. Contrary to popular belief, unconventional yeasts have a similar, and in some cases even higher, ethanol production capacity.
Titratable acidity and pH
Titratable acidity
There are numerous types of acids in beer that are considered the main flavor chemicals. They are classified as organic and inorganic acids. To a greater or lesser extent they come from raw materials used, compounds produced in the fermentation process and substances released after yeast autolysis. Organic acids are also abundant in grapes and include mainly tartaric, malic and citric acids. Such acids also include lactic, succinic and acetic acids, which are formed during the alcoholic fermentation of grape must. The content of organic acids determines the acidity of the finished product [9].
Table 2 clearly shows that the introduction of grape marc, pulp and must into beers resulted in an increase in titratable acidity. This may suggest that such an addition contributes naturally occurring organic acids as well as provides yeasts with more substrates. As a result, production of acid compounds both from substances in wort and from grapes increases. In the variants without any addition and those containing grape pulp and 20% of grape must, the lowest acidity value was observed for S. cerevisiae Safale US-05 compared to the other two yeast strains. The highest acidity value was observed in the samples produced by the strain M. pulcherrima MG970690 (Table 2). It may indicate different abilities of these yeasts to produce acidic metabolites. Literature describes some studies on musts fermented using the strain M. pulcherrima in which there was a slight reduction in acidity compared to S. cerevisiae used as a control. The acidity of wines is determined by the ratio of tartaric acid to malic acid. M. pulcherrima exhibits an ability to degrade the latter, which influences the acidity value. As to acetic acid, both species produce similar amounts [11]. In limited oxygen supply D. bruxellensis produces little or no acetic acid. It also exhibits the ability to convert tartaric acid into lactic acid, which may also reduce acidity [6]. However, in our study, no considerable differences in titratable acidity were found for beers attenuated with D. bruxellensis 3429 compared to other strains (Table 2). The results obtained show that non-Saccharomyces yeasts exhibit similar capacity to produce acid compounds as the strain S. cerevisiae Safale US-05.
pH
In the brewing industry pH of wort greater than 5.2 is desirable as it causes a strong extraction of hop tannins. It also contributes a sustained and intense bitterness. A slightly acidic pH favors creating appropriate conditions for yeast. It also affects the final color and taste of the product [3, 13]. Grapes are rich in organic acids and their pH is much lower than that of traditional beers (4.3–4.6). In beer wort, the pH drops during the fermentation process and then remains constant [2]. The results obtained for beers without the addition of grapes confirm the above statement. In these samples a decrease in pH was observed as compared to the non-fermented samples. On the other hand, in the variants with the addition of grape marc, pulp and must the value of this parameter increased (Tables 1, 2). The obtained results are similar to the values obtained for fruit beers analyzed by Nardini and Garaguso [14] in which pH was within the range of 3.56–4.87. The value of this parameter for ale type beer with the addition of 200 g of grapes per 1 L of wort was 4.02. The obtained results may be influenced by different yeast abilities to utilize nitrogen compounds or to produce acidic metabolites. It should be noted that a low pH can prevent the growth of undesirable microorganisms, which is an additional advantage.
Free amino nitrogen (FAN)
Free amino nitrogen (FAN) is a term defined as the sum of amino acids, ammonium ions and low molecular weight peptides that can constitute a source of nitrogen for yeast in the fermentation process. The FAN level in wort depends on the quality of the malt used and the correctness of the malting and mashing process. FAN content in finished beer is also influenced by the use of this element by yeast. Nitrogen from wort is absorbed by yeasts for their growth through the synthesis of proteins and other cellular compounds. FAN is also used by yeast cells to produce a range of metabolic products, especially higher alcohols which affect the flavor and stability of beer. Too high level of free amino nitrogen can make beer cloudy. Conversely, too low level of it can delay the fermentation process and causes the formation of undesirable compounds. High nitrogen content in wines is caused by the production of amino acids, as a result of proteolysis of grape must [4].
The data presented in Table 2 show different levels of free amino nitrogen in the tested samples. In beers without and with 20% content of grape marc and pulp fermented using the yeast strains S. cerevisiae Safale US-05 and M. pulcherrima MG 970,690, there was an increase in the content of free amino nitrogen compared to the wort (Tables 1, 2). The greatest increase was observed in the samples produced by the yeast S. cerevisiae Safale US-05. Perhaps it was related to the process of autolysis of yeast cells which takes place at the end of fermentation or the lack of adequate amount of nitrogen. In such circumstances compounds in the form of amino acids are released. Yeasts utilize different sources of nitrogen during the fermentation process and not all amino acids are metabolized [15]. Concerning the yeast D. bruxellensis 3429, no such phenomenon was observed (except for beer with 40% addition of grape pulp) (Table 2). According to Colomer et al. [16], the yeast D. bruxellensis use a variety of compounds as their nitrogen source. Moreover, these yeasts are able to assimilate nitrates which are largely transferred to wort from hops. This is confirmed by the fact that the strain D. bruxellensis 3429 showed the highest consumption of FAN in all variants compared to the samples before fermentation. Beers with a higher content of grape must, pulp and marc (40%) exhibited a relatively higher, proportionally to the initial content, FAN assimilation by yeast (Table 2). It was probably also influenced by the pH values (Table 2) which had an impact on the quantitative consumption of nitrogen material by yeasts used for fermentation [4].
Color
The color of beer depends primarily on the selection and proportion of grains used in the production of wort. The greater the addition of darker malts, the darker the color of the drink becomes. The color of beer is also determined by the reactions that occur during the malting process. Dye compounds are formed as a result of the Maillard browning reaction and, in some cases, caramelization and pyrolysis. These processes depend on the temperature, amino acids and sugar content in the grains used. Yeasts affect the color of beer indirectly. They show the ability to adsorb color compounds on the cell surface. To a small extent, the color of beer comes from the oxidation of polyphenols contained in malt and hops [17]. The color of grapes is influenced by the phenolic composition, especially the level of anthocyanins, their derivatives and tannins. The content of the aforementioned compounds is particularly high in grape peels [18].
The addition of grape marc, pulp and must contributed to a significant increase of color in the analyzed samples (Table 2). The main source of dye compounds in grapes is their peel. It constituted a significant part of marc added to the samples. Therefore, darker color were obtained for these variants, depending on the size of the addition. The values obtained for beers after fermentation were lower than for non-fermented samples (Table 2). It is likely that during the fermentation process some of dye compounds decomposed [19]. The color of the beer fell during fermentation. It is caused by decoloration of some substances caused by the decrease in pH, and precipitation of highly colored compounds or absorption in the yeast cells [20].The greatest decrease of color was observed for the samples obtained with the use of the yeasts M. pulcherrima MG970690. A smaller decrease was observed in beers fermented by the strain D. bruxellensis 3429, and the smallest one for S. cerevisiae Safale US-05 (Tables 1, 2). Both the yeasts M. pulcherrima and D. bruxellensis are characterized by good enzymatic activity. These abilities may have contributed to the decomposition of compounds that gave color to unfermented samples. This resulted in a higher decrease of color for these yeasts than for the control yeasts, i.e., S. cerevisiae Safale US-05. Used non-Saccharomyces yeasts showed no adverse effect on color. On the other hand, the introduction of grape marc had a positive effect on this parameter, contributing to the unusual carmine color of the tested beers.
Organic acids
Organic acids in wort, grape pulp, marc and must
Beer acidity is related to the presence of organic acids, hydrogen ions and CO2 (present in non-degassed beer). Its value ranges from 1.2 to 3.4 mL of NaOH/100 mL of beer [10]. It gives lightness and freshness to beers and wines. The main organic acids found in grapes are tartaric acid and malic acid. Although these compounds have a similar chemical structure, they are synthesized in fruits from glucose using different metabolic pathways. What especially affects the ratio of the two acids are differences in the acidity of various grapevines. Tartaric acid is usually present in grapes in concentrations of 5–10 g/L., while the content of L-malic acid in ripe fruit usually ranges between 2 and 6.5 g/L. Its particularly large content (25 g/L) can occur in grapes harvested in cold climates, as low temperatures slow down the acid respiration process [21]. The organic acid content of grapes depends on the climate, region, season and grape variety [22].
Table 3 shows the profile of organic acids in non-attenuated samples. It was observed that the highest content values of tartaric and malic acid occurred in grape marc. L-malic acid accumulates mainly in the peel of the fruit, much less in grape pulp and must. This tendency may be subject to change during grape ripening and technological treatment [23, 24]. The lowest malic acid content among the tested samples was definitely found in wort (Table 3). Greater addition of grape must, pulp and marc to the wort resulted in higher content of malic acid in the samples. Although citric acid is present in grapes only in trace amount (0.5–1 g/L), it plays an important role in biochemical and metabolic processes (Krebs cycle). It delays the development of yeast cells without inhibiting their growth completely. L-tartrates are resistant to degradation by microorganisms during the fermentation process, while malic and citric acids can be partially metabolized by yeast and bacteria, which reduces the acidity of wine [25]. The highest amounts of citric acid were observed in grape marc, while the lowest in grape must (Table 3). This proves that the highest content of this compound occurs in the grape peel. Acetic acid in small amounts (< 500 mg/L) positively affects the complexity of the taste and aroma of wine. It plays an important role in the production of acetate esters which give drinks a fruity character. However, too high an amount of it contributes to the sour aftertaste and undesirable smell of drinks [26]. A small amount of acetic acid was found in the analyzed samples (Table 3). The only exception was grape marc. The grape marc introduced into the wort was fresh; therefore, it was not possible that it had been acidified. Another component produced by yeast during the fermentation process is succinic acid (1,4-butanedioic acid). In combination with fumarate, it participates in lipid metabolism and the Krebs cycle [26]. Succinic acid is produced in the fermentation process, hence its presence in grape must, pulp and marc was not found (Table 3).
Organic acids in beers
Most of organic acids found in beer come from the deamination of amino acids by yeast or they are products of carbohydrate metabolism in the glycolysis pathway. The developing yeast cells use the amino group, NH2, from amino acids. It is used by them to build their own proteins, and the organic acids devoid of the amino group created in this way remain in beer. As a result, in addition to higher alcohols obtained in a similar cycle of reactions, a wide range of organic acids is formed. They, together with the higher alcohols, create the so-called bouquet of flavor and aroma of the drink. They contribute to lowering the pH during fermentation and affect the taste, aroma, color, stability, acceptability and quality of the product [27, 28]. Knowing the level of organic acids in beverages provides important information to monitor the fermentation process [29].
Table 4 shows the profile of organic acids in the tested beers The lowest content of organic acids was definitely found in beers without any additions, while the highest in samples with grape marc (40%). The exception was malic acid, the highest amounts of which were recorded in beers with pulp (40%). The lowest malic acid content was found in beers fermented with the yeast D. bruxellensis 3429 (Table 4). This shows that this yeast proved to be the most effective in breaking down malic acid, the content of which in the non-attenuated samples is presented in Table 3. The strain S. cerevisiae Safale US-05 was less effective in decomposing malic acid (Tables 3, 4). Saccharomyces yeasts exhibit different degradation abilities of L-malic acid during alcoholic fermentation (up to 3 g/L). The ability depends, among others, on the growth temperature of these microorganisms [30]. The cultures S. cerevisiae show the ability to decompose L-malic acid in the presence of glucose in amounts even up to 48% [31]. Unlike L-malic acid, tartaric acid is resistant to degradation by microorganisms during the fermentation process [25]. Our research also showed no considerable differences in the tartaric acid content in non-fermented samples and beers (Tables 3, 4). Analyzing the profile of the remaining organic acids, significant amounts of succinic acid were found (Table 4). Citric acid was present in all analyzed samples and remained at a relatively similar level. The only exception was beer with the addition of grape marc in which much larger amounts of this component were found. A similar tendency was found in the case of lactic acid (Table 4). Yeasts are capable of producing small amounts of acids, e.g., lactic, succinic, citric or acetic acids, which may lead to an increase in the acidity of wine/beer compared to its level in must/wort.
Sugars
Sugars in wort, grape pulp, marc and must
Glucose and fructose are the main sugars in grapes. Usually, they are in equal proportions, but in the case of overripe berries, the share of fructose is higher. It also predominates in grapes covered with Botrytis cinerea. The content of sugar depends on a grape variety, ripeness and health condition of grapes. During the fermentation process, glucose is processed much faster; therefore, the residual sugar in the wine consists of mainly fructose. The actual concentration of glucose and fructose in grape must is from 80 to 130 g/L for each of the sugars separately. In addition, grapes also contain trace amounts of sucrose (2–10 g/L), rhamnose (up to 0.4 g/L) and arabinose (up to 1.5 g/L) [26]. In the examined grape must, grape pulp and grape marc, a comparable content of glucose and fructose was found (Table 5). The highest glucose content was noted in the grape pulp (Table 5). A significant amounts of maltose, glucose and fructose were found in the wort. It also contained sucrose (Table 5). Not all sugars in the wort were fermented. These ingredients give the beer body and sometimes sweetness [27, 32]. The fructose content in the wort is in the range of 1.0–1.5 g/L [33]. In the analyzed wort, the concentration of this sugar was equal 4.82 g/L. In turn, glucose was present in the amount of 11.4 g/L (Table 5). The obtained glucose concentration in the wort is similar to the values reported in the literature, indicating the range of this parameter between 8 and 10 g/L [33]. Introducing grape must, marc and pulp into the wort increased the amount of glucose and fructose in the tested variants (Table 5). Among the sugars in a typical wort, maltose is present in the highest amount [33, 34]. Maltose in the tested wort exceeded the level reported in the literature (33–54 g/L) [33]. Most likely this was caused by the type of malt used.
Sugars in beers
All the samples, except for the variant with 20% addition of grape pulp, inoculated with D. bruxellensis 3429, were characterized by a similar, low level of glucose (Table 6).
In the variants without the addition of grapes, glucose was completely attenuated (Table 6), which proves that non-Saccharomyces yeast showed similar glucose fermentation efficiency to S. cerevisiae Safale US-05. Slight differences occurred in beers with the addition of grape must (Table 6). The obtained results are within the range presented in the literature for glucose (0–8 g/L) in beers after fermentation [35]. The obtained results suggest that, depending on the composition of the environment, the yeast species used in the study assimilate carbohydrates in different ways. D. bruxellensis compared to S. cerevisiae, are characterized by a slower rate of growth and the consumption of sugars, as well as higher or equal mass loss [6]. The data shown in the graphs (Fig. 1) confirm the information in the literature. Between D. bruxellensis 3429 and S. cerevisiae Safale US-05, the difference in fermentation rate was insignificant. Moreover, both S. cerevisiae and D. bruxellensis yeasts have variable maltose fermentation abilities [6]. In fermented beers without the addition of grapes, the highest content of maltose was found in variants inoculated with D. bruxellensis 3429 (Table 6). A similar tendency was noted in beers with the addition of grape must, grape pulp and grape marc (Table 6). The highest content of maltose was found in beers with the addition of grape marc, inoculated with D. bruxellensis 3429 (Table 6). In beers inoculated with D. bruxellensis 3429 yeast without the addition of grapes, as well as beers with a 20% share of grape must and grape marc, the maltose concentration was twice as high as compared to beers fermented with S. cerevisiae Safale US-05 yeast (Table 6). This could be due to the higher amount of fermenting compounds derived from grapes or a lower fermentation capacity of maltose by the yeast D. bruxellensis 3429. Glycerol is the third most abundant product of yeast metabolism, after ethanol and carbon dioxide. This compound is characterized by a sweet taste and is largely responsible for the full flavor of fermented beverages. Its typical content in beers is in the range of 1–3 g/L [3, 36]. Microorganisms produce glycerol mainly due to its protective properties against osmotic and thermal stresses [37]. Glycerol is a by-product of yeast metabolism; therefore, it was not found in the wort (Table 5). Its content in particular beers was significantly diversified (Table 6). Beers inoculated with D. bruxellensis 3429 yeast, without the addition of grapes, showed a higher content of glycerol, compared to S. cerevisiae Safale US-05 (Table 6). Literature data indicate a higher content of glycerol in beers fermented with non-Saccharomyces yeast, compared to traditional brewer’s yeast [38]. The highest content of glycerol was found in beers with the addition of grapes, inoculated with S. cerevisiae Safale US-05 (Table 6). This yeast produced a greater amount of this compound in beers as compared to non-Saccharomyces yeast. Presumably, the higher presence of glycerol in the samples with the addition of grapes was due to the addition of more sugars with the grape must, pulp and marc. Comparing the course of the dynamics of fermentation with the use of sugars by the tested yeasts, it was found that despite the different adaptation times of the strains to the environment, all the tested yeasts carried out the fermentation satisfactorily. The non-Saccharomyces showed a similar ability to attenuate and utilize the ingredients contained in the wort as S. cerevisiae. This proves that both S. cerevisiae Safale US-05 and the non-Saccharomyces strains are suitable for the fermentation of beer. Despite the longer fermentation time by M. pulcherrima MG970690 strain and lower mass loss compared to the other trials, this yeast used most of the available sugars in the fermenting media (Fig. 1, Table 6). The addition of grape marc to the wort caused the introduction of naturally occurring simple sugars to the samples. They were not fully fermented by yeast. Although the yeast S. cerevisiae Safale US-05 and D. bruxellensis 3429 had the shortest and similar adaptation time to environmental conditions in the fermenting broth with the addition of grape marc, the yeast S. cerevisiae Safale US-05 fermented twice as much maltose compared to D. bruxellensis 3429.
In conclusion, despite the different adaptation times to the environment, all tested yeasts conducted the fermentation satisfactorily. This proves that both S. cerevisiae Safale US-05 and the non-Saccharomyces strains are suitable for the fermentation of beer. The yeast S. cerevisiae Safale US-05 produced a comparable amount of alcohol in the beers compared to the strains D. bruxellensis 3429 and M. pulcherrima MG970690. The addition of grape marc, grape pulp and grape must to the wort contributed to the increase of this parameter, which was probably the result of introducing more sugars into the wort, which could be consumed by yeast during the fermentation process. The non-Saccharomyces yeast was characterized by a similar ability to assimilate the sugars contained in the wort and those provided with the grape marc. These strains showed a similar glucose fermentation performance to S. cerevisiae Safale US-05. The highest content of maltose was found in beers with the addition of grape marc, inoculated with D. bruxellensis 3429. In beers without the addition of grapes, with a 20% share of grape must and marc, the value of maltose was twice higher than in beers fermented with yeast S. cerevisiae Safale US-05. This could be due to the greater amount of fermenting compounds derived from grapes or the lower fermentation capacity of maltose by the yeast D. bruxellensis 3429. The introduction of grape marc, pulp and grape must into the beers increased the titratable acidity. This may suggest that the addition of grapes provides naturally occurring organic acids and more substrates for the growth of yeasts. There is an increased production of acidic compounds, both from substances in a wort and in grapes. The obtained results show that the non-Saccharomyces yeast is characterized by similar acid production capacity as the S. cerevisiae Safale US-05 strain. D. bruxellensis 3429 yeasts were characterized by the highest consumption of FAN, compared to the samples before fermentation. This strain was also the most effective in breaking down malic acid.
Conclusion
The research has shown that the fermentation performed by strain D. bruxellensis 3429 is similar to S. cerevisiae Safale US-05. Along with the increase in the content of grape must, marc and pulp in the samples, the amount of alcohol, titritable acidity and color in beers increased. The utilization of L-malic acid in the fermentation process was most effective for strain Dekkera bruxellensis 3429. The unconventional yeast used in the study is capable of producing beers without and with the addition of grape marc, pulp and must with parameters similar to beers obtained using S. cerevisiae Safale US-05. The conducted research provides a lot of new information on beers with the addition of grape must, pulp, and marc, which were obtained with the use of non-Saccharomyces yeast. However, more research is needed to better understand the metabolism of the yeast used in the study.
Data availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Olewnicki D (2018) Uprawa winorośli w Polsce w świetle danych statystycznych. Rocz Nauk Stowarzyszenia Ekon Rol i Agrobiznesu 20:139–145
Gómez-Brandón M, Lores M, Insam H, Domínguez J (2019) Strategies for recycling and valorization of grape marc. Crit Rev Biotechnol 39:437–450. https://doi.org/10.1080/07388551.2018.1555514
Esslinger HM (2009) Handbook of brewing: processes, technology, markets. Wiley-VCH, Weinheim
Hill AE, Stewart GG (2019) Free amino nitrogen in brewing. Fermentation 5:22. https://doi.org/10.3390/fermentation5010022
Willaert R (2012) Biochemistry of beer fermentation. In: Simpson BK, Nollet LML, Toldrá F, Benjakul S, Paliyath GHY (eds) Food biochemistry and food processing. John Wiley and Sons, Sussex, pp 627–653
Blomqvist J, Passoth V (2015) Dekkera bruxellensis—spoilage yeast with biotechnological potential, and a model for yeast evolution, physiology and competitiveness. FEMS Yeast Res 15:fov021. https://doi.org/10.1093/femsyr/fov021
Pawlikowska E, Kregiel D (2017) Niekonwencjonalne drożdże Metschnikowia pulcherrima i ich zastosowanie w biotechnologii. Postępy Mikrobiol 56:405–415
Meneghin MC, Bassi APG, Codato CB et al (2013) Fermentative and growth performances of Dekkera bruxellensis in different batch systems and the effect of initial low cell counts in co-cultures with Saccharomyces cerevisiae. Yeast 30:295–305. https://doi.org/10.1002/yea.2959
Tang K, Li Q (2017) Biochemistry of wine and beer fermentation. In: Pandey A, Sanroman MA, Du G, Soccol CRDCG (eds) Current developments in biotechnology and bioengineering. Elsevier, Amsterdam, pp 281–304
Garrett O (2012) The oxford companion to beer. Oxford University Press, New York
Vicente J, Ruiz J, Belda I et al (2020) The genus Metschnikowia in enology. Microorganisms 8:1038. https://doi.org/10.3390/microorganisms8071038
Steensels J, Daenen L, Malcorps P et al (2015) Brettanomyces yeasts—from spoilage organisms to valuable contributors to industrial fermentations. Int J Food Microbiol 206:24–38. https://doi.org/10.1016/j.ijfoodmicro.2015.04.005
Li H, Liu F, Kang L, Zheng M (2016) Study on the buffering capacity of wort. J Inst Brew 122:138–142. https://doi.org/10.1002/jib.286
Nardini M, Garaguso I (2020) Characterization of bioactive compounds and antioxidant activity of fruit beers. Food Chem 305:125437. https://doi.org/10.1016/j.foodchem.2019.125437
Smith BD, Divol B (2016) Brettanomyces bruxellensis, a survivalist prepared for the wine apocalypse and other beverages. Food Microbiol 59:161–175. https://doi.org/10.1016/j.fm.2016.06.008
Colomer MS, Funch B, Forster J (2019) The raise of Brettanomyces yeast species for beer production. Curr Opin Biotechnol 56:30–35. https://doi.org/10.1016/j.copbio.2018.07.009
Shellhammer TH (2009) Beer color. In: Bamforth CW (ed) Beer: a quality perspective. Academic Press, San Diego, pp 213–227
Jensen JS, Demiray S, Egebo M, Meyer AS (2008) Prediction of wine color attributes from the phenolic profiles of red grapes (Vitis vinifera). J Agric Food Chem 56:1105–1115. https://doi.org/10.1021/jf072541e
Ruta LL, Farcasanu IC (2019) Anthocyanins and anthocyanin-derived products in yeast-fermented beverages. Antioxidants 8:182. https://doi.org/10.3390/antiox8060182
Kunze W (2004) Technology of Brewing and Malting (3rd International English Edition). The Research and Teaching Institute for Brewing in Berlin (VLB). VLB’s Publishing Department, Berlin
Volschenk H, Van Vuuren HJJ, Viljoen-Bloom M (2006) Malic acid in wine: Origin, function and metabolism during vinification. S Afr J Enol 27:123–136. https://doi.org/10.21548/27-2-1613
Redzepovic S, Orlic S, Majdak A et al (2003) Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int J Food Microbiol 83:49–61. https://doi.org/10.1016/S0168-1605(02)00320-3
Coloretti F, Zambonelli C, Castellari L et al (2002) The effect of DL-malic acid on the metabolism of L-malic acid during wine alcoholic fermentation. Food Technol Biotechnol 40:317–320
Jackson RS (2008) Wine science: principles and applications. Academic press, San Diego
Moreno-Arribas MV, Polo MC (2009) Wine chemistry and biochemistry, 1st edn. Springer, New York
Ribéreau-Gayon P, Dubourdieu D, Donèche B, Lonvaud A (2006) Handbook of enology, volume1: the microbiology of wine and vinifications. John Wiley & Sons, New York
Briggs DE, Brookes PA, Stevens R, Boulton CA (2004) Brewing: science and practice. Woodhead Publishing Limited Cambridge, London
Santalad A, Teerapornchaisit P, Burakham R, Srijaranai S (2007) Capillary zone electrophoresis of organic acids in beverages. LWT-Food Sci Technol 40:1741–1746. https://doi.org/10.1016/j.lwt.2007.01.007
Esteves VI, Lima SSF, Lima DLD, Duarte AC (2004) Using capillary electrophoresis for the determination of organic acids in Port wine. Anal Chim Acta 513:163–167. https://doi.org/10.1016/j.aca.2003.12.036
Vilela-Moura A, Schuller D, Mendes-Faia A, Côrte-Real M (2008) Reduction of volatile acidity of wines by selected yeast strains. Appl Microbiol Biotechnol 80:881. https://doi.org/10.1007/s00253-008-1616-x
Rainieri S, Zambonelli C, Giudici P, Castellari L (1998) Characterisation of thermotolerant Saccharomyces cerevisiae hybrids. Biotechnol Lett 20:543–547. https://doi.org/10.1023/A:1005389309527
Oliver G, Colicchio T (2011) The Oxford companion to beer. Oxford University Press, New York
Hough JS (1991) The biotechnology of malting and brewing. Cambridge University Press, Cambridge
Serrano R (1977) Energy requirements for maltose transport in yeast. Eur J Biochem 80:97–102. https://doi.org/10.1111/j.1432-1033.1977.tb11861.x
Bamforth CW (2005) Beer, carbohydrates and diet. J Inst Brew 111:259–264. https://doi.org/10.1002/j.2050-0416.2005.tb00681.x
Zhao X, Procopio S, Becker T (2015) Flavor impacts of glycerol in the processing of yeast fermented beverages: a review. J Food Sci Technol 52:7588–7598. https://doi.org/10.1007/s13197-015-1977-y
Klein M, Swinnen S, Thevelein JM, Nevoigt E (2017) Glycerol metabolism and transport in yeast and fungi: established knowledge and ambiguities. Environ Microbiol 19:878–893. https://doi.org/10.1111/1462-2920.13617
Contreras A, Hidalgo C, Henschke PA et al (2014) Evaluation of non-Saccharomyces yeasts for the reduction of alcohol content in wine. Appl Environ Microbiol 80:1670–1678. https://doi.org/10.1128/AEM.03780-13
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Compliance with ethics requirements
All authors were compliant and followed the ethical guidelines, according to the requirements of European Food Research and Technology.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cioch-Skoneczny, M., Sral, A., Cempa, A. et al. Use of red grape pulp, marc and must in the production of beer. Eur Food Res Technol 249, 1059–1072 (2023). https://doi.org/10.1007/s00217-022-04195-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04195-5