Abstract
Trace metals, particularly zinc, influence the growth and metabolism of yeast. In the literature the recommended concentration of zinc in pitching wort is > 0.15 mg/L; lower concentrations cause fermentation problems and reduce in consequence final beer quality. The aim of this study was the exploration of changes in bioavailability (available zinc), which was never considered before, and in the mass balance of total zinc during malting process and wort production. Therefore, the work comprised two parts: (1) investigating the effect of malt modification on zinc content and bioavailability of, respectively, produced malt depending on malt modification by varying the steeping degree (38–48%) in the malting process and (2) examining the effect of zinc losses and changes in bioavailability in the by-products (spent grain and hot break) by performing brewing trials up to pitching wort. Zinc was measured by atomic absorption spectroscopy. We applied a specific extraction scheme to evaluate first the bioavailability of zinc in brewing-related samples. In the malting process, total zinc losses increased with greater modification level of the malt samples, although bioavailability increased simultaneously. Spent grain was the primary zinc loss by-product (98%) in the brewing process. The distribution of the binding forms of zinc in hot break and spent grain was significantly altered with an increase in the less water-soluble binding forms of zinc.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Zinc is a necessary trace metal with an important function in the growth and metabolism of yeast during the fermentation process [1]. Several studies have confirmed the fundamental role of zinc in yeast fermentation and defined an initial, sufficient concentration of available zinc (bivalent cation) in pitching wort between 0.10 and 0.15 mg/L [2,3,4,5,6]. It is known that metal ions like zinc in solution can interact with other ingredients leading to various binding forms [7]. Therefore, a part of total zinc in the pitching wort is coordinated on suspended matter and useless for the yeast cells, so only a certain percentage is available for zinc uptake. This part is defined as the bioavailable fraction of zinc.
There exist several binding forms of total zinc such as exchangeable (water soluble), carbonated (ionic interactions with anions), organically bound, occluded on iron oxides and residual zinc [8]. Therefore, a part of the total zinc could get lost in the brewing process by an increasing percentage of less available zinc binding forms like the organically, the occluded on iron oxides or the residual fraction during the wort production [7, 9]. Regarding the zinc uptake by yeast cells, the percentage of the exchangeable and the carbonated form is important; these both can be seen as bioavailable [6]. Nevertheless, the common analytical method is the determination of total zinc by atom absorption spectroscopy (AAS) technique after microwave digestion regardless of the binding forms [10]. This technology cannot differentiate the several binding forms and, therefore, it is in principal inappropriate in details.
Considering the possible chemical interaction, zinc can be in ionic form as hydrate in solution (exchangeable), coordinated at organic anions such as carbonate or inorganic anions such as sulfate anions (carbonated), complexed in proteins (organically), occluded on oxides of iron (occluded on iron oxide), generally absorbed in insoluble organic or inorganic particles or entrapped in silicate minerals (residual) [7, 9]. Studies have shown that the distribution of zinc in its different binding forms depends on the pH-value, the concentration of suspended matter and interactions with other ions [11, 12]. To characterize the different binding forms, defined mediums are used with varied extraction affinities for different zinc links in the organic system. By conducting these extraction steps sequentially, the distribution of the binding forms of zinc in the organic samples can be evaluated [7, 13]. The described sequential extraction procedure was already used for bioavailability studies on metals in soil research [8, 13,14,15]. In this study, we applied such a method to analyze first the bioavailability by determining the binding forms of zinc in malt, spent grain and hot break depending on the distribution of their binding forms.
Especially bioavailable zinc has an important function in the metabolism of yeast [1]. Its uptake by the yeast cells is dominated mainly by two transporter proteins encoded in the ZRT1 and ZRT2 genes [16, 17]. After uptake by the cell, zinc takes part in yeast metabolism. It is a structural component of several proteins and enzymes including alcohol dehydrogenase [18]. It was shown in the literature that if the concentration of available zinc exceeds the recommended level in wort (100–150 µg/L), it is toxic to the yeast cells in higher concentrations (450 µg/L) by interfering the heme enzymes [19].
In the malting process, malt is produced from barley and the zinc partly remains in the grains. Liu et al. showed an accumulation of zinc in rootlets and shoots [20]. Less is known about the loss of total zinc in the malting process and the influence by technological process parameters during malt production. However, it is expected that a certain percentage of total zinc content in barley, which is transferred to rootlets and shoots, depends on the malt modification.
In the brewing process, malt is the main raw material and it is the most important zinc source in pitching wort [10]. During the wort production, most of the total zinc is released into the by-products spent grains and hot break [10, 21,22,23]. Hop plays only a small role as zinc source, due to comparable small amounts in brewing [10, 23]. Further investigation showed that first the zinc content is high in the liquid part of mash (0.48–0.58 mg/L) at the beginning of the mashing process and afterwards it was successively decreased down to 0.09–0.13 mg/L in pitching wort [24].
To date, the mass balance of total zinc over the brewing process has been well studied, but less is known about changes in the distribution of the binding forms of zinc during the brewing process. We expect that the total zinc loss in the brewing process depends on the changes in the binding forms of zinc in the wort production. Consequently, the distribution of the binding forms could be the key to understand total zinc losses in the brewing process.
Materials and methods
Chemicals
Zinc standard for atomic absorption spectroscopy (AAS; TraceCert), hydrochloric acid, sodium hydroxide, nitric acid and ammonium acetate were obtained from Sigma-Aldrich (Steinheim, Germany). Acetic acid, ethylenediaminetetraacetic acid (EDTA), oxalic acid and ammonium nitrate were purchased from Merck (Darmstadt, Germany). Ammonia and ascorbic acid were purchased from VWR (Darmstadt, Germany).
Malting experiments
Barley malt samples of 500 g were produced in the micromalting system of the Chair of Brewing and Beverage Technology, (TU Munich, Freising, Germany) from a common malting spring barley variety [harvest year 2017, Germany, crude protein 9.5% dry matter (d.m.)]. Steeping was performed in periods of 5 h of wet steeping, 17 h of air rest (95% humidity), 4 h wet of steeping and 20 h of air rest (95% humidity). Germination was done by decreasing the temperature from 18 to 14.5 °C (vegetation time of 6 days). Steeping degree was varied at levels of 38%, 40%, 42%, 44%, 46% and 48% to achieve different modification levels of the resulting malt samples. Kilning followed a standardized MEBAK procedure (MEBAK R-110.00.008 [2016-03]) [25]. Samples and sample points were steeping water after the wet steeping steps, the malting and root losses after the cleaning step at the end of the kilning process and the final malt. The crude protein (calculated Kolbach index), the soluble nitrogen, and the extract were measured according to MEBAK (2016) [25] to indicate the level of malt modification. Analyzed values are shown in Table 1. All samples were produced in three biological replicates.
Brewing experiments
Brewing trials were performed at the pilot brewery of the Chair of Brewing and Beverage Technology (TU Munich, Freising, Germany). Lager beer (12°P) was produced at a scale of 80 L. A total of 10.36 kg of barley malt (grist:water ratio = 1:4) was used and standardized hopping was performed with pellets of type 90 and variety Hallertauer Perle at the beginning and end of boiling [calculated 25 international bitterness units (IBU)]. Wort separation was performed in a lauter tun and wort cooling in a whirlpool. The zinc mass balance was investigated in the brewhouse up to the wort cooling step. Brewing trials were performed in three biological replicates.
All raw materials, products and by-products were collected and the zinc content, moisture content, volume or mass were analyzed to evaluate the mass balance of zinc. Sampling was done up to the pitching wort to guarantee that a zinc amount sufficient for fermentation was based on its concentration in pitching wort. Pitching wort was not fermented, because the study only wanted to investigate the mass balance of zinc until this production step in the beer production.
Sample preparation for quantitation of zinc
Solid samples (0.5 g) of the malting experiments were prepared by microwave digestion with 7 mL of nitric acid and 3 mL of hydrogen peroxide after drying in a Mars 6 microwave (CEM GmbH; Kamp-Lintfort; Germany). Zinc content of steeping water was measured directly.
Solid samples (1 g) of the brewing trials were dried in a drying chamber and incinerated using a Heraeus 10013 muffle furnace (Thermo Fisher Scientific; Waltham; USA) until a constant mass was reached. Then, the ash was solubilized using 50 mL of 0.5% nitric acid and measured by AAS-technique. Liquid samples were measured directly.
Quantitation of moisture content
Moisture content was measured according to MEBAK 2016 [25].
Quantitation of zinc
Zinc was measured by AAS analysis using an iCE 3000 series device (Thermo Fisher Scientific; Waltham; USA) with a 5 cm titanium burner and a CuZn hollow cathode lamp (Thermo Fisher Scientific; Waltham; USA), and at a determination wavelength of 213.9 nm.
Incinerated and digested solid samples and water samples were analyzed by external calibration (calibration range: 0.1–1.0 mg of Zn/L). Wort samples were determined using the standard addition method; the added concentrations were 0.1, 0.5, and 1.0 mg of Zn/L.
Determination of bioavailability of zinc
Analysis of the bioavailability of zinc was analyzed according to Zeien and Brümmer [8]. The sequential extraction scheme is defined in Table 2.
We exclude the extraction step of occluded zinc in manganese oxide, because Förstner [7] showed that this binding form of zinc is partly solubilized by acetate solutions and completely integrated in EDTA-solutions. Therefore, we would expect that this fraction to be negligible. The ratio of solid sample to extraction medium was set according to Zeien and Brümmer [8].
Statistical analysis
Statistical analysis was performed using the software JMP® Pro 12 software (SAS Institute GmbH; Heidelberg; Germany). Results are shown as the average ± standard deviation. ANOVA (Tukey test) at a significance level of 0.05 was applied for average comparison.
Results and discussion
Mass balance of total zinc throughout the malting process
The malting process converts raw grain into malt in three main stages: steeping, germination and kilning. During the production of malt, proteolytic, amylolytic and cytolytic changes take place in the metabolic and proteomic profile, which also lead to changes in mineral contents. Accumulation of metals in the by-products (rootlets, sprouts) during the malting process has been demonstrated previously [20].
In the first part of our study, we investigated the loss of total zinc in the malting process according to various malt modifications using different steeping degrees (38%, 40%, 42%, 44%, 46% and 48%). Variations in the malt modification level are shown in Table 1. According to MEBAK [25], reference values for soluble nitrogen range between 550 and 700 mg/100 g of d.m. at a crude protein content of 9.0–11.5% (congress mash procedure) and for extract (pale malt) 79–82%. In particular, the proteolytic modification represented by soluble nitrogen increases with a higher malt modification [25]. Based on the malt modification levels, we expected an effect on total zinc amount or bioavailability of zinc in malt. In this study, we analyzed total zinc mass balance throughout the malting process by different malt modification levels caused by variation of the degree of steeping (38%, 40%, 42%, 44%, 46%, 48%) to evaluate its influence on the total zinc loss. Figure 1 shows the mass balance of the total zinc amount, throughout malting, using a steeping degree of 44% as an example.
The first small release of zinc in the malting process occurred after the steeping step into the steeping water. Zinc in soluble binding forms is partly transferred to the water phase during the wet steeping steps. After kilning, the dried grains are cleaned using a combination of agitation and sieving. Here, rootlets, sprouts, husk fragments, and dust are removed from the final malt and the sum of all partitioned material is defined as malt loss. The accumulation of zinc in by-products has been shown in the literature [20].
Using the sample with a steeping degree of 44% as an example, the zinc content was 30.34 ± 0.88 µg/g of d.m. in the barley and 23.87 ± 0.19 µg/g of d.m. in the product malt sample. The losses were 0.03 ± 0.01 mg/L into the steeping water and 57.55 ± 2.11 µg/g of d.m. in the malt loss following the cleaning step. Consequently, we might expect that zinc accumulates in grown germ (part of the malt kernel) during the germination stage as well as in the rootlets (malting loss). This effect has been shown in previous comparable studies regarding the changes in zinc content in growing barley [20, 26], but in these surveys, the accumulation of zinc could also be influenced by mineral uptake from the soil.
Table 3 shows the mass balance of zinc at a steeping degree of 44% over the malting process normalized to the final dry matter of malt. The total output of zinc in malt and by-products was less than the total input from barley. This finding was probably a result of release effects such as losses in the cleaning device. Water used for steeping had no detectable concentration of zinc. The concentration of zinc normalized to final malt weight in grains was reduced by 21% during the malting process at a steeping degree of 44%.
We also wanted to investigate whether the malt modification level affected zinc transfer from barley into malt in the malting process. Figure 2 shows the effect of steeping degree (the malting parameter that affected the malt modification) on the final concentration of zinc in malt and on the total zinc loss, which represents the sum of the percentage output of the steeping water and the malt loss (cleaning process).
Considering the final total zinc content in malt, the malt modification level caused by steeping degree had a small influence (nearly 1.5 µg/g of d.m.). Generally, zinc concentration showed a declining trend at higher steeping degrees, but only the values at steeping degrees of 38% and 48% differed significantly.
The total zinc loss increased with steeping degree which could be explained by an increasing malt loss caused by a more intensive formation of rootlets and shoots. Therefore, low levels of malt modification led to higher zinc transfer efficiency from barley into malt. Furthermore, higher malt modification levels are known to enhance the degradation of protein in barley grains and increase the transfer of nitrogen to sprouts and germs [27, 28]. Regarding the effects of the intensity of the malting process caused by steeping degree on zinc loss, we can assume that the small declining trend in total zinc content at a higher steeping degree was caused by bioaccumulation in roots and germs, especially bound at formed proteins. Changes in zinc distribution within the barley grain to roots and germs during the malting process have been reported in previous studies [20, 26].
Generally, it is shown that the malt modification caused by the steeping degree had only a small effect on the final total zinc content, but it had a strong effect on zinc efficiency (zinc loss) in the malting process.
Bioavailability of zinc in the malting process
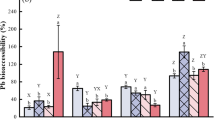
The total content of zinc in the malt samples is a global parameter, because zinc ions can interact with different chemical partners [7, 9] and thus leads to the effect that not all of the zinc is available for uptake by the yeast cells. Therefore, it is important to evaluate the bioavailability of zinc in malt to evaluate the efficiency of zinc solubility. Even though the effect of the steeping degree on the total content of zinc in the final malt was small, the main aim of this study was to investigate first the effects of biotransformation of zinc. Therefore, we analyzed the distribution of zinc in different binding forms (i.e., exchangeable, carbonated, organic, weak occluded, strong occluded, and residual) in barley and malt samples. Because of the small differences on total zinc contents in the produced malts, we focused the malt variations with the lowest (38%) and highest (48%) steeping degree, which had a significantly different total zinc content. The results are presented in Fig. 3.
Generally, the percentage of exchangeable zinc in the final malt is increased toward the barley grains, regardless of the total zinc amount. Therefore, the bioavailability of zinc is enhanced during the malting process, while the organically bound-zinc fractions is reduced. Previous studies have shown an increase in the bioaccessibility of zinc after model digestion of malted cereals and this effect was thought to be caused by the decrease of phytate during the germination process [29,30,31]. Minerals such as zinc can interact with the phytic acid and partly bind on the molecule. Therefore, a reduction in phytate leads to a decreased carbonated zinc fraction. Generally, the malting process undergoes enzymatically protein degradation influenced mainly by the steeping degree [27, 28] and thus affects the organically bound fraction. Fewer possibilities for interaction reduce the amount of zinc complexed in or adsorbed on organic matter in the final malt. The weak and strong occluded fraction on iron oxide are also decreased during the malting process, but generally, both fractions play a minor role in the total zinc distribution. Zinc was not detected in the residual fraction.
Further, we analyzed the binding forms of the lowest and highest modified malts (steeping degrees of 38% and 48%) to evaluate the effect of germination intensity on the bioavailability of zinc in the final malt. The higher steeping degree increased the percentage of exchangeable zinc by 5 percentage points. Further, the carbonated and organically bound-zinc phases were reduced by 6 percentage points. This effect could be caused by increased protein degradation (Table 1) and thus decreased binding potential for zinc, as discussed above.
In conclusion, we demonstrated that the malting process improved the bioavailability of zinc in the barley grains that the improvements are affected by the malting conditions.
Mass balance of total zinc in the production of wort
The second aim of our study was to analyze the release of total zinc throughout the brewhouse processes. Therefore, we first investigated the mass balance of total zinc in the brewing process up to pitching wort. The input and output percentages of all raw materials, by-products and process products are presented in Fig. 4.
The main source of total zinc is malt (99.5%); the hop used in the wort boiling process play only a minor role (0.5%). The zinc concentrations and the normalized values (towards pitching wort) are shown in Table 4. Previous studies report the total zinc concentration of barley malt samples to be within a range of 18 to 25 mg/kg [10, 23, 32] and those of hop samples to range from 37 to 43 mg/kg [10, 23, 33]. The malt used in this study had a comparable total zinc concentration (Table 4), but the hops showed a lower total zinc content. Zinc was not detectable in the water used for the brewing trials. The main release of total zinc was into the spent grain (98.1%), which is comparable to that in previous studies [10]. Although zinc has a relatively high bioavailability in malt used for brewing trials (Fig. 5), most of the zinc potential was already released during the mashing process. Therefore, we can assume that first exchangeable and carbonated zinc is solubilized into water and afterwards directly bound by precipitation of organic or inorganic polymer fractions which leads to the important release of total zinc by spent grain. We would expect that the effect depends on the mashing program; that is, the defined temperature and time steps that affect the intensity of the process and on the raw materials used. The step of wort boiling (whirlpool) also leads to total zinc output in hot break (0.9%). Solubilized zinc (exchangeable and carbonated) is likely also released by protein precipitation. In our trials, the entire output processes resulted in an available zinc concentration in pitching wort of 0.05 mg/L, which represents a transfer rate of only 1% of the total zinc into wort. Therefore, mashing and wort boiling processes are highly inefficient in terms of total zinc yield from malt to pitching wort.
Bioavailability of zinc over the production of wort
In the previous section, we showed that, despite its good bioavailability in malt, total zinc is mainly released into spent grain or hot break. Therefore, we investigated the changes in bioavailability by analyzing the distribution of the binding forms of zinc in spent grain and hot break. Analyzing both by-products is necessary to determine the main molecular processes responsible for the total zinc loss in the mashing and wort boiling processes. Results are shown in Fig. 5.
The exchangeable zinc fraction was substantially decreased in hot break (7%) and spent grain (11%) compared with the malt (81%). Both by-products are separated out of the brewing process which means that the composition of these components is mostly insoluble in water. Exchangeable zinc is a water-soluble fraction and thus it is greatly decreased in the by-products analyzed here. Considering that zinc solubilization in the brewing process is highly inefficient (see Mass balance of total zinc section), we would expect that most of the soluble zinc (exchangeable and carbonated) of malt would be converted into other binding forms. This hypothesis was confirmed by a strongly increased percentage of the organically bound-zinc fraction in hot break and spent grain. Therefore, we proved that the exchangeable zinc in malt is first solubilized and afterwards it undergoes direct biotransformation reactions to other binding types. In addition to direct precipitation as zinc phosphate or zinc oxalate, another possible transformation is adsorption on high-molecular-weight molecules such as precipitated polymers, formed melanoidins, polyphenol complexes or insoluble polysaccharides. Comparing all zinc binding forms, the organically bound zinc was the major group in hot break and spent grain. Therefore, we can assume that zinc is mostly coordinated on precipitated proteins or released by precipitation of proteins containing zinc as structural ion. Zinc in the residual fraction appears in the hot break and spent grain. Consequently, zinc might be entrapped in strongly insoluble in water polymers such as high-molecular-weight silicates.
The bioavailability of zinc is heavily changed during the mashing and wort boiling process. Total zinc undergoes several biotransformation processes and we can assume, therefore, that changes in distribution of the binding forms of the mineral are responsible for its solubilization efficiency in the brewing process.
Conclusion
The study investigated the mass balance of total zinc and changes in bioavailability by determining the binding forms during the malting and the brewhouse processes. We first analyzed the distribution of zinc according to its binding forms (exchangeable, carbonated-bound, organically bound, occluded on amorphous Fe-oxide, occluded on crystalline iron oxide and residual zinc) in barley, barley malt, hot break and spent grain.
In the malting process, total zinc was mainly released in the malt loss (rootlets of germinated barley). This total zinc loss depended on the malt modification level and ranged between of 9% and 15%. Higher steeping degrees, (i.e., a more intensive malting procedure) increased the total zinc loss. However, the final concentration of total zinc in the malt samples was less affected by malt modification level. Bioavailability of zinc is improved over the malting process, because the exchangeable fraction of zinc, which is needed for yeast fermentation, is strongly enhanced. In a comparison of steeping degrees of 38% and 48%, the bioavailability of zinc was increased at the higher steeping degree. Thus, the intensity of the malting process is mainly responsible for increasing the bioavailability of zinc regardless of the total amount of zinc in the malt.
Within the brewing process, total zinc was mainly released by spent grain; only 1% of the total amount of zinc in the malt was transferred to the pitching wort, which is highly inefficient. Further, we analyzed the distribution of the binding forms of zinc in hot break and spent grain to investigate the molecular effects of total zinc loss. Compared with the malt used in the brewing trials, the exchangeable zinc fraction was substantially decreased, and the carbonated bound and organically bound fractions were simultaneously increased in the by-products. We would assume that total zinc undergoes biotransformation reactions and is mainly released from the brewing process by precipitated proteins. The changes in the binding forms of zinc may be the main cause of total zinc loss in the brewing process.
Considering our overall results, we analyzed the binding forms of zinc in malt, spent grain and hot break samples to investigate the molecular transformation that are responsible for total zinc loss in the brewing process. The distribution of the binding forms of zinc is sufficient for the solubilization efficiency of zinc in the brewing process. A higher percentage of exchangeable zinc in the malt may increase the concentration of zinc in the pitching wort. The malting process is sufficient to maximize the bioavailability of zinc in the malt and the pitching wort to optimize fermentation of the yeast. As an alternative way to optimize fermentation, if it is possible by country legislation, supplementation of zinc should be directly conducted to pitching wort, because of the high losses during mashing and wort boiling as well as critical changes in bioavailability at these two process steps.
References
Vecseri-Hegyes B, Fodor P, Hoschke A (2005) The role of zinc in beer production I. Wort production. Acta Aliment 34:373–380. https://doi.org/10.1556/AAlim.34.2005.4.5
Bromberg SK, Bower PA, Duncombe GR, Fehring J, Gerber L, Lau VK, Tata M (1997) Requirements for zinc, manganese, calcium, and magnesium in wort. J Am Soc Brew Chem 55:123–128. https://doi.org/10.1094/ASBCJ-55-0123
De Nicola R, Walker GM (2011) Zinc interactions with brewing yeast: impact on fermentation performance. J Am Soc Brew Chem 69:214–219. https://doi.org/10.1094/ASBCJ-2011-0909-01
Jones RP, Greenfield PF (1984) A review of yeast ionic nutrition. Part I. Growth and fermentation requirements. Process Biochem 19(48–52):54–58
Mochaba F, O’Connor-Cox ESC, Axcell BC (1996) Effects of yeast quality on the accumulation and release of metals causing beer instability. J Am Soc Brew Chem 54:164–171. https://doi.org/10.1094/ASBCJ-54-0164
Blackwell KJ, Singleton I, Tobin JM (1995) Metal cation uptake by yeast. A review. Appl Microbiol Biotechnol 43:579–584. https://doi.org/10.1007/BF00164757
Foerstner U (1983) Types of binding of heavy metals in sediments and sludges: Sorption/mobilization, chemical extraction and bioavailability. Fresenius’ Z Anal Chem 316:604–611
Zeien H, u, Brümmer G. (1989) Chemische extraktion zur bestimmung von schwermetallbindungsformen in böden. Mitteilungen der Deutschen Bodenkundlichen Gesellschaft 59:505–510
Hodgson JF (1968) Chemistry of the micronutrient elements in soils. Advan Agron 15:119–159 (A.G. Norman, editor. Academic)
Wietstock PC, Kunz T, Waterkamp H, Methner FJ (2015) Uptake and release of ca, cu, fe, mg, and zn during beer production. J Am Soc Brew Chem 73:179–184. https://doi.org/10.1094/ASBCJ-2015-0402-01
Salomons W, Mook WG (1980) Biogeochemical processes affecting metal concentrations in lake sediments (Ijsselmeer, The Netherlands). Sci Total Environ 16:217–229. https://doi.org/10.1016/0048-9697(80)90106-0
Iu KL, Pulford ID, Duncan HJ (1981) Influence of waterlogging and lime or organic matter additions on the distribution of trace metals in an acid soil. Ii. Zinc and copper. Plant Soil 59:327–333. https://doi.org/10.1007/BF02184204
Han X, Li X, Uren N, Tang C (2011) Zinc fractions and availability to soybeans in representative soils of northeast china. J Soils Sediments 11:596–606. https://doi.org/10.1007/s11368-011-0336-5
Chahal D, Sharma B, Singh P (2005) Distribution of forms of zinc and their association with soil properties and uptake in different soil orders in semi-arid soils of Punjab, India. Commun Soil Sci Plant Anal 36:2857–2874. https://doi.org/10.1080/00103620500306031
Khoshgoftarmanesh AH, Afyuni M, Norouzi M, Ghiasi S, Schulin R (2018) Fractionation and bioavailability of zinc (zn) in the rhizosphere of two wheat cultivars with different zn deficiency tolerance. Geoderma 309:1–6. https://doi.org/10.1016/j.geoderma.2017.08.019
Zhao H, Eide D (1996) The yeast zrt1 gene encodes the zinc transporter protein of a high-affinity uptake system induced by zinc limitation. Proc Natl Acad Sci U S A 93:2454–2458. https://doi.org/10.1073/pnas.93.6.2454
Zhao H, Eide D (1996) The zrt2 gene encodes the low affinity zinc transporter in saccharomyces cerevisiae. J Biol Chem 271:23203–23210. https://doi.org/10.1074/jbc.271.38.23203
Biosca JA, Fernandez MR, Larroy C, Gonzalez E, Pares X (2002) Description and metabolic functions of alcohol dehydrogenases in Saccharomyces cerevisiae. Some aspects of metabolic engineering applied to brewing. Cerveza Malta 39:27–38
Witham IJ (1963) Zinc toxicity in brewer’s yeast. Nature 197:1113. https://doi.org/10.1038/1971113a0
Liu DJ, Pomeranz Y, Robbins GS (1975) Mineral content of developing and malted barley. Cereal Chem 52:678–686
Kreder GC (1999) Yeast assimilation of trub-bound zinc. J Am Soc Brew Chem 57:129–132. https://doi.org/10.1094/ASBCJ-57-0129
Passaghe P, Bertoli S, Tubaro F, Buiatti S (2015) Monitoring of some selected heavy metals throughout the brewing process of craft beers by inductively coupled plasma mass spectrometry. Eur Food Res Technol 241:199–215. https://doi.org/10.1007/s00217-015-2445-7
Poreda A, Bijak M, Zdaniewicz M, Jakubowski M, Makarewicz M (2015) Effect of wheat malt on the concentration of metal ions in wort and brewhouse by-products. J Inst Brew 121:224–230. https://doi.org/10.1002/jib.226
Schneiderbanger H, Knöpfle M, Jakob F (2017) Zinkgenerierung im rahmen des reinheitsgebotes. In: Brauwelt. pp 58–61
Jakob F (2006) Brautechnische analysenmethoden. Selbstverlag der MEBAK, Freising-Weihenstephan
Gonzalez A, Lobo MC (2013) Growth of four varieties of barley (Hordeum vulgare L) in soils contaminated with heavy metals and their effects on some physiological traits. Am J Plant Sci 4:1799–1810. https://doi.org/10.4236/ajps.2013.49221
Narziß L (1989) Der stand der mälzereitechnologie. Brauwelt 129:939–940
Narziß L, Hellich P (1966) Die keimung mit fallenden temperaturen. Brauwelt 106:885–894
Edney MJ, Rossnagel BG, McCaig R, Juskiw PE, Legge WG (2011) Reduced phytate barley malt to improve fermentation efficiency. J Inst Brew 117:401–410. https://doi.org/10.1002/j.2050-0416.2011.tb00486.x
Platel K, Eipeson SW, Srinivasan K (2010) Bioaccessible mineral content of malted finger millet (Eleusine coracana), wheat (Triticum aestivum), and barley (Hordeum vulgare). J Agric Food Chem 58:8100–8103. https://doi.org/10.1021/jf100846e
Tontisirin K, Nantel G, Bhattacharjee L (2002) Food-based strategies to meet the challenges of micronutrient malnutrition in the developing world. Proc Nutr Soc 61:243–250
Jacobsen T, Lie S, Hage T (1981) Wort quality and the zinc content of malt. In: Proc. Congr. - Eur. Brew. Conv. 18:97–104
Hautke P, Snajdar J, Sittova M (1984) Copper and zinc residues on hops and their fate during the course of the brewing process. Monatsschr Brauwiss 37:270–276
Acknowledgements
We are grateful to Cajetan Geißinger and Dr. rer. nat. Michael Kupetz for performing the malt analysis. Further, we thank Dr.-Ing. Johannes Tippmann for the support for the brewing trials.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
The authors confirm that this manuscript has not already been published nor is it under consideration for publication elsewhere. This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution for the Special Issue: The chemistry behind malt and beer production—from raw material to product quality.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nobis, A., Berg, B., Gastl, M. et al. Changes in bioavailability of zinc during malting process and wort production. Eur Food Res Technol 249, 157–165 (2023). https://doi.org/10.1007/s00217-022-04141-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04141-5