Abstract
Certain polyunsaturated fatty acids with n-3 double bonds are essential nutrients for the human body and are part of the bilayer of cell membranes or precursors of tissue hormones. The most abundant dietary n-3 fatty acids in human nutrition are α-linolenic, eicosapentaenoic, and docosahexaenoic acid and can be taken up through dietary sources such as vegetable oils or fish or, alternatively, dietary supplements with high levels of n-3 fatty acids. In previous studies, considerable variation of lipid patterns and quantities of n-3 fatty acids were observed. In this study, 33 dietary supplements from the German market, based on fish-, krill-, microalgae, and plant oil, have been analyzed. Lipid profiling (LC–MS) revealed triacylglycerols as the dominant lipid species in most samples. However, krill oil was rich in phospholipids and samples containing fatty acid concentrates featured abundant fatty acid ethyl esters and diacylglycerols. Furthermore, total lipid profiles showed considerable variance depending on the lipid sources (e.g., fish or plant oil), which was also apparent in fatty acid analysis. The contents of n-3 fatty acids ranged between 150 and 570 mg/g capsule content (GC–MS) and vitamin E (α-tocopherol and tocopheryl acetate) were found in quantities ranging from 1.2 to 86.1 mg/g capsule content (HPLC–UV/Vis). While our analyses indicated a good agreement between labeled and present quantities of total n-3 fatty acids and vitamin E for the majority of samples, significant differences in agreement between individual fatty acids were observed, as well as frequent mismatches between declared and present vitamin E derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatty acids (FA), as part of the lipid fraction, are a principal component of most food items. Besides their caloric values, some FAs exhibit additional biological effects. Certain polyunsaturated FAs (PUFA), specifically the n-3 FAs, are essential as they cannot be synthesized by the human body. They are part of the lipid bilayer of cellular membranes and precursors of different messenger substances like eicosanoids which, as tissue hormones, influence inflammatory processes in the body [1]. They are especially important for a proper development of the vision and the brain of fetuses and newborns [2]. Furthermore, an increased intake of n-3 FAs can reduce the risk of cardiovascular diseases [3].
In food, n-3 FAs are found mainly in the form of α-linolenic acid in vegetable oils such as linseed oil (45–60% of the total FAs) or as long-chain polyunsaturated FAs (LC-PUFA) in the form of eicosapentaenoic (EPA) and docosahexaenoic acid (DHA) in sea fish [for example, with 18% EPA and 11% DHA in the lipids of anchovies (also see Fig. 1)] [4, 5]. The European Food Safety Authority recommends an intake of 250 mg of EPA and DHA to maintain regular cardiac function and 2–4 g to benefit of other effects like regulation of the blood pressure. Adults on average consume 400–500 mg EPA and DHA a day from food [6]. To ensure a sufficient intake of n-3 FAs and to reach the additional benefits of the n-3 FAs, many people consume nutritional supplements with high concentrations of the FAs of interest. Sales of dietary supplements have been constantly increasing in Germany over the last few years [7]. In recent surveys, only 30 and 17% of participants in Germany and USA, respectively, stated that they did not take any nutritional supplements at all [8].
Usually, the dietary supplements with high amounts of n-3 FAs consist of encapsulated oil, but they can also be sold in bottles with oils (mostly aromatized) or even gums for kids. Besides fish oil, lipids from alternative sources, such as krill, microalgae, or linseed, are offered [9]. Furthermore, it is known that products can contain concentrates enriched in the contents of LC-PUFA compared to the aforementioned “natural” sources [10, 11]. A common process to obtain n-3 FA fish oil concentrates is based on the transesterification of the FAs to the respective ethyl esters (EE) followed by purification of the PUFA-EE fraction, e.g., by chromatographic or distillation methods [10] achieving purities of up to 90% EPA and DHA [12]. These ethyl ester can subsequently either be used for pharmaceutical applications or be re-esterified to triacylglycerols (TG) for food applications [12]. In Germany, supplements can only be sold if, with recommended daily intake of the supplement in question, the contents of ethyl esters of EPA and DHA are not exceeding 600 and 300 mg, respectively [13].
The occurence of the n-3 FAs as different lipid classes also affects their bioavailability. Various studies give reason to assume that n-3-fatty acids are better utilized by the human body when they are present as TGs in comparison to EEs, even though there are also studies that show no significant difference between these two lipid species [14, 15]. This difference may be caused by varying efficiencies of the respective lipases which hydrolyze the bound FAs before they get absorbed in the small intestine. After absorption into the intestine, the free FAs are re-esterified back to TGs. Free FAs resulting from TGs are simply re-esterified to the likewise absorbed MG residue. This could be more difficult and therefore inefficient for FAs resulting from EE, since an additional glycerol derivative must be provided for the FAs for esterification to the TG [16, 17].
Similarly, higher bioavailability of n-3 FAs in krill oil compared to fish oil was noted in a publication by Ulven et al. [18]. Since most FAs in krill oil are bound as phospholipids instead of as TGs, it is assumed that the bioavailability may be increased due to the emulsifying properties of the phospholipids and therefore increased lipase activity [16].
The different lipid classes are not only affecting the bioavailability but also the oxidative stability of oils. There are various studies showing both prooxidant and antioxidant properties of phospholipids in different experiments. Antioxidant effects may be, e.g., caused by complexing of metals or changing the location of other antioxidants. Prooxidant effects as observed in bulk oils might be evoked through forming colloids, which provoke the interaction with metals [19]. Bandarra et al. observed antioxidant effects of phospholipids in fish oil and synergistic effects with α-tocopherol [20]. LC-PUFAs bound as TGs were found to be less likely to oxidize compared to ethyl esters showing lower peroxide values and p-anisidine values in oxidized fish oil [12].
The EPA and DHA contents of nutritional supplements of different regions have already been compared, showing various outcomes [21,22,23,24,25,26]. Studies with products from the US analyzed by Shim et al. showed that more than 90% of the investigated samples contained less EPA and DHA than stated by the manufacturer [22]. On the other hand, products from Finland analyzed by Damerau et al. met their label claim quite well, suggesting potential regional differences between retail samples [24]. Only little data are available for the German market: Kutzner et al. as well as Koch et al. previously analyzed the lipid classes and the n-3 FA contents of three and ten products, respectively, from the German market. They found that lipid class and FA distributions strongly differed depending on the source of the oil and the use of n-3 FA concentrates [9, 23].
To stabilize the oils rich in n-3 PUFAs, often antioxidants are added [10], especially vitamin E derivatives like α-tocopherol. It is known that tocochromanols delay lipid oxidation by stopping radical chain reactions in different vegetable oils but also animal-derived fats and oils [27]. Depending on the type and amount of vitamin E derivatives used, the oxidative stability of the products can differ. Hamilton et al. showed that lipid oxidation in fish oil is delayed by adding tocopherols and shows decreasing antioxidative effects for δ, γ, and α-tocopherol [28]. Also, interactions with different tocochromanol derivatives, other antioxidants, or the presence of phospholipids can influence and amplify the antioxidative effect [20, 29]. So far, no data are available on the presence and contents of vitamin E derivatives in PUFA-rich food supplements from the German market.
To evaluate the composition and quality of nutritional supplements containing high amounts of n-3 FAs from the German market, 33 products were bought retail and analyzed in detail regarding their lipid compositions and quantities of LC-PUFAs and tocochromanols by LC–MS, GC–MS, and LC–UV/Vis. Doing so, we were able to identify considerable differences in compositions between individual samples and highlight disagreements between the information provided to the consumer and actual compositions and contents.
Materials and methods
Samples
The 33 n-3 FA dietary supplements were purchased in drugstores and pharmacies in Erlangen (Germany) or in German online pharmacies between November and December 2020. Of the 33 samples 27 contained fish oil, two linseed oil, one krill oil, one microalgae (from Schizochytrium sp.), and two samples contained fish and plant oils (linseed, sunflower, pumpkin seed, wheat germ, evening primrose, rosemary, and rice bran) as source of n-3 FAs and other essential FAs, according to the label.
Chemicals
Methyl myristate (≥ 99%), ( ±)-α-tocopherol (≥ 96%), DL-α-tocopheryl acetate (≥ 96%), dioxane (anhydrous, 99.8%), trilinoleate (> 98%), rhodium-tris-(triphenylphosphine)-chloride (Wilkinsons catalyst), Supelco 37 Component FAME Mix, sodium methanolate (25%) in methanol, acetic acid (≥ 99.7%), isopropanol, acetonitrile, formic acid, ammonium formate (all LC–MS grade), BHT (99%), and tert-butyl methyl ether (≥ 99.8%) were from Sigma-Aldrich (Steinheim, Germany). Trioleate (99%) was supplied by ACROS Organics and n-hexane (HPLC grade) was supplied by VWR (Ismaning, Germany). Dichloromethane and methanol (both HPLC grade) were from Acros Organics (Geel, Belgium). PC 17:0/17:0 (99%) was from Avanti Polar Lipids (Alabaster, AL/USA) and TG 18:1/18:1/18:1-glycerol-D5 (98%) was from Larodan (Solna, Sweden). Ethyl acetate was from Carl Roth (Karlsruhe, Germany). Silica gel 60 (0.04–0.063 mm) was from Macherey–Nagel (Düren, Germany).
Analysis of lipid extracts by LC–MS
Sample aliquots of ca. 10 mg were weighed into amber screw cap vials and dissolved in dichloromethane/methanol (50 µg/mL BHT) 2:1 (v/v). Of this stock solution, 10 µL were aliquoted (corresponding to 0.1 mg) into another amber glass vial, and 2 µg each of PC 17:0/17:0 (ISTD 1) and TG 18:1/18:1/18:1-glycerol-D5 (ISTD 2) were added as internal standards before the solvent was removed using a gentle stream of nitrogen. The residue was re-dissolved in 1 mL of mobile phases A and B 1:2 (v/v), filtered through a 0.2 µm PTFE syringe filter and used for LC–MS analysis.
Lipids were analyzed using an NCP-3200RS microflow LC system (Dionex/Thermo Fisher) coupled to a timsToF Pro ion mobility-mass spectrometer (Bruker, Bremen/Germany). Water/acetonitrile (40:60, v/v) and acetonitrile/isopropanol (10:90, v/v), both containing 0.1% formic acid and 10 mM ammonium formate, were used as mobile phases A and B, respectively. A Triart C18 column (150 mm x 0.5 mm, 1.9 µm particle size; YMC, Dinslaken/Germany) held at 50 °C was used for separation using the following gradient: Starting at 30%, the ratio of B was increased to 80% within 7 min and further to 95% within 16 min. After 2 min of hold time at 95% B starting conditions were re-established within 2 min and the system was re-equilibrated for 6 min. The flow rate was 30 µL/min.
The timsToF Pro was used with positive electrospray ionization with end-plate offset, capillary voltage, nebulizer pressure, dry gas flow, and dry temperature set to 500 V, 4500 V, 2 bar, 6 L/min, and 200 °C, respectively. The mass range for MS1 and MS2 data acquisition was set to m/z 100–1550 and the ion mobility range from 1/K0 0.8 to 1.9 using a ramp time of 100 ms and the internal charge control set to 5E06. The Parallel Accumulation and Serial Fragmentation (PASEF) mode was used for MS/MS using a collision energy of 30 eV over the whole mass range. Sodium formate cluster and ESI LC/MS tuning mix (Agilent) were used for mass and mobility calibration, respectively. Mass calibration for every run was achieved by introducing sodium formate calibrant at the beginning of every sample run using a six-port valve. The LC–MS data were processed using Data Analysis 6.0 for manual peak inspection and MetaboScape 2022b (both Bruker, Bremen/Germany) for (semi-) automated data processing, respectively. Lipid annotations in MetaboScape were manually checked for plausibility, taking into account MS/MS spectra and (relative) retention times within homologue series. The data table was gap filled (1/5th of each feature’s lowest detected intensity) and imported into MetaboAnalyst 5.0 for further analysis [30]. The data were normalized by ISTD 2, log transformed, and mean-centered prior to analysis by Principal Component Analysis.
Transesterification of lipids for GC–MS analysis of fatty acid methyl esters
Each sample was prepared and measured as a duplicate from two capsules. The samples were converted to fatty acid methyl ester (FAME) as described previously by Christie [31] and Berdeaux et al. [32]. To 10 mg aliquots of the lipid samples, 200 µg of the internal standard TG 18:0/18:0/18:0-D12 (ISTD 4), 1 mL tert-butyl methyl ether and 2 mL of 0.5 M sodium methanolate in methanol were added. After mixing the sample, it was heated at 50 °C for 15 min. Afterward, 150 µL acetic acid and 2 mL ultrapure water were added and the FAMEs were extracted twice with 2 mL n-hexane. The solvent was evaporated and the residue was re-dissolved in 1 mL of hexane. An aliquot (8 µL) of the solution was transferred to another vial, the solvent was evaporated and 1 mL of n-hexane added. Because of their varying content of n-3 FAs, some samples had to be diluted differently to be quantified. To the final dilutions, 50 µL of the solution of the internal standard 14:0 ethyl ester (ISTD 3) of 0.1 mg/mL were added before the samples were analyzed by GC–MS. The synthesis and purification of the ISTD 3 and ISTD 4 is described in the supporting information.
Determination of declared n-3 fatty acid and vitamin E concentration
The weight of the capsule content was determined in order to be able to calculate the concentrations of the n-3 FAs or vitamin E from the declared amount per capsule. The capsules were weighed, cut open, and the lipid fraction was extracted first with hexane and then ethyl acetate. After the capsule shells were dried, their weight was determined and the capsule content calculated as follows. The determination was performed in triplicate for each sample
GC–MS analysis of fatty acid methyl esters
For GC–MS analysis, a Trace GC Ultra with an AS 3000 auto-sampler and DSQ II mass spectrometer (Thermo Scientific) were used. Samples (1µL) were injected using a split/splitless injector in splitless mode onto a VF-23 ms column (60 m × 0.25 mm; 0.25 μm; Agilent Technologies (Santa Clara, California). Helium (purity 5.0) was used as a carrier gas at a constant flow rate of 1.0 mL/min. The temperature program of the oven started at 50 °C and was heated up at a rate of 12 K/min to 170 °C (held for 7 min), then at 10 K/min to 210 °C (held for 5 min), and finally at 15 K/min to 250 °C (held for 10 min). The temperatures of the ion source and transfer line were set at 250 °C and 300 °C, respectively, and electron ionization at 70 eV was used. Quantification of selected n-3 FAs (ALA, EPA, and DHA) was performed in SIM-Mode monitoring with m/z 74, 81, 87, 101, 292, 298 as qualifiers and m/z 79 (n-3 FAMEs), 88 (14:0-FAEE), and 302 (18:0-FAME d4 as quantifiers for the respecting fatty acid (all dwell time 100 ms). To support the identification of the FAs, every sample was measured additionally in full scan using a mass range of m/z 50–650. The FAs were identified by comparing to reference spectra and elution order of the Supelco 37 Component FAME Mix.
The n-3 FAs were quantified using a six-point external calibration using Supelco 37 Component FAME Mix (0.5; 1; 5; 10; 15 and 20 μg/mL) using the SIM-Mode, where 18:3n-3 was used for quantification for 18:3n-3 and 22:6n-3 was used for the external calibration for the quantification of 20:5n-3 and 22:6n-3 in the sample. Due to the different response factors of EPA and DHA, the measured EPA was divided by a factor of 1.08 (determined using single compound standards). The instrumental limits of detection (Signal-to-Noise ratio (S/N) = 3) and limits of quantification (S/N = 10) for 18:3n-3 the LOD and LOQ were at 2.6 and 8.7 ng/mL which corresponds to a concentration of 34 or 110 ng/mg of the FA in the matrix (at the regular dilution of the sample). For 20:5n-3 and 22:6n-3, the LOD and LOQ were at 3.2 and 11 ng/mL which corresponds to a concentration of 42 or 140 ng/mg of the FA in the matrix (for the regular 8:1050 dilution of the sample). Coefficient of determination (R2) of the calibration curve was always 0.995 or better. Recovery of the ISTD 4 on average was 85% (Median 82%). Accuracy with single standards (created by methylation of the free fatty acid) was 87% for ALA-ME, 114% for EPA-ME, and 111% for DHA-ME (n = 3). The precision of the method showed a coefficient of variation around 7% for the n-3 FAs for the repeatability (n = 6) and a coefficient of variation of maximum 5% for the interday reproducibility (n = 3). The authors do not know the method used by the manufacturers to quantify their content of n-3 FAs. The official method by the European Pharmacopoeia 9.7: 2.4.29. Composition of fatty acids in oils rich in omega-3-acids uses a very similar alkaline transesterification method and a quantification by GC-FID[33].
Quantification of vitamin E in samples
Sample aliquots of ca. 10 mg were weighed into screw cap vials and dissolved in 1 mL of dichloromethane as stock solutions. An aliquot of every stock solution was transferred into another vial, the solvent was evaporated using a gentle stream of nitrogen, and the residue was re-dissolved in 1 mL of HPLC solvent with a target analysis concentration of 0.1 mg/mL. For dissolving the sample a mixture of the Eluent A [methanol/water 40:60 (v/v)] and Eluent B [isopropanol/methanol 90:10 (v/v)] in a ratio of 1:2 (v/v) was used. Prior to HPLC analysis, the samples were filtered through 0.2 µm PTFE syringe filters.
The samples were analyzed using a Smartline HPLC system with pump 2050, auto-sampler 3950, and 2850 diode array detector (all Knauer, Berlin/Germany). Sample injection (100 µL, full loop fill) was done by the auto-sampler. An EC Nucleosil C18 column (125 mm × 4 mm, 5 µm particle size; Macherey–Nagel, Düren/Germany) was used for the separation. The analysis was performed using the following method: after 2 min at 20% B, the proportion of eluent B was increased to 90% over the course of 8 min. This ratio was held for 10 min. Within 2 min, the starting conditions were re-established and the system was re-equilibrated for 3 min. The flow rate was 1 mL/min. Two detector channels were set to 292 nm and 286 nm for detection of α-tocopherol and α-tocopheryl acetate, respectively.
Quantification was achieved using an external calibration of ( ±)-α-tocopherol and DL-α-tocopheryl acetate in HPLC solvent at concentrations of 0.5, 1, 2, 5, 7, and 10 µg/mL. Some of the samples with lower vitamin E contents had to be reanalyzed at higher analysis concentrations for the analytes to fit within this calibration range. The instrumental limits of detection (LOD) (S/N = 3) and limits of quantification (LOQ) (S/N = 10) for α-tocopherol and α-tocopheryl acetate were 0.04 and 0.12 µg/mL as well as 0.05 and 0.16 µg/mL. This corresponds to LOQs of 1.2 mg/g and 1.6 mg/g capsule content for α-tocopherol and α-tocopheryl acetate, respectively. The coefficient of determination (R2) of the calibration curve was always 0.9995 or better.
Results and discussion
Lipid profiling of supplements by reversed-phase liquid chromatography coupled to mass spectrometry (LC-timsToF MS)
Analysis of the lipid samples by reversed-phase microflow LC coupled to high-resolution ion mobility and mass spectrometry (LC-timsToF MS) allowed the detailed investigation of the molecular composition of lipids present in the samples. Furthermore, it directly allows to identify the dominant lipid class(es) in the samples, an important information given the different stabilities and bioavailabilities reported for polyunsaturated FAs in their different forms of supplementation [12, 15, 34]. In total, close to 300 lipids were annotated on a species level in the samples (see Supplementary Table 2). However, particularly, the triacylglycerol species consisted of numerous combinations of different FAs (as indicated by MS/MS spectra), making the number of potentially present molecular species significantly larger. Triacylglycerols were detected in almost all samples, while FA ethyl esters and abundant diacylglycerols were particularly detected in FA concentrates. Furthermore, in the single krill oil sample (sample 2), different families of phospholipids (PC, PC-O, PE, and LPC) were detected.
In general, four different sample groups could be distinguished:
a) Products based on fish oils
These samples were characterized by triacylglycerols as the dominant lipid class. Among these products, fish oils sourced from salmon and cod (according to labels) were dominating, although most samples did not specify the source (see Supporting Table 1). These samples generally showed a highly complex TG distribution with frequently 150 or more TG species. In contrast to the samples mentioned below, no addition of (re-esterified) PUFA concentrates was apparent in this group.
b) Products based on plant oils
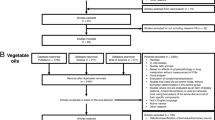
Four samples contained lipids based on plant oils, particularly linseed and flaxseed oils rich in α-linolenic acid. The two products indicating the use of exclusively plant oils featured a TG composition with relatively few prominent peaks (< 50 species), while the remaining two were mixed with fish oils and had a more complex lipid pattern (Fig. 2).
LC-timsToF MS base peak chromatograms of selected samples showing the distribution of triacylglycerol (TG), phosphatidyl choline (PC), diacylglycerol (DG), and fatty acid ethyl esters (EE) in these samples. ISTD 1 and ISTD 2 denote the internal standards PC 17:0/17:0 and TG 18:1/18:1/18:1-glycerol-D5, respectively. The signal marked by an asterisk is the calibrant
c) Products based solely or partly on fatty acid concentrates
Eight samples were found to be based on n-3 FA concentrates, to varying degrees. As mentioned above, these concentrates are usually prepared by enriching ethyl esters of DHA and EPA. The ethyl esters, usually used for pharmaceutical applications, are sometimes directly incorporated into the supplements, or re-esterified to triacylglycerols, which is more common in food applications and also considered the higher quality form based on stability and bioavailability [14, 35, 36]. In LC–MS, the addition of fatty acid concentrates was either apparent by the presence of abundant FA ethyl ester peaks (sample 17) or, in the case of re-esterified products, high abundance of early eluting triacylglycerols with two or even three DHA/EPA chains (Fig. 2). In addition, re-esterified samples contained abundant peaks of highly unsaturated diacylglycerols, which are a by-product of re-esterification (Fig. 2).
d) Other products
One product was based on krill oil, which contained the PUFAs almost exclusively bound to phospholipids, particularly phosphatidyl choline, while the TG fraction was highly saturated. There are studies suggesting that PL-bound PUFAs might have higher bioactivity and efficacy compared to triacylglycerols or ethyl esters, but there are currently only limited human studies to confirm this [18, 37]. One further product was based on microalgae lipids characterized by early eluting, highly unsaturated triacylglycerols. Finally, two more products were based on cod liver oil, and featured additional abundant and late eluting long-chain TG species with ≥ 58 carbons and ≤ three double bonds (ECN ≥ 52, e.g., TG 18:1_20:1_20:1) which were not observed in the other animal-based products.
LC–MS proved very useful to distinguish these patterns: With the elution largely based on the length and degree of unsaturation of the acyl chains, FA ethyl esters eluted first in the chromatogram followed by PC species, and both groups overlapped with DGs. Given the complex composition (C44–C68, 0–18 double bonds) and large range of equivalent chain numbers (ECN, 26–56), the TGs eluted over a wide range of the chromatogram, with homologues with higher ECN, i.e., longer acyl chains with a lower number of double bonds eluting later than shorter, more unsaturated homologues. Accordingly, the incorporation of re-esterified triacylglycerols from PUFA concentrates was apparent by abundant peaks in the early parts of the TG retention time range. In fact, TG 20:5/20:5/20:5 and particularly TG 22:6/22:6/22:6 were only observed in significant abundance in such samples.
The distinct differences in lipid composition were also clearly visible from the clustering of samples in the Principal Component Analysis scores plot (Fig. 3). Here, the samples based on fish oil (green circles) formed one cluster, while samples with different compositions were found on different parts of the plot. Samples 29 and 32, which were based on linseed oil (blue triangles), had the most positive scores in PC1 and PC 2, which was also reflected in highly positive loadings for triacylglycerols abundant in linseed oil, particularly TG 54:8 and TG 52:6 species, where MS/MS spectra suggested the presence of 18:3 FA chains. Noteworthy, samples 8 and 9, which consisted of mixtures of linseed/flaxseed oil with other plant and fish oils, were clearly separated, clustering more closely with the fish oil samples (Fig. 3). Samples containing FA concentrates (red squares) were not found as one cluster but separated widely over PC1 and PC2. Sample 17, which was the single sample based on pure FA ethyl esters plotted away from the other samples, and samples 5 and 33 were found at most negative PC 2 scores. As could be expected, negative loadings on PC 2 were found particularly for highly unsaturated TG (e.g., TG 64:16, TG 66:18) or DG (e.g., DG 42:11, DG 44:12) species. Noteworthy, sample 11 plotted away from the other fish oils but did not exhibit a clear signature of highly unsaturated DG or FA ethyl esters but only of some highly unsaturated TG species (such as TG 60:12), and therefore could not be unequivocally assigned to be fortified with concentrates. Finally, within the remaining samples (black diamonds), samples 3 and 4 (based on cod liver oil) plotted relatively closely to the fish oil samples, while sample 2 (krill oil) and sample 30 (microalgae oil) were clearly separated.
Principal Component Analysis scores plot based on lipid profiles from LC–MS analysis showing the clustering of samples based on fish oil (green circles), (re-esterified) concentrates (red squares), linseed oil (and mixtures thereof with fish oil, blue pyramids), and other compositions (black diamond). The insert in lower left shows the crowded fish oil region enlarged. Sample 31 was analyzed under slightly different conditions and is not included in the PCA
Generally, our findings of strongly varying lipid compositions in our samples corresponded well with the previous findings reported for dietary supplements from the German and other markets [9, 24].
Noteworthy, comparing our interpretations from LC–MS profiling of the lipid distributions with the labeled compositions, we noticed that the declaration on the products only partly reflected the actual contents. In fact, samples 1, 5, 11, 15, 17, 28, 31, and 33, where the lipid compositions and/or clustering in PCA suggested the addition of concentrates, were all bar sample 28 simply labeled as fish oil (Supporting Table 1). This means for consumers it is currently near impossible to know what exactly they are consuming when buying “fish oil” food supplements. Considering the aforementioned differences in stability and bioavailability of PUFAS as ethyl esters and/or from triacylglycerols structured differently to the natural distribution in fish oil, this raises questions if current nutritional label requirements are sufficient [14, 35, 36].
Fatty acid patterns of nutritional supplements
Of the 33 investigated products, 27 contained only fish oil and only a few were based on linseed (2), krill (1), or microalgae oil (1) or fish oil mixed with plant oil (2). Even though all of the dietary supplements exhibited high amounts of n-3 FAs, they showed very different FA patterns depending on the type of oil and the manufacturing process. The analyzed marine oils differed from vegetable oils in their long-chain FAs and EPA and DHA instead of ALA as the main source of n-3 FAs. Products based on fish oil showed a larger variety of FA methyl esters in the GC–MS chromatogram compared to the samples based on plant oils. The major FAs of the fish oil generally ranged from C14 to C22 with zero to six double bonds, with 16:0, 18:0, 18:1n-9, 18:2n-6, and the n-3 FAs 20:5n-3 and 22:6n-3 as most abundant FAs. In addition, a large number of minor FAs such as 15:0 and other odd chain FAs, isoprenoid FAs (phytanic acid), or lower abundant PUFAs such as 18:4n-3 could be detected. In products based on linseed oil, a drastically different FA pattern was observed: As expected, these products mainly contained C16 and C18 FAs and no LC-PUFAs such as 20:5n-3 and 22:6n-3 could be detected. The principal source of n-3 FAs in these samples was 18:3n-3 (Fig. 4). However, there were also differences in the FA pattern within the marine oils. Krill and microalgae oil showed similarities in the pattern of FAs, but differences in the relative distribution. Similar to Kutzner et al. we observed that krill and microalgae oil contained relatively more saturated FAs such as 14:0 and 16:0 and less EPA and DHA than fish oil [9]. Krill oil for example showed higher amounts of 14:0, 16:0, 16:1n-7, as well as 18:4n-3. Still, the main sources of n-3 FAs in all of the marine oils were 20:5n-3 and 22:6n-3. However, it should be considered that within the samples investigated, only one product was based on microalgae and krill oil, respectively.
Also, within the fish oil samples, differences in the FA pattern occurred in relation to the fish species or the processing of the fish oil. Compared to other samples containing sea fish oil, cod liver oil (samples 3 and 4) contained remarkably high amounts of 20:1n-9 and 22:1n-11 (approximately 18 and 10% in sample 3), which had also been apparent in lipid class analysis (see above). Fish oil concentrates exhibited distinctly higher relative proportions of 20:5n-3 and 22:6n-3 (sample 33, Fig. 4) and thus less of saturated or monounsaturated FAs than “natural” fish oil, as also described by Kutzner et al. [9].
LC–MS and GC–MS chromatograms both exhibited more signals for samples based on fish oil than on plant oil or the product with fish oil concentrates. Through the larger variety of FAs in the matrix, also a broader spectrum of TGs was obtained. However, in general, the lipid class pattern cannot be predicted by the FA pattern. Krill and fish oil for example showed similar FA patterns, but completely different lipid class distributions. As fish oil shows a large variety of different TGs, krill oil exhibits only few TGs and abundant PCs which contain the LC-PUFAs (Fig. 2). Also, samples containing concentrates cannot be identified with certainty by the relative distribution of FAs. Some samples like sample 33 (Fig. 4) with a high relative proportion of n-3 FAs suggest the use of concentrates, but other samples containing concentrates exhibit a regular distribution of FAs.
Contents of polyunsaturated fatty acids and comparison with declaration
For the determination of the n-3 FA content and the comparison to the declaration, the samples were analyzed by GC–MS and FAs of interest quantified by an external calibration. Only the n-3 FAs, that were labeled on the respecting package, were considered for quantification, i.e., 18:3n-3, 20:5n-3 and 22:6n-3 (Table 1). The contents of these FAs in the nutritional supplements ranged from approximately 150 to 570 mg/g capsule content. On average, n-3 FAs made up close to a third of the capsule contents (311 mg/g), with a mean value of 296 mg/g of 18:3n-3, 165 mg/g of 20:5n-3, and 139 mg/g of 22:6n-3 detected in the samples where the respective FA was labeled. Approximately one-third of the products contained at least the declared content of the sum of labeled n-3 FAs, but every sample contained at least 80% of the declared content. The largest deviation was found in sample 22 with quantities exceeding the declared amount almost twofold (186% of declared value).
Remarkably, the ratio between measured and declared amount of n-3 FAs differed significantly, when looking at the FAs individually. While the products on average contained 116% of their declared EPA content, they “only” reached 99% of their DHA label claim. The largest deviation could be observed in sample 31 containing high amounts of 20:5n-3 (224% of declared amount) and only 81% of the declared contents of 22:6n-3.
Previous literature reports on compliance of dietary supplements with labeled contents are highly ambiguous. On one hand, less than one-third of the samples analyzed by Shim et al. (bought in Australia) contained more than 80% of their label claim for DHA. On the other hand, Damerau et al. reported similar results compared to us. The majority of samples contained the labeled contents, or close to the labeled contents, with a median of 95% of declared n-3 FAs for products of the Finnish market (vs. a median of 99% for our products)[24]. From the German market, a small sample size of three dietary supplements had been analyzed by Kutzner et al. and ten by Koch et al. and did generally also meet the expectation of n-3 FA contents [9, 23].
Interestingly, the observation in our samples of higher quantities of EPA than DHA in relation to their declaration (median of 103% EPA and 95% DHA) is in contradiction to the reports of Damerau et al., where median values of 95% for EPA and 109% DHA were found. The reason for this mismatch is unclear, but it makes a general higher oxidative susceptibility of DHA (compared to EPA) unlikely as the reason for the lower values found in our samples.
The eight samples containing fish oil concentrates (as identified by LC–MS analysis) showed approximately 8% higher n-3 FA content than the average (Table 1) with DHA contents being more than 50% higher. However, concentrates did not show differences in meeting the label claim.
Quantitative determination of vitamin E derivatives
To the majority of n-3 FA supplements, vitamin E was added by the manufacturers. In addition to their vitaminizing effect some derivatives show antioxidant properties potentially delaying the onset of lipid oxidation and improving the stability of the products [27].
Noteworthy, almost all products contained the declared amount of tocochromanols (Fig. 5) but a number of samples were labeled incorrectly regarding the type or presence of tocopherols (Table 2).
Declared and measured contents of declared n-3 fatty acids (left) and vitamin E derivatives (right) of nutritional supplements with high contents of n-3 fatty acids. Dashed lines indicate the ranges for samples containing 120, 110, 100, 90 and 80%, respectively, of the declared contents of n-3 fatty acids or vitamin E. Vitamin E content of sample 5 (86.1 mg/g) is not displayed (Table 2)
Close to all samples contained quantifiable contents of one of the vitamin E derivatives (31/33), most frequently α-tocopherol (23/33) (Table 2). Two samples did not contain detectable contents, while two products contained both derivatives, but only one derivative with quantifiable contents. However, more than a quarter of the products were not labeled correctly. Four products contained a different derivative as indicated and five products did not indicate the content or presence of detected vitamin E derivatives at all (Table 2).
The average measured concentration of α-tocopherol and tocopherol acetate in the capsule contents was 13.8 mg/g and ranged between 1.2 and 86.1 mg/g (Table 2). When only considering the vitamin E content and neglecting whether the right derivative was indicated, 31 of 33 products agreed with the information provided on the label. Most of the samples contained a large excess of vitamin E leading to an average ratio of measured to declared content of 143% (excluding sample 12), with a maximum of 354% in sample 27. Sample 12 had a content of 11.7 mg/g α-tocopherol, but only 0.1 mg/g of α-tocopherol were indicated (ratio of 11,700%) due to a mistake in labeling (as confirmed by the supplier). The two samples not meeting the aimed content of vitamin E contained 85 and 87% of the labeled amounts, respectively.
To the best of our knowledge, there are currently no data available on the composition and quantity of vitamin E added to PUFA supplements. Sunarić et al. already determined the added vitamin E content in different dairy products, infant formulas, and plant milks by HPLC with fluorescence detection [38]. Because the products differ strongly in their matrix and especially in the amount and type of lipids the vitamin E content is not directly comparable to the products of our interest. Most dairy products with a maximum of 20% of fat contained less than 1 µg/g α-tocopherol—which were probably naturally occurring in the fat fraction—and no quantifiable contents of α-tocopherol acetate. Products with declared contents of vitamin E observed by Sunarić et al. contained up to 57 µg/g vitamin E, which is approximately 250 times less concentrated than in our samples. In contrast to most of our samples, their samples contained both tocopherol and tocopheryl acetate, but they also reported inadequate labeling.
Tocochromanols can act as radical scavenger and delay lipid oxidation by reacting preferentially but are usually structurally altered in the process. It was therefore assumed that samples with lower contents of LC-PUFAs could also show lower contents of tocochromanols (and vice versa), if the lack of n-3 FAs is caused by lipid oxidation not just due to a lower addition to the formulation. Since the contents of n-3 fatty acids meet their label claim quite well, it is not possible to make valid statements regarding possible correlations of the vitamin E content and lack of n-3 fatty acids. In further studies, it might be interesting to analyze supplements at the end of their shelf life or during storing experiments promoting lipid oxidation and investigate vitamin E, n-3 fatty acid contents, as well as lipid oxidation products. Furthermore, it would be interesting to check the antioxidant effect of tocopheryl acetate compared to other tocochromanols, since it is often used but not expected to exhibit any antioxidative effect, and hydrolysis to α-tocopherol is unlikely in the lipid-rich medium [39, 40].
Conclusions
In our study we investigated 33 dietary supplements with high contents of n-3 FAs from the German market regarding their lipid classes, FA contents, and vitamin E derivatives and contents. The products were based on different types of oils, e.g., fish oil from different species, krill oil, microalgae oil, or linseed oil and manufacturing processes like creating concentrates with higher concentrations of the desired FAs.
Depending on their ingredients and manufacturing, the dietary supplements showed different principal lipid classes and FA pattern. Products based on different ingredients can exhibit similar FA pattern but completely different lipid classes, e.g., fish oil with mainly TGs, krill oil containing high amounts of PCs, or fish oil concentrates with high concentrations of EEs and DGs. Even though the use of concentrates can influence the bioavailability and oxidative stability, only one of eight products suspected to contain concentrates were labeled accordingly, which prevents consumers from identifying what kind of products they are buying.
The observed products on the German market generally do not show a major deficiency of the indicated n-3 FAs and should therefore be suitable to support a sufficient intake of n-3 FAs. Regarding the content of vitamin E, almost every product contained either α-tocopherol or tocopheryl acetate and contents agreed well with their claimed contents of vitamin E. However, numerous samples were declared to contain the wrong derivative and one contained exceedingly high concentrations vitamin E. There was no significant correlation between the kind of derivative or amount of vitamin E and the content of n-3 FAs or accordance to their label claim. The influence of the different derivatives and contents of vitamin E could be potentially better observed in products that are at the end of their shelf lives or during storing experiments forcing lipid oxidation.
Data availability
All data to support the findings of the manuscript are presented in the main document and the supporting information.
Abbreviations
- ALA:
-
α-Linolenic acid
- DG:
-
Diacylglycerol
- DHA:
-
Docosahexaenoic acid
- ECN:
-
Equivalent chain number
- EE:
-
Ethyl ester
- EPA:
-
Eicosapentaenoic acid
- FAEE:
-
Fatty acid ethyl ester
- FAME:
-
Fatty acid methyl ester
- GC–MS:
-
Gas chromatography–mass spectrometry
- ISTD 1:
-
Internal standard PC 17:0/17:0
- ISTD 2:
-
Internal standard TG 18:1/18:1/18:1-glycerol-D5
- ISTD 3:
-
Internal standard 14:0 fatty acid ethyl ester
- ISTD 4:
-
Internal standard TG 18:0/18:0/18:0-D12
- LC–MS:
-
Liquid chromatography–mass spectrometry
- LC-PUFA:
-
Long-chain polyunsaturated fatty acid
- LC-UV/Vis:
-
Liquid chromatography–UV/Vis detection
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- n-3 FA:
-
n-3 Fatty acid
- PC:
-
Phosphatidyl choline
- PUFA:
-
Polyunsaturated fatty acid
- S/N:
-
Signal-to-noise ratio
- TG:
-
Triacylglycerol
References
Husted KS, Bouzinova EV (2016) The importance of n-6/n-3 fatty acids ratio in the major depressive disorder. Medicina 52(3):139–147
Connor WE (2000) Importance of n-3 fatty acids in health and disease. Am J Clin Nutr 71:171S-S175
Ludwig DS, Willett WC, Volek JS, Neuhouser ML (2018) Dietary fat: from foe to friend? Science 362(6416):764–770
Alasalvar C, Taylor T (2002) Seafoods—quality, technology and nutraceutical applications, Berlin, Heidelberg
Belitz H-D, Grosch W, Schieberle P (2008) Lebensmittelchemie.
EFSA assesses safety of long-chain omega-3 fatty acids (2012) European Food Safety Authority https://www.efsa.europa.eu/en/press/news/120727 (accessed 21.03.2022)
Umsatz mit Nahrungsergänzungsmitteln in Deutschland in den Jahren 2014 bis 2020 (2021) IQVIA https://de.statista.com/statistik/daten/studie/1040811/umfrage/umsatz-mit-nahrungsergaenzungsmitteln-in-deutschland/ (accessed 24.02.2022)
Welche Arten von Nahrungsergänzungsmitteln haben Sie in den letzten 12 Monaten eingenommen? (2021) Statista https://de.statista.com/prognosen/722012/umfrage-zum-konsum-von-nahrungsergaenzungsmitteln-in-deutschland (accessed 24.02.2022)
Kutzner L, Ostermann AI, Konrad T, Riegel D, Hellhake S, Schuchardt JP, Schebb NH (2017) Lipid class specific quantitative analysis of n-3 polyunsaturated fatty acids in food supplements. J Agric Food Chem 65(1):139–147
Rubio-Rodríguez N, Beltrán S, Jaime I, Diego SM, Sanz MT, Carballido JR (2010) Production of omega-3 polyunsaturated fatty acid concentrates: a review. Innov Food Sci Emerg Technol 11(1):1–12
Killeen DP, Marshall SN, Burgess EJ, Gordon KC, Perry NB (2017) Raman spectroscopy of fish oil capsules: polyunsaturated fatty acid quantitation plus detection of ethyl esters and oxidation. J Agric Food Chem 65(17):3551–3558
Sullivan Ritter JC, Budge SM, Jovica F, Reid A-JM (2015) Oxidation rates of triacylglycerol and ethyl ester fish oils. J Am Oil Chem Soc 92(4):561–569
Nahrungsergänzungsmittel mit Zusatz von Fettsäure-Ethylestern aus verestertem Fischölkonzentrat (BVL 12/01/004) (2012) Bundesamt für Verbraucherschutz und Lebensmittelsicherheit
Neubronner J, Schuchardt JP, Kressel G, Merkel M, Schacky C, Hahn A (2011) Enhanced increase of omega-3 index in response to long-term n-3 fatty acid supplementation from triacylglycerides versus ethyl esters. Eur J Clin Nutr 65(2):247–254
Nordøy A, Barstad L, Connor WE, Hatcher L (1991) Absorption of the n-3 eicosapentaenoic and docosahexaenoic acids as ethyl esters and triglycerides by humans. Am J Clin Nutr 53(5):1185–1190
Schuchardt JP, Hahn A (2013) Bioavailability of long-chain omega-3 fatty acids. Prostagland Leukot Essent Fat Acids 89(1):1–8
Ghasemifard S, Turchini GM, Sinclair AJ (2014) Omega-3 long chain fatty acid “bioavailability”: a review of evidence and methodological considerations. Prog Lipid Res 56:92–108
Ulven SM, Holven KB (2015) Comparison of bioavailability of krill oil versus fish oil and health effect. Vasc Health Risk Manag 11:511–524
Cui L, Decker EA (2016) Phospholipids in foods: prooxidants or antioxidants? J Sci Food Agric 96(1):18–31
Bandarra NM, Campos RM, Batista I, Nunes ML, Empis JM (1999) Antioxidant synergy of α-tocopherol and phospholipids. J Am Oil Chem Soc 76(8):905–913
Kleiner AC, Cladis DP, Santerre CR (2015) A comparison of actual versus stated label amounts of EPA and DHA in commercial omega-3 dietary supplements in the United States. J Sci Food Agric 95(6):1260–1267
Shim SM, Santerre CR, Burgess JR, Deardorff DC (2003) Omega-3 fatty acids and total polychlorinated biphenyls in 26 dietary supplements. J Food Sci 68(8):2436–2440
Koch E, Kampschulte N, Schebb NH (2022) Comprehensive analysis of fatty acid and oxylipin patterns in n3-PUFA supplements. J Agric Food Chem 70(13):3979–3988
Damerau A, Ahonen E, Kortesniemi M, Puganen A, Tarvainen M, Linderborg KM (2020) Evaluation of the composition and oxidative status of omega-3 fatty acid supplements on the Finnish market using NMR and SPME-GC–MS in comparison with conventional methods. Food Chem 330:127194
Hamilton K, Brooks P, Holmes M, Cunningham J, Russell FD (2010) Evaluation of the composition of omega-3 fatty acids in dietary oil supplements. Nutr Diet 67(3):182–189
Ritter JCS, Budge SM, Jovica F (2013) Quality analysis of commercial fish oil preparations. J Sci Food Agric 93(8):1935–1939
Seppanen CM, Song Q, Saari Csallany A (2010) The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J Am Oil Chem Soc 87(5):469–481
Hamilton RJ, Kalu C, McNeill GP, Padley FB, Pierce JH (1998) Effects of tocopherols, ascorbyl palmitate, and lecithin on autoxidation of fish oil. J Am Oil Chem Soc 75(7):813–822
Wang J, Han L, Wang D, Sun Y, Huang J, Shahidi F (2021) Stability and stabilization of omega-3 oils: a review. Trends Food Sci Technol 118:17–35
Pang Z, Zhou G, Ewald J, Chang L, Hacariz O, Basu N, Xia J (2022) Using MetaboAnalyst 5.0 for LC–HRMS spectra processing, multi-omics integration and covariate adjustment of global metabolomics data. Nat Protoc 17(8):1735–1761
Christie WW (1994) Gas chromatography and lipids. Ayr, Scotland
Berdeaux O, Márquez-Ruiz G, Dobarganes M (1999) Characterization, quantitation and evolution of monoepoxy compounds formed in model systems of fatty acid methyl esters and monoacid triglycerides heated at high temperature. Grasas Aceites 50(1):53–59
(2019) 2.4.29. Composition of fatty aids in oils rich in omega-3-acids. In: European Pharmacopoeia 9.7
Lawson LD, Hughes BG (1988) Human absorption of fish oil fatty acids as triacylglycerols, free acids, or ethyl esters. Biochem Biophys Res Commun 152(1):328–335
Ritter JCS (2012) Chemical measures of fish oil quality: oxidation products and sensory correlation
Martín D, Terrón A, Fornari T, Reglero G, Torres CF (2012) Oxidative stabilization of ultra-high omega-3 concentrates as ethyl esters or triacylglycerols. Food Res Int 45(1):336–341
Lordan R, Redfern S, Tsoupras A, Zabetakis I (2020) Inflammation and cardiovascular disease: are marine phospholipids the answer? Food Funct 11(4):2861–2885
Sunarić S, Lalić J, Spasić A (2017) Simultaneous determination of alpha-tocopherol and alpha-tocopheryl acetate in dairy products, plant milks and health supplements by using SPE and HPLC method. Food Anal Methods 10(12):3886–3901
Burton G, Traber M (1990) Vitamin E: antioxidant activity, biokinetics, and bioavailability. Annu Rev Nutr 10:357–382
Bundesinstitut für Risikobewertung (2004) Verwendung von Vitaminen in Lebensmitteln. Berlin
Acknowledgements
The authors are grateful to Rudolf und Henriette Schmidt-Burkhardt Stiftung for the financial support.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AZ and SH: conceived the study. Sample preparation, data collection, analysis, and interpretation were performed by AZ, LV, and SH. The first draft of the manuscript was written by AZ and SH and subsequently reviewed and edited by all authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Compliance with ethics requirements
No human or animal studies have been conducted for this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zartmann, A., Völcker, L. & Hammann, S. Quantitative analysis of fatty acids and vitamin E and total lipid profiling of dietary supplements from the German market. Eur Food Res Technol 249, 1035–1048 (2023). https://doi.org/10.1007/s00217-022-04193-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04193-7