Abstract
Honey, a natural healthy liquid bee product, is rich in amino acids, vitamins, and other essential nutrients. Different origin honeys also varied in organic acids. The objective of this study is to establish an efficient solid-phase extraction-gas chromatography–mass spectrometry (SPE-GC–MS) method to eliminate interference of sugar and other impurities for accurately estimating 22 organic acids in honey by optimizing extraction, purification, derivatization, and gas chromatography–mass spectrometry (GC–MS) analytical conditions. After being extracted, purified and derivatized, organic acids in honey were qualified and quantified by GC–MS. This method was evaluated experimentally, and the results showed that, within a certain concentration range, the standard curve linear relationship was satisfactory (R2 > 0.9942), and the target organic acid recovery rate was 86.74% ~ 118.68%. Besides, precision (relative standard deviation, RSD = 2.98% ~ 13.42%), detection limit (LOD = 0.002 ~ 0.2 mg kg−1), and quantification limit (LOQ = 0.008 ~ 0.5 mg kg−1) met the target requirements. Also, based on this analytical method, the organic acids in five types of honey (acacia, jujube, vitex, canola and linden honey) were estimated. Notably, they all contained 22 different kinds of organic acids, and significant differences (p < 0.05) in the organic acid content and composition among different honey varieties were observed. PCA analysis showed that the five honeys could be differentiated based on the content of 22 organic acids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Honey is a sweet and pure natural product produced by honeybees (Apis mellifera) that collect nectar from flowers and honeydew from plant secretions or the excretion of insects that suck plants before brewing them into nutritious food. Its main components include water, sugar, minerals, vitamins, organic acids, polyphenols, etc. [1, 2]. The specific chemical composition and sensory properties of honey depend on the type of flowers visited by the bees, climatic conditions in which the specified plants grow, and different storage conditions of honey [1, 3]. The composition and concentration of organic acids in honey are majorly from nectar, honeydew, or generated through glucose and fructose metabolism during brewing [4, 5]. These organic acids mainly include gluconic, lactic, citric, malic, succinic, and pyroglutamic acid [1, 6, 7]. In addition, honey also includes some aliphatic dicarboxylic acids, and adipic, suberic, azelaic, and sebacic acid fatty acids [8]. In conclusion, there are three main sources of organic acids in honey, those contained in the nectar or honeydew collected back by the bees themselves, those produced by the metabolism of substances in the honeycomb, and some organic acids secreted by the bees themselves. These organic acids account for about 0.57% of the total honey produced [1] and can usually play the role of bacteriostasis, antioxidation and other potential activities in honey [1, 9]. Also, organic acids in honey are in a position to regulate honey pH and influence honey flavor and texture [10]. In addition, the organic acids in honey can also be used as marker for the identification and distinction between different types of honey. The main organic acid in fir honey is galacturonic acid rather than glucuronic acid found in most honeys; therefore, galacturonic acid can be used as a marker of authenticity in fir honey [11]. Aliphatic organic acids may serve as markers for authenticity identification of bracatinga honeydew honey and nectar honey [12]. Therefore, the determination of the type and content of organic acids in honey is important to the study of honey quality characteristics as well as authenticity identification.
The sugars account for about 95% of the dry matter (DM) content in honey, which could interfere with the accurate quantification of organic acids. Therefore, there is a requirement to eliminate the sugar interference during the pretreatment to quantify organic acids. Solid-phase extraction (SPE) is a pretreatment technology that can effectively remove sugar interference and improve the detection accuracy of organic acids in honey [13]. Suárez-Luque et al. studied the contents of maleic acid, malic acid, fumaric acid, succinic acid, and citric acid in honey by passing them through a Waters Accell Plus QMA SPE column [14]. Derivatization, as a sample pretreatment approach, could change the chemical properties of samples to achieve better retention and detection on GC [15]. In this study, some organic acids that are highly polar and nonvolatile were difficult to be separated by GC. Thus, the derivatization treatment was used to reduce the polarity of organic acids and make them evaporable, which could be quantified accurately by GC–MS.
To date, extraction and detection methods of organic acids in honey could be categorized into enzymatic, capillary electrophoresis (CE), and chromatography [ion chromatography (IC), liquid chromatography (LC), and GC]. The enzymatic method is usually used to calculate non-aromatic organic acid content in honey [16]. It has high specificity, but only one organic acid can be detected once [9]. Capillary electrophoresis (CE), a vital separation and detection technique of organic acids, avoids the matrix effect, has low cost, and short time. This technique detects 14 different aliphatic organic acids within 9 min [7]. However, it has poor reproducibility and limited sensitivity [9]. Chromatographic techniques, particularly GC and LC, have been widely used to determine unique organic acids due to their high sensitivity and good reproducibility. Previously, L-malic acid, maleic acid, succinic acid, citric acid, D-malic acid, shikimic acid, and quinic acid in bee products were estimated through HPLC [6, 17]. GC is generally suitable for estimating volatile organic acids, such as aromatic acids, but it is not appropriate for aliphatic organic acids. Therefore, to analyze aliphatic organic acids, enhancing their volatility by reducing their polarity through derivatization is key. After derivatization, GC–MS or GC–FID could be employed to analyze non-aromatic organic acids in honey [9, 18, 19]. Jurado-Sánchez [20] analyzed 29 categories of organic acids in food and beverage using SPE pretreatment and GC–MS, which showed excellent LOD (0.5 ~ 1000 µg kg−1) and precision (RSD ≤ 6.2%). However, in the two honey samples used in that research, only five organic acids (acetic, malic, succinic, fumaric, and citric) were detected. Compared with LC–MS, GC–MS allows better separation of target compounds, has better system stability and reproducibility, and has a wider linear range. In addition, GC–MS has a library of ion fragmentation spectra, which is more conducive to the qualitative and quantitative analysis of unknown compounds [21]. Moreover, organic acids, which belong to polar substances, are notoriously difficult to retain on reversed-phase columns and are not durable and are unstable using normal-phase columns in liquid-phase systems. Thus, it is suitable to be analyzed using a gas-phase separation system.
In summary, this study has established an analytical method for 22 different types of organic acids in honey through optimizing pretreatment. SPE conditions to eliminate sugar interference, and derivative, chromatography, and spectrometry conditions. In this study, a method was established to compare the effects of different solid-phase extraction cartridges, and the cartridges with the best retention effect was selected for optimization of various extraction conditions. Then the extracts were subjected to a two-step derivatization reaction: one for derivatization of residual sugars in the extracted compounds to avoid them from being derivatized in the next step and the other for derivatization of organic acid compounds to facilitate gas-phase separation. The two-step derivatization method further reduced the interference of impurities during instrumental detection and ensured accurate quantification of compounds. Finally, the gas-phase separation conditions and mass spectrometry qualitative and quantitative ion fragments of each target organic acid were committed to complete the quantification of organic acid compounds. Furthermore, this method has been verified, and organic acids of five types of honey were estimated in practice.
Materials and methods
Samples
Seventeen honey samples from five types (acacia, jujube, vitex, canola, and linden) were collected from an apiary for this research (Table S1) and subsequently stored at 4 °C before use. Five types of honey are common large-scale single-flower honey in China. The surrounding area is covered with large areas of targeted plant for at least 3–4 km around during the blooming period, and no other plants co-exist at the same time due to the special geographical environment of China, which allows China to produce several large monofloral honey species such as acacia, jujube, litchi, vitex, canola, buckwheat, Sapium discolor, and linden honey. We collected the honey samples during the blooming period of the specific nectar plant, thus ensuring the unity and authenticity of the samples.
Preparation of standard solution
Detailed information on 22 standard products of organic acids is shown in Table S2. They were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. Consequently, we prepared standard stock solution, mixed standard stock solution, mixed standard working solutions of 22 organic acids, and their internal standard stock and working solutions, using methanol as solvent. All the solutions were stored at – 20 °C away from light for later use.
Sample preparation
A 1.0 g honey sample was weighed using an electronic balance (Germany Sartorius Co., Ltd., BSA124S) and then placed into a 50 mL centrifuge tube [if the sample had crystals, we removed crystallization using a 40 °C water bath (Shanghai Heng Technology Co., Ltd., TUS-200P)]. Then, we dissolved the sample in 10 mL deionized water (prepared by USA Millipore Company, Milli-Q), followed by centrifugation (1-15PK centrifuge, Sigma-Aldrich Co., Ltd.) at 8000 r/min for 15 min. Next, all supernatant was diluted to 50 mL volume with deionized water and mixed uniformly. After that, 5 mL of solution was taken out to the new centrifuge tube and mixed into 5μL of each of two types of internal standard (DL-malic acid-2,3,3-d3、citric acid-2,2,4,4-d4), then adjusted to pH = 7.5 using 0.1 mol L−1 NaOH.
Purification
Solid-phase extraction column (Waters Company, Oasis® MAX) was activated and balanced using 10 mL methanol and 10 mL deionized water, respectively. It was used for the extraction of the prepared honey solution, which was then rinsed with 10 mL deionized water and eluted with 8 mL methanol solution (pH = 0.4), and the eluent was collected. Lastly, the eluent was concentrated with nitrogen to about 1 mL volume, transferred to a 1.5 mL centrifuge tube, and then concentrated to dryness using a vacuum concentrator (Beijing Geim Technology Co., Ltd., cv200) (1300 rpm/min, 35 °C).
Derivatization
First, the samples were derivatized using methoxyamine hydrochloride. The samples that were concentrated to dryness were mixed with 20 µL of 50 mg mL−1 methoxyamine hydrochloride in pyridine solution through vortexing for 30 s. The resulting mixture was derivatized in a metal bath at 40 °C for 30 min. Next, we employed the silylation derivatization method. Consequently, 180 µL of MSTFA was added into the mixture, followed by vortexing for 30 s, 30 min derivatization at 37 °C, and centrifugation at 14,000 r/min for 10 min at 4 °C. The supernatant was transferred into the sample bottle for further analysis.
GC conditions
Column: DB-5 MS UI (Agilent Technologies, Santa Clara, CA) (30.0 m × 0.25 mm × 0.25 µm, thickness); carrier gas was high-purity helium (He); oven temperature: 60.0 °C; inlet temperature: 250 °C; pressure: 57.5 kPa; total flow: 16.0 mL min−1; column flow: 1.0 mL min−1; linear velocity: 36.5 cm s−1; purge flow: 5.0 mL min−1; injection mode: splitless injection; injection volume: 1 µL; column temperature program: 60.0 °C for 2 min, 10.0 °C min−1 heating rate to 250.0 °C for 2 min, and 20.0 °C min−1 heating rate to 325.0 °C for 5 min.
MS conditions
Ion source (EI) temperature: 200 °C; ion energy: 70 eV; interface temperature: 280 °C; detector voltage: 0 kV; solvent delay: 6 min; SCAN mass range: 45–600 m/z; monitoring mode: SIM. The parameters are demonstrated in Table S3. For mass spectrometry quantification, the organic acids were divided into two main categories: one for malic acid structure and the other for citric acid structure, based on the structure retention time of the two internal standards. To avoid simultaneously detecting too many ions at one time and affecting the quantitative accuracy, the compounds were divided into two groups for acquisition according to the structure of the internal standard.
Statistical analysis
All samples were measured with three sub-samples analyzed in parallel; SPSS 26.0 was used to analyze molecular variance, and SIMCA 14.1 was used for PCA analysis. All results were expressed as mean ± standard deviation.
Results
Selecting sample solution pH value using solid-phase extraction
Based on the Henderson–Hasselbach equation, the pH value of the matrix should at least be two pH units higher than the pKa value of the target compound to ensure 99% ionization of weak acid compounds. In this experiment, the target organic pKa value was 2.42 ~ 5.19; hence, the pH value of the honey sample solution should be higher than 7.19. However, in a high pH environment, some saccharides in honey could exist as anions [22, 23], which are adsorbed on the anion solid-phase extraction column and subsequently compete with the target organic acids for retention. Hence, the efficiency of the extraction process is seriously weakened. In summary, the pH of the substrate was chosen to be slightly higher than pKa by two units in this study to ensure complete ionization of the weak acid and to avoid interference from the ionized sugar. Therefore, the pH of the honey solution of the upper sample was kept at 7.5.
Selecting eluent pH value using solid-phase extraction
When eluting weak acidic compounds, the pH value of the solvent should at least be two units lower than the target compound pKa value to ascertain that 99% of the weak acidic compounds are in a neutral state. Only in such state, weak acidic compounds can be eluted from the anion exchange column. In this experiment, the lowest pKa of the target compound was 2.42, so the pH value of the eluent was defined as less than 0.42. As the eluent needs dryness in the later derivatization stage, methanol was selected as the eluting solvent. Meanwhile, considering that the pKa value of Waters Oasis MAX column is 0 ~ 14, the pH value of methanol solution was adjusted to 0.4 using HCl.
Selecting internal standards
This study used the internal standard method to analyze the endogenous organic acids in honey. It could eliminate errors caused by operation and loading volume and correct matrix effects, improving the accuracy of quantitative results. However, the internal standard is expensive, and the corresponding internal standards are hardly found for some unique target compounds. Thus, substances possessing similar structures to the target compounds are commonly chosen as the internal standards. Notably, all the target organic acids in our study with a carboxyl molecule structure. Based on their retention time, DL-malic acid-2,3,3-d3 and citric acid-2,2,4,4-d4, which also have a carboxyl molecule structure, were selected as internal standards (Table S4). The DL-malic acid-2,3,3-d3 (chemical purity: 98%, isotope purity: 97.9%) was used as the internal standard of different acids, including L-( +) lactic, 3-hydroxypropionic, malonic, benzoic, propanedioic, malic, glycolic, succinic, fumaric, oxalic, and adipic acid. On the other hand, citric acid-2,2,4,4-d4 (chemical purity: 97%) was selected as the internal standard for DL-pyroglutamic, DL-3-phenyllactic, suberic, trans-aconitic, DL- tartaric, azelaic, shikimic, citric, α-ketoglutarate, quinic, and gluconic acids.
Selecting the solid-phase extraction (SPE) column
Target organic acids are all weakly acidic compounds (Table S2), which could be extracted using strong anion exchange columns. Therefore, we used Waters Oasis MAX, Bond Elutes SAX, and Cleanert SAX (Table S5) to extract and purify the organic acids in honey and subsequently compared the extraction and retention effects of 22 organic acids. Figure 1A demonstrates the extraction and purification effects on different organic acids in honey using three SPE columns. It shows that Waters Oasis MAX is superior to the other two SPE columns in extracting. Waters Oasis MAX is a mixed anion exchange column with both ion exchange retention mode and reverse-phase retention mode. It had good adsorption on weak acidic substances and could remove some compounds such as sugar and strong ionic substances. Therefore, the Waters Oasis MAX SPE column is the best to extract and purify organic acids from honey solution.
Optimizing the sample preparation method. A Comparison of target organic acids in honey purified using three solid-phase extraction columns: when other experimental conditions remained constant, we compared the extraction and retention effects of three kinds of solid-phase extraction columns: Waters Oasis MAX, Bond Elutes SAX, and Cleanert SAX. B For comparing the response values of target organic acids at different elution volumes, the Waters Oasis MAX was used as a solid-phase extraction column. Subsequently, by having all other conditions constant, we compared the response values of target organic acids under different eluent volumes
Selecting the eluent volume
The eluent (methanol solution, pH = 0.4) volume effect was investigated based on the Water Oasis Max SPE column. When the methanol solution equaled or was greater than 8 mL, the target organic acid could be eluted fully. Thereafter, the response value of the target organic acids remained constant by increasing the eluent volume. Therefore, we chose 8 mL of methanol solution to elute the target organic acids (Fig. 1B).
Selecting derivative agents
A derivative reagent is a substance used to modify a compound to obtain another compound with the desired properties. The feature of this new compound is suitable for GC or LC analysis. The silylation derivatization method is mainly used to detect short-chain organic acids through GC–MS. Its derivatives have a substantial volatility and good stability and show narrow and symmetrical peaks suitable for GC analysis of non-volatile samples. The most commonly used silylation derivative reagent is the trimethylsilyl, among which N-methyl-N-trimethylsilyl trifluoroacetamide (MSTFA) is the best. In its derivatives, the MSTFA has advantages like high reactivity, wide derivative range, and good volatility [24,25,26,27]. Christou et al. studied different analytical conditions to estimate ten free organic acids through GC–MS, focusing on the derivatization (trimethyl silylation) conditions and the stability of the derivatives formed [28].
Because of reducing sugars with cyclized and open-loop structures in honey, multiple interference peaks of sugars would be produced if directly derived through silanization (not conducive for later processing [29]). Therefore, methoxyamination was first introduced before silylation to protect the aldehydes and ketones carbonyl groups in reducing sugars from cyclization (methoxyamination–silylation derivatization) [30, 31]. Moros et al. studied the effect of three commonly used silylation reagents (MSTFA reagents, BSTFA reagents containing 1% TMCS, and MSTFA reagents containing 1% TMCS) in plasma derivatization [32]. The results showed the MSTFA containing 1% TMCS provided a high signal. This study obtained a highly efficient derivatization reaction technique by optimizing the derivatization conditions of methoxyamination and silylation processes.
Briefly, we used the methoxyamine hydrochloride in pyridine solution as the derivatization reagent in the first step (methoxyamination derivatization). Then, in the second step (silylation derivatization), MSTFA was used as the derivatization reagent. Subsequently, we studied different silylation conditions (silylation reagent dosage, derivatization temperature, and derivatization time). In the first step of this experiment, derivatization reduces the peak of carbohydrate isomers; hence, this step is not the critical factor influencing target organic acids detection. Previously, studies found that the suitable temperature for methoxyamination derivatization was 40 °C for 30 min [25, 32].
Selecting an ideal derivative dosage
Ideal derivatization of the target compounds should be derivatized thoroughly within the stipulated time and temperature; thus, the dosage of derivatization reagent becomes the crucial factor during derivatization. The derivatization reagent is usually excessive to fully derivatize the target compounds, but it will increase impurity peaks unfavorable for analysis [33, 34]. Here, we compared the effect of silylation reagents (MSTFA) volume (100 µL, 150 µL, 180 µL, and 200 µL) on derivatization. When the volume of the derivatization reagent increased from 100 µL to 150 µL, the organic acid value increased. Of note, the best effect was achieved when the derivatization reagent was 150 ~ 180 µL. Furthermore, even when the derivatization reagent reached 200 µL, no significant increase in the content of organic acids was detected. Therefore, the silylation reagents used in this experiment were 180 µL (Fig. 2A).
Optimizing derivatization conditions. A The effect of the volume of silylation reagent on the derivatization of the target organic acids (100 μL,150 μL,180 μL, and 200 μL). B The effect of temperature on the derivatization of target organic acids (30 °C, 37 °C,50 °C, and 70 °C). C The impact of derivatization time on the derivatization of target organic acids (10 min,30 min,1 h, and 2 h). D The derivative standing time effect on the derivatization of the target organic acids (0 h,4 h,8 h,12 h, 24 h, and 48 h)
Derivatization temperature and time selection
Completing the silylation derivatization process also depends on the reaction temperature and time. In our study, silylation reagents were derivatized in a shaker at 30 °C, 37 °C, 50 °C, and 70 °C temperatures, and the derivatization response values were compared. The quantitative analysis showed that the derivatization was incomplete at 30 ℃ because the response values of all target organic acids were low. At 37 °C, the response value was already high, and it decreased as temperature increased, which could be due to the decomposition of derivatives. In addition, due to derivative instability, the silylation time should not be too long [32]. The derivatization efficiency at 10 min, 30 min, 1 h, and 2 h was evaluated at 37 °C, using a derivatization reagent of 180 µL and vortexed for 30 s (Fig. 2B). At 10 min, the derivatization process was incomplete as the silylation reagent did not completely contact the sample. After 10 min, derivatization continued as the reaction time increased. At 30 min, the derivative content was stable. Subsequently, as reaction time increased, the derivative content remained constant (Fig. 2C). Therefore, the best derivation time is 30 min.
Stability of derivatives
The stability of the derivatives is a critical factor during optimization. Before MS analysis, derivatives must remain stable in the automatic sampler. Commonly, the derivatives could remain stable for some time, but the derivatives would be decomposed as standing time extended, so it will affect the accuracy of the measurement. Thus, this experiment investigated the peak area and retention time of derivatives in different time periods using the same instruments. After derivatization of organic acids, the derivatives did not degrade within 24 h, but degraded after 24 h. Hence, as demonstrated in Fig. 2D, all derivatives should be detected within 24 h after the batch determination.
Optimizing GC–MS conditions
The working solution mixed with organic acid standard substance was concentrated and dried using a vacuum concentrator. After a two-step derivatization reaction, the whole scanning in the mass range (45 ~ 600 m/z) was carried out through GC–MS. Then, the GC–MS conditions were optimized. Finally, we determined the optimal temperature rising program. Figure 3 illustrates the total ion current diagram.
Total ion current diagram showing 22 kinds of organic acids. 1: L-( +)-lactic acid, 2: glycolic acid, 3, oxalic acid, 4: 3-hydroxypropionic acid, 5: propanedioic acid, 6: benzoic acid, 7: succinic Acid, 8: fumaric acid, 9: propanedioic acid, 10: malic acid, 11: adipic acid, 12: DL-pyroglutamic acid, 13: α-ketoglutaric acid, 14: 3-phenyllactic acid, 15: tartaric acid, 16: suberic acid, 17: trans-aconitic acid, 18: azelaic acid, 19: shikimic acid, 20: citric acid, 21: D-(-)-quinic acid, 22: gluconic acid
Selecting an MS scanning mode
The SIM GC–MS mode provided a high signal and enhanced the selectivity and sensitivity suitable for quantitative analysis. Even if two similar targets with different quantitative ions cannot be separated by chromatography, quantitative analysis for the two similar substances could still be achieved under the SIM mode. Therefore, GC–MS SCAN mode was used for qualitative analysis of target organic acids, whereas the GC–MS SIM mode was applied in quantitative analysis. The single standard working solutions of 22 kinds of organic acids were diluted into 10 μg mL−1, concentrated and dried using a vacuum concentrator, and used for derivatization. Through SCAN mode, a full scan was carried out at a range of 45 ~ 600 m/z. Figure S1 illustrates typical mass spectra. Consequently, characteristic ions of each target organic acid derivatives were determined per the mass spectra. Notably, we established the SIM mode for optimizing the GC–MS technique using characteristic ions and retention time. Table S3 shows the SIM mode parameters.
Standard curve
The ratio of the peak area of the quantitative ion of the target component to the peak area of the quantitative ion of the corresponding internal standard was taken as Y-axis. Moreover, the target component concentration was taken as X-axis to help draw a standard curve. Table 1 demonstrates the linear relationship and correlation coefficient. The LOD of GC–MS is the corresponding value when the signal-to-noise ratio of quantitative ion chromatographic peak is three. The LOQ is the corresponding value when the signal-to-noise ratio of quantitative ion chromatographic peak is ten [35]. Therefore, low LOD and LOQ values are not recommended for high content endogenous compounds, and LOD and LOQ should be set based on the target component content range. Table 1 illustrates the LOD and LOQ of the 22 organic acids as 0.002 ~ 0.2 mg kg−1, and 0.008 ~ 0.5 mg kg−1, respectively. Compared to the others, the LOD of quinic acid was the lowest, whereas DL-pyroglutamic acid was the highest.
Recovery rate and intra-day accuracy of the GC–MS method
A standard working solution of low, medium, and high concentration gradients was mixed with honey samples, and internal standard working solution of organic acids was added. Then, the mixture was extracted as step in 2.4, analyzed per the optimized GC–MS method, and the recovery rates were calculated. As shown in Table 2, the recovery rates of 22 organic acids ranged from 86.74% to 118.68%, and the relative standard deviations ranged from 2.34 to 13.42%, which met the required analytic conditions [36].
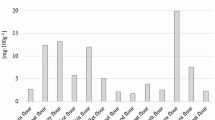
Detecting 22 organic acids in five types of honey
Table 3 demonstrates the content of organic acids in five types of honey. There are significant variations in the organic acid content among the different types of honey. Jujube honey had the highest content of organic acids (850.74 mg kg−1), followed by linden honey (644.19 mg kg−1), and acacia honey had the lowest content (409.19 mg kg−1). However, there was no significant difference (p < 0.05) between canola and vitex honey.
Discussion
Table 3 shows 22 organic acids whose content significantly differed. Compared to other organic acids, the contents of gluconic acid in the five types of honey were higher, but different honey varieties showed significant differences (p < 0.05). The lowest glucomic acid content was 198.47 mg kg−1 (acacia honey), whereas the highest content was 473 mg kg−1 (jujube honey), which was approximately 2.5 times higher than that in acacia honey. No significant difference (p < 0.05) was shown between acacia and vitex honey, and between linden and canola honey. Of note, the gluconic acid in honey is the product formed during glucose degradation and is mainly caused by oxidase or bacteria [37].
The content of tartronic acid in vitex honey was 10.53 mg kg−1, which was significantly higher than that in the other four types of honey (0.45 ~ 5.06 mg kg−1). Notably, it was 23 times higher than that in acacia honey (0.45 mg kg−1). In the human body, the tartronic acid could inhibit carbohydrate transformation into fat and prevent coronary heart disease [38]. However, it cannot be synthesized by the human body and can only be obtained from diet [39].
The adipic acid in honey may be a pheromone of bees [40] and it was detected in citrus honey by Alissandrakis [41]. In our research, the content of adipic acid in five types of honey was 1.03–2.98 mg kg−1 and that in suberic acid is 0.81–1.53 mg kg−1. The content of suberic acid in canola honey was significantly higher than that of other honeys, but the other four honeys showed no significant difference with each other.
The shikimic acid content shows a significant difference (p < 0.05) among the different types of honey. It was 16.17 mg kg−1 in linden honey, which is higher than that of the other four types of honey (0.38 ~ 1.37 mg kg−1). In vitex honey, it was the lowest (0.38 mg kg−1). Katarína et al. [17] estimated the quinic and shikimic acid contents in single-origin and forest honey from Slovakia. shikimic acid was not detected in single-origin honey, but was detected in mixed forest honey whose content was 111.6 µg g−1. However, quinic acid was not detected. In this experiment, quinic acid and shikimic acid were detected in all honey samples, and the quinic and shikimic acid contents in linden honey were the highest. Notably, shikimic acid is the starting material for the secondary metabolism in plants. The shikimic acid pathway generates chorismic acid that can synthesize tryptophan and then to be converted into prebenzoic acid, which could be turned into phenylalanine and tyrosine [42]. Shikimic acid and its derivatives have numerous pharmacological activities, including antibacterial, antitumor, anticerebral ischemia, antithrombosis, etc. [43].
The content of quinic acid in five honey samples is 0.38–12.67 mg kg−1. The average quinic acid content in linden honey was 12.67 mg kg−1, which was significantly higher than that of the other four types of honey and 33.34 times higher than that of vitex honey. The average content of quinic acid in canola honey was 8.77 mg kg−1, second only to linden honey, significantly higher than that in acacia, vitex, and jujube honey (0.38 ~ 1.84 mg kg−1). Quinic acid, a particular alicyclic acid, is widely found in higher plants and microorganisms and often coexists with shikimic acid. Like shikimic acid, quinic acid is also a precursor for synthesizing aromatic amino acids. Moreover, quinic acid is a potent antioxidant activity compound and could be used as a natural preservative [44].
To distinguish the five monofloral honeys obtained from China according to the 22 organic acids, PCA analysis was performed using SIMCA 14.1, and the cumulative variance rate could reach more than 80% when four principal components were selected. The score plots of the five honeys' principal components are shown in Fig. 4, which can distinguish the five monofloral honeys obtained from China according to the 22 organic acids. From Fig. 4, we know that under the coordinate system of principal component 1 and principal component 2, all honey samples can be separated according to each monofloral species, except for the sample of vitex honey 3 (JTM3), which cannot be separated from linden honey. In addition, three samples of canola honey were separated and more scattered in Fig. 4. This may be related to the fact that the samples were collected from three different oilseed canola production areas in China. That is, geographical factors may also lead to differences in organic acids in honey, which is also reflected in vitex honey in Fig. 4. Both vitex honey 1 (JTM1) and vitex honey 2 (JTM2) were collected from Hebei Province, China, while vitex honey 3 (JTM3) was collected from Henan Province, China, and the figure shows that JTM1 and JTM2 were more aggregated and JTM3 was independent, outside. These will be focused on in our future study.
Conclusions
Taken together, this study has established a novel analytical method that detects various organic acids based on SPE-GC–MS in SIM mode using an internal standard. This high-throughput technique could be used to estimate 22 different kinds of organic acids in honey simultaneously. Here, the organic acid extraction and derivatization conditions have been optimized, and their reliability in detecting organic acids through GC–MS has been comprehensively evaluated. The overall LOD in this method is 0.002 ~ 0.2 mg kg−1 with an LOQ = 0.008 ~ 0.5 mg kg−1 and the recovery rate 86.74 ~ 118.68%. This study verifies that this method shows a good linear relationship within a certain concentration range and has satisfactory recovery, precision, sensitivity and reproducibility, and short detection time. Generally, this research provides a solid basis for analyzing organic acids in honey. Lastly, the determination of organic acids in the five different types of honey revealed that gluconic acid content and proportion are highest in five types of honey, and the organic acid composition differs in different types of honey, which not only affects the sensory and activity of different honeys, but also can be used to distinguish various honeys.
References
da Silva PM, Gauche C, Gonzaga LV, Costa ACO, Fett R (2016) Food Chem 196:309–323
Boussaid A, Chouaibi M, Rezig L, Hellal R, Donsì F, Ferrari G, Hamdi S (2018) Arab J Chem 11(2):265–274
Gulfraz M, Ifftikhar F, Asif S, Raja GK, Asad MJ, Imran M, Abbasi K, Zeenat A (2010) African J Biotechnol 9(41):c–6906
Kalaycıoğlu Z, Kaygusuz H, Döker S, Kolaylı S, Erim FB (2017) LWT 84:402–408
Khan SU, Anjum SI, Rahman K, Ansari MJ, Khan WU, Kamal S, Khattak B, Muhammad A, Khan HU (2018) Saudi J Boil Sci 25(2):320–325
Xiaoling Z, Fei Y, Jie Y, Xiao X, Hong W, Rui L (2010) Chin J Chromatogr 28(10):945–949
Brugnerotto P, Della Betta F, Gonzaga LV, Fett R, Oliveira Costa AC (2019) J Food Compos Anal 82:103243
Wilkins AL, Lu Y (1995) J Agric Food Chem 43(12):3021–3025
Mato I, Huidobro JF, Simal-Lozano J, Sancho MT (2006) Crit Rev Anal Chem 36(1):3–11
Suto M, Kawashima H, Nakamura Y (2020) Food Anal Methods 13(12):2249–2257
Daniele G, Maitre D, Casabianca H (2012) Rapid Commun Mass Spectrom 26(17):1993–1998
Seraglio SKT, Bergamo G, Brugnerotto P, Gonzaga LV, Fett R, Costa ACO (2021) Food Chem 343:128449
Nayik GA, Nanda V (2015) Eur Food Res Technol 240(6):1091–1100
Suárez-Luque S, Mato I, Huidobro JF, Simal-Lozano J, Sancho MT (2002) J Chromatogr A 955(2):207–214
Mohd MA (2012) Advanced gas chromatography progress in agriculture biomedical and industrial applications. InTechOpen, London
Tourn ML, Lombard A, Belliardo F, Buffa M (1980) J Apic Res 19(2):144–146
Hroboňová K, Lehotay J, Čižmárik J (2007) J Liq Chromatogr Relat Technol 30(17):2635–2644
Horváth K, Molnár-Perl I (1998) Chromatographia 48(1–2):120–126
Pilz-Guther D, Speer K (2004) Dtsch Lebensm-Rundsch 100(3):84–87
Jurado-Sánchez B, Ballesteros E, Gallego M (2011) Talanta 84(3):924–930
Kivilompolo M, Obůrka V, Hyötyläinen T (2007) Anal Bioanal Chem 388(4):881–887
Cai Y, Liu J, Shi Y, Liang L, Mou S (2005) J Chromatogr A 1085(1):98–103
Cailong H, Dan L, Xiaolei L (2011) J Changchun Univ 21(10):56–59
Uçaktürk E, Özaltin N, Kaya B (2009) J Sep Sci 32(11):1868–1874
Gallart-Ayala H, Chéreau S, Dervilly-Pinel G, Bizec BL (2015) Bioanalysis 7(1):133–146
Pleik S, Spengler B, Schäfer T, Urbach D, Luhn S, Kirsch D (2016) J Am Soc Mass Spectrom 27(9):1565–1574
Yi W, Ping Z, Shengxiu W, Min H, Yuanyuan S, Tong Z (2017) J Chin Mass Spectrom Soc 38(06):620–629
Christou C, Gika HG, Raikos N, Theodoridis G (2014) J Chromatogr B 964:195–201
Xiyue X, Xiaoqi S, Hua W, Yichao W (2015) Chemistry 78(07):602–607
Villas-Bôas SG, Mas S, Åkesson M, Smedsgaard J, Nielsen J (2005) Mass Spectrom Rev 24(5):613–646
Kanani H, Chrysanthopoulos PK, Klapa MI (2008) J Chromatogr B 871(2):191–201
Moros G, Chatziioannou AC, Gika HG, Raikos N, Theodoridis G (2017) Bioanalysis 9(1):53–65
Kind T, Tolstikov V, Fiehn O, Weiss RH (2007) Anal Biochem 363(2):185–195
Dunn WB, Broadhurst D, Ellis DI, Brown M, Halsall A, O’Hagan S, Spasic I, Tseng A, Kell DB (2008) Int J Epidemiol 37(suppl 1):i23–i30
Taverniers I, De Loose M, Van Bockstaele E (2004) TrAC, Trends Anal Chem 23(8):535–552
Brodie R, Hill H (2002) Chromatographia 55(1):S91–S94
Ruiz-Argüeso T, Rodriguez-Navarro A (1973) Microbiology 76(1):211–216
Xu L, Li Y, Wang Y (2016) Food Res Dev 37(11):93–96
Yuqing Y, Zhiwei Q, Xiuyan Z, Ming X (2014) North Hortic 10:19–21
Tan ST, Holland PT, Wilkins AL, Molan PC (1988) J Agric Food Chem 36(3):453–460
Alissandrakis E, Daferera D, Tarantilis P, Polissiou M, Harizanis P (2003) Food Chem 82(4):575–582
Kangjian Z, Mingsheng B, Tan Z, Xihan M, Jinming G (2001) Sci Silvae Sinicae 37(6):45–51
Yongzhen Y, Guiyuan L, Zhu’nan N (2007) Chin Arch Tradit Chin Med 02:386–387
Pero RW, Lund H, Leanderson T (2009) An international journal devoted to pharmacological and toxicological evaluation of natural product derivatives. Phytother Res 23(3):335–346
Acknowledgements
This project was financially supported by China Agriculture Research System of MOF and MARA, grant number (CARS-44-KXJ8), National Project of Risk Assessment for Quality and Safety of Special Agro-products, grant number PRC (GJFP2020005), The Agricultural Science and Technology Innovation Program, grant number (CAAS-ASTIP-2020-IAR), Department of Science and Technology of Guizhou Province, grant number (2019)2293, (2019)2270, Guizhou Academy of Agricultural Sciences, grant number (2020)16, and China Agriculture Research System of MOF and MARA, grant number (CARS-44-SYZ8).
Funding
National Project of Risk Assessment for Quality and Safety of Special Agro-products, GJFP2020005, Guizhou Science and Technology Department, (2019)2293, (2019)2270, The Agricultural Science and Technology Innovation Program, CAAS-ASTIP-2020-IAR, China Agriculture Research System of MOF and MARA, CARS-44-KXJ8,CARS-44-SYZ8, and Guizhou Academy of Agricultural Sciences, (2020) 16.
Author information
Authors and Affiliations
Contributions
LS: conceptualization, methodology, writing—original draft preparation, writing—review and editing, project administration. FS: methodology, software, validation, data curation, writing—original draft preparation. XH: software, validation, formal analysis, investigation, resources. YC: methodology, validation, formal analysis, resources, visualization. YY: validation, formal analysis, investigation, visualization. DY: formal analysis, visualization, writing—review and editing. JZ: conceptualization, data curation, writing—review and editing. XW: conceptualization, data curation, writing—review and editing, supervision, project administration.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, L., Shi, F., He, X. et al. Establishment and application of quantitative method for 22 organic acids in honey based on SPE-GC–MS. Eur Food Res Technol 249, 473–484 (2023). https://doi.org/10.1007/s00217-022-04146-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04146-0