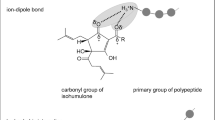
Abstract
The aim of this study was to investigate the amino acid composition and the amount of individual glycation compounds in hot trub formed during boiling of wort prepared from different malts. Compared to the initial amino acid composition of the used malts, some Maillard reaction products (namely MG-H1, pyrraline) and hydrophobic amino acids (leucine, isoleucine, valine, phenylalanine) accumulated in the hot trub, whereas hydrophilic amino acids remained in the boiled wort. For MG-H1, a threefold increase was observed during wort boiling, whereas the other Maillard reaction products, namely CML, CEL, pyrraline and maltosine increased only slightly (1.1–2-fold). Furosine as a hallmark for peptide-bound Amadori compounds showed a small decrease. The results suggest that mainly glycated amino acids derived from small dicarbonyl compounds such as methylglyoxal and glyoxal are formed during wort boiling. Furthermore, the studies indicate that the modification of the protein structure as a result of the Maillard reaction has an influence on the hydration of the denatured proteins during the wort boiling process, thus affecting the coagulation process and, therefore, precipitation of the hot trub. The work carried out contributes to the understanding of the chemical reactions influencing the amino acid and Maillard reaction product transfer from malt to beer.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the course of beer production, wort boiling, along with malt roasting and mashing, represents the last of three central heating steps, which serves a variety of different technological purposes. In addition to sterilization of the wort, evaporation of undesired substances, aromatization by dissolving hop constituents, adjustment of the desired extract content, inactivation of enzymes, the precipitation of trub substances (hot trub), which are subsequently separated from the hot wort in the whirlpool, is one of the central functions of process step [1]. The extent of heat-induced protein precipitation not only influences yeast growth during fermentation [2,3,4], but also has a direct impact on the clarity as well as storage and foam stability of the finished beer [5]. Besides denaturation, the Maillard reaction (also referred to as glycation or nonenzymatic browning) is the most important chemical reaction in food in the presence of reducing sugars and amino compounds, such as proteins, peptides, or amino acids. Early on, the Maillard reaction was associated with beer production. In particular, the Maillard reaction has an influence on the color of beer due to the formation of water-soluble melanoidins [6,7,8,9] as end products of the glycation cascade. Furthermore, the influence of Maillard reaction products on the aroma formation [10,11,12] and storage stability [13] of beer was in focus. In contrast to the formation of low-molecular-weight compounds, such as hydroxymethylfurfural or 3-deoxyglucosone, the changes in amino acid-derived Maillard reaction products (free, peptide-bound and protein-bound MRPs; see Fig. 1) during the various stages of beer production have been little studied. The investigations carried out for this purpose refer to the ingredient brewing malt [14, 15] and the final product beer [16]. To the best of our knowledge, studies on the influence of individual brewing steps on the MRP composition do not exist to date. For brewing malt, no correlation between the color value (given in EBC units) and the content of specific advanced glycation end products (AGEs) was observed. Likewise, no direct correlation between MRP contents and beer styles could be determined, which was attributed to the variations in malt selection, but also to the large variance in process control [16]. In terms of quantity, the Amadori rearrangement products (ARPs) N-ε-maltulosyllysine and N-ε-fructosyllysine as well as the AGEs pyrraline and MG-H1 were most strongly represented in the beer [16]. The comparison of the MRP contents in malt and beer indicates that the content of MG-H1 increases in the progression of the brewhouse work, which Hellwig et al. (2016) attributed to a neoformation of methylglyoxal (MGO) during the brewing process [14].
The increase in beer color intensity during wort boiling [17], which is attributed to the formation of melanoidins [18], indicates that wort boiling in particular has an influence on MRP contents due to the high thermal load. In addition to the formation of MRPs, hot trub formation must also be considered. Thermal denaturation and subsequent coagulation of the proteins, which is influenced by disulfide and thiol groups [3], leads to precipitation of the hot trub and, thus, to the removal of proteins from the wort [1, 3]. An increased kilning temperature leads to lower deposition of high-molecular nitrogen during the wort boiling [1]. Per hectoliter, 0.2–0.4 kg of hot trub is produced [19, 20], which corresponds to a dry mass of 40–80 g [1, 3, 21]. In addition to phenols, lipids, tannins, minerals and hop constituents, proteins make up the main part of the hot trub with a content of 40–70% [20, 22]. No further information is yet available on the exact composition of the precipitated protein. The aim of this work was to investigate the effect of wort boiling on the composition of amino acids and MRPs. This is done by comparing contents of selected MRPs before and after wort boiling. To fully understand the system, the by-product hot trub is analyzed in more detail. On the basis of these investigations, the protein content of the precipitate will be characterized in more detail and, on the other hand, information on the behavior of the MRPs during protein denaturation and coagulation can be obtained. Furthermore, the investigations will provide information on how glycation in the aqueous environment modifies the protein structure. The investigation contributes to further elucidating the transfer of individual MRPs and amino acids from the starting material malt into beer. The influence of individual process steps, especially that of the most intense heating step under aqueous conditions, the wort boiling, will be highlighted. Changes in amino acid spectrum can furthermore have an effect on the sensory properties of the beer as a result of the metabolization by the yeasts to fusel alcohols. Moreover, beer-typical parameters such as mouthfeel and foam stability depend significantly on the protein and, thus, on the extent and character of protein precipitation in the hot trub.
Materials and methods
Chemicals
Unless otherwise stated, chemicals were purchased from commercial suppliers without further purification: NaCl (≥ 99.5%, Fisher Scientific), NaOH (97%, VWR chemicals), HCl (≥ 37%, VWR chemicals), H2SO4 (> 96%, VWR chemicals), heptafluorobutyric acid (HFBA, 99%, Alfa Aesar), nonafluoropentanoic acid (NFPA, 97%, Sigma Aldrich), pepsin (3839 U/mg protein, Sigma Aldrich), pronase E (4000 PU/mg, Merck), leucine aminopeptidase (18 U/mg protein, Sigma Aldrich), prolidase (553 U/mg protein, Sigma Aldrich), acetonitrile (HPLC–MS/MS grade, VWR), tris-(hydroxymethyl)-aminomethane (TRIS, > 99.9%, Carl Roth), bidistilled water, N-ε-benzoyllysine, CML, [2H2] CML and furosine (PolyPeptide). The following substances were synthesized according to methods stated in the literature: Pyrraline [23, 24], MG-H1 [25], CEL [25], maltosine [26], pentosidine [27]. The syntheses of [13C6,15N2] pyrraline, [13C6] MG-H1 and [13C6,15N2] maltosine were performed in the same manner but using [13C6,15N2] lysine (pyrraline and maltosine) and [13C6] arginine (MG-H1) instead of the unlabeled amino acids. For the synthesis of [13C3] CEL, [13C3] pyruvic acid was used instead of unlabeled pyruvic acid.
Sample material
Eight commercially available spray-dried and liquid malt extracts, obtained from Weyermann (Bamberg. Germany) and Brewferm (Beverlo, Belgium) with indicated EBC values varying from 8 to 600 were used for the studies. All commercial malt extracts were pre-mashed and without hop addition.
Kjeldahl protein
Nitrogen contend was determined using the method of Kjeldahl. The raw protein content was calculated from the nitrogen content by multiplication using the factor 5.88 for barley protein [28].
EBC value
The color values of the worts and boiled worts given in EBC units were measured photometrical at 430 nm after centrifugation and membrane filtration (0.45 µm) of the samples. The EBC value is calculated using formula: EBC = E430 nm × 25 × dilution factor.
ATR-IR spectroscopy
ATR-IR measurements were carried out with a D5 ATR Thermo Scientific. Spectra were recorded in the range from 4000 to 600 cm−1 by co-adding 5 scans at 4 cm−1 spectral resolution. The measurement of the solid, dry samples was carried out after fixing on the crystal surface and previous measurement of a background spectrum. All samples were measured five times, averaged, and normalized to the band of C–H bending vibrations at 1450 cm−1.
Simulation of the wort boiling process
Model worts (with a volume of 4–10 L) were prepared by dissolving a known amount of malt extract with deionised water to an original gravity of 16°Plato. The pH value of the model wort was measured and adjusted to a value between 5.2 and 5.4 with 6 M HCl or 1 M NaOH following by an addition of sodium chloride to set a constant ionic strength of 0.1. For the simulation of the wort boiling process, the prepared model mashes were stirred in an open vessel at 30 °C for 15 min to ensure complete dissolution of the malt extracts. Thereafter, the temperature was increased to 100 °C and kept there for 60 min. After 60 min, the heat source was removed, and the hot wort was set aside to cool to room temperature. The separation of the raw hot trub from the hot wort was done by vacuum filtration. The separated raw hot trub was washed to remove impurities from the wort by thoroughly suspending the raw hot trub in 0.1 M sodium chloride solution and subsequent centrifugation and separation of the supernatant. This washing step was repeated two times with sodium chloride solution, two times with deionised water and finally one time with bidistilled water. The purified hot trub was suspended in bidistilled water and freeze-dried to gain a fine powder, which was milled to guarantee homogeneity. For each wort with defined EBC value, the whole process was done in duplicates.
Preparation of the high-molecular-weight fractions
For the analysis of the wort protein fraction and the boiled wort protein fraction, the samples were dialyzed using a cellulose membrane (MWCO 3.5 kDa, Carl Roth) at 4 °C against deionized water over a period of 96 h. The water was changed seven times during this period and the retentates were lyophilized.
Acid and enzymatic hydrolysis and amino acid analysis
Total and protein-bound amino acids and Maillard reaction products were analyzed after acid [14] and enzymatic [29, 30] hydrolysis according to the methods stated in the literature. All hydrolysis and related measurements were performed at least in duplicates. Acid hydrolysis was performed by dissolving an appropriate amount of sample corresponding to 10 mg protein in 2.0 mL 6 M HCl, overlaying with nitrogen gas and incubating for 23 h at 110 °C in a closed test tube in a sand bath. The hydrolysate was filtered, and an aliquot of 1.0 mL was evaporated to dryness in vacuo, suspended in 1.0 mL of the loading buffer for the amino acid analysis (0.12 N lithium citrate, pH 2.20) and filtrated through a 0.45 µm membrane filter.
For the enzymatic total hydrolysis, an appropriate amount of sample corresponding to 2–3 mg protein was suspended in 1.0 mL 0.02 M hydrochloric acid and 50 µL 0.02 M hydrochloric acid containing 356 U of pepsin was added. The mixture was incubated at 37 °C for 24 h in a drying oven. Afterwards, 250 µL of 2 M TRIS/HCl buffer, pH 8.2 and 50 µL of 2 M TRIS/HCl buffer containing 400 PU of pronase E were added. After a second incubation at 37 °C over a period of 24 h, 0.4 U (4 µL) leucine aminopeptidase and 1 U (10 µL) of prolidase were added and the whole mixture was incubated again over 24 h at 37 °C. The hydrolysate was filtrated and frozen for storage. For amino acid analysis, 300 µL of the enzymatic hydrolysate was freeze-dried and the residue was dissolved in 1.0 mL of the loading buffer for the amino acid analysis (0.12 N lithium citrate, pH 2.20).
Analysis of CML, CEL, MG-H1 and maltosine
For analysis of the Maillard reaction products CML, CEL, MG-H1 and maltosine, 10 µL of an internal standard mixture (containing isotope-labeled MG-H1, CEL, CML and maltosine) and 44 µL concentrated NFPA were added to 600 µL of the filtrated enzymatic hydrolysate. The mixture was applied to a solid phase extraction (SPE) cartridge (Chromabond HLB 60 µm, 200 mg), which was prepared prior by consecutively flushing with 2.0 mL of methanol, 2.0 mL of methanol/10 mM NFPA (50/50, v/v), and 4.0 mL of 10 mM NFPA [29]. After addition of the sample, it was washed by adding 4.0 mL methanol/10 mM NFPA (5/95, v/v) to the cartridge. The sample was eluted from the SPE cartridge with 5.0 mL methanol/10 mM NFPA (90/10, v/v) and evaporated under a nitrogen flow. The evaporated sample was redissolved in 50 µL bidistilled water, centrifuged and applied to HPLC–MS/MS. The elution system for CML, CEL, MG-H1and maltosine contained eluent A water and B acetonitrile each containing 10 mM NFPA as a counter ion. The gradient program was as follows: 0 min, 90% A; 10 min, 80% A; 16 min, 70% A; 20 min, 10% A; 30 min, 10% A; 30.5 min; 90% A; 45.5 min, 90% A) at a flow rate of 0.2 mL/min. A Kinetex C18, 2.1 × 50 mm, 1.7 µm column was used for separation. Quantitation of the MRPs was done in multiple reaction monitoring (MRM) mode according to the parameters shown in Table S1. The MRPs CML, CEL, MG-H1 and maltosine were analyzed on an Agilent 1200 series chromatographic system connected to an Agilent 6410 mass spectrometer with electrospray ion source (ESI-MS, all from Agilent, Waldbronn, Germany). The working conditions were 350 °C gas temperature, gas flow 11 L/min, nebulizer pressure 35 psi, and capillary voltage 4000 V.
Analysis of pentosidine
For the analysis of pentosidine, 400 µL of the acid hydrolysate was evaporated to dryness in vacuo and dissolved in 200 µL bidistilled water before membrane filtration. Prior to injection, 10 µL internal standard solution containing 0.8 µM N-ε-benzoyllysine was added to 40 µL of each sample. The elution system contained eluent A water and B acetonitrile each containing 0.1% HFBA as a counter ion. The gradient program was as follows: 0 min, 90% A; 10 min, 80% A; 16 min, 70% A; 20 min, 10% A; 30 min, 10% A; 30.5 min; 90% A; 45.5 min, 90% A at a flow rate of 0.2 mL/min. A Kinetex C18, 2.1 × 50 mm, 1.7 µm column was used for the separation and for the analysis, the same chromatographic system and mass spectrometer as mentioned above were used. Detection of pentosidine and N-ε-benzoyllysine was done in MRM mode according to the parameters shown in Table S2.
Analysis of pyrraline
For the determination of pyrraline, 400 µL of the enzymatic hydrolysate was freeze-dried and redissolved in 200 µL bidistilled water followed by a membrane filtration (0.45 µm). Quantitation of pyrraline was done via HPLC–DAD with a Eurospher C18, 250 × 4,6 mm column at a detection wavelength of 297 nm and 254 nm according to the method stated in literature [31]. The elution was isocratic using a 10% ethanolic solution containing 7.5 mM sodium pentansulfonic acid set to pH 3 with propionic acid. Detection of pyrraline was done by comparison of the retention time and UV spectra with a standard.
Analysis of furosine
For the analysis of furosine, 400 µL of the acid hydrolysate was evaporated to dryness in vacuo and dissolved in 200 µL bidistilled water before membrane filtration. Quantitation of furosine was done by HPLC–DAD with an Eurosphere C18, 250 × 3 mm column and a detection wavelength of 280 nm. 0.1% HFBA in water (eluent A) and acetonitrile (eluent B) were used as the elution system. The gradient program started at 0 min, 95% A; 20 min, 85% A; 25 min, 60% A; 27 min, 5% A; 35 min, 5% A at a flow rate of 0.8 ml/min and 25 °C column temperature. Identification of furosine was done by comparison of the retention time and UV spectra with a standard.
Results and discussion
Technological effects on the formation of hot trub and its amino acid composition
To understand how the composition of amino acids and Maillard products and the total amount of glycation products change over the wort boiling step, it is essential to understand the formation and composition as well as the factors influencing the formation of the hot trub. This is because hot trub and its formation in the brewing process have a direct influence on the protein content, which remains in the hot wort and, thus, also passes into the final product, the beer. It is unclear whether hot trub precipitation leads to a statistically uniform decrease of amino acids and Maillard products from the hot wort, or whether precipitation of the hot trub leads to discrimination against certain protein sections, thus differentiating the amino acid and Maillard product composition between wort, boiled wort and hot trub. To make up a balance of the protein components, it is important to know the influence of brewing process parameters on the amount of hot trub formed and its protein content. Wort boiling was investigated using laboratory-scale model experiments (volume 4–10 L). Worts were prepared by dissolving commercially available malt extracts in the technologically relevant EBC range of 16–130 EBC and, as an example, also with a roasted malt with an EBC value of 551 EBC. The brewing mixtures were standardized in terms of original wort content, starting pH, salt concentration and boiling time to achieve greater comparability and the greatest possible reproducible hot trub formation [3]. For optimal hot trub separation, the pH was kept between 5.2 and 5.4 [3]. Furthermore, it was found that an increase in the original wort content leads to an extended hot trub formation due to the greater amount of protein present, but the percentage protein transfer from the malt extract to the hot trub remains unaffected (data not shown). Therefore, the original gravity of 16°Plato was chosen to achieve a high hot trub yield without being out of line when compared with realistic brewing conditions. Moreover, in view of the microbrewing scene that has grown over the last few years [32] and, thus, the emerging popularity of strong alcoholic beer styles such as India pale ales, high original wort contents are no longer unusual. To simplify the system, hops were not added, as it can be assumed that they have only a minor influence on the composition of the Maillard reaction products due to the low thermal load during the production of hops. In addition, the use of hops leads to a significant increase in the degrees of freedom of the experiment and, thus, complicates the evaluation of the amino acid balance and the conclusions about the composition of the malt. Furthermore, it should be mentioned that the results presented here are not transferable to wheat malts (results not shown). Color represents a characteristic component of beer and belongs to the style-defining properties. Thus, the determination of color strength and, consequently, the selection of malts is an intrinsic part of the development of a beer recipe. Achieving one and the same color value on the EBC scale can be done in different ways, depending on the desired sensory impression. Either directly using weakly to moderately kilned malts or by blending light malts with small amounts of roasted color malts. In practice, a combination of both approaches is usually used; in the study carried out here, the two extreme cases mentioned were considered for clarification.
The relationship between the EBC value indicated for the respective malt on the hot trub yield and the crude protein content of the hot trub is shown in Fig. 2A and B. For better comparability, all contents and yields were normalized to a mash volume of 1 L. A distinction is made between the already mentioned cases of using light to medium kilned malts (16–130 EBC), referred to as single malt worts, and the use of roasted malt (551 EBC) in variable proportions in combination with the light malt (16 EBC), referred to as malt mixture worts. When using light to medium kilned malt extracts, the hot trub yield is in the range of 300–500 mg dry mass/L wort depending on the EBC value, which is comparable to the literature [1, 3, 21], but relatively low, probably due to the lack of hop addition and due to the reference to the higher volume of the unboiled wort compared to the boiled wort. The crude protein content of the hot trub was in the range of 73–85% and around 30% for the hot trub of the roasted malt. Overall, the hot trub formation shows only a weak dependence on the color strength. The small decrease in hot trub formation resulting for a malt with an EBC value of 130 may be attributed to improved solubility and stabilization in solution due to an increased degree of glycation [33]. Furthermore, it is likely that the extent of proteolysis during mashing also has a direct effect on the size and denaturation stability of the dissolved proteins and protein fragments. The use of roast malt results in a significantly increased hot trub separation, with a concomitant decrease in the protein content of the precipitate (Fig. 2B). Even in moderate and technologically significant amounts of 5–10% roast malt addition [34], this leads to a fourfold increase in total hot trub deposition. A comparison of the theoretically expected hot trub quantity, which is calculated from the proportion of malt extracts and the hot trub quantities of the unmixed malt extracts, shows that the use of roasted malt leads to a higher hot trub precipitation than expected. Thus, the roasted malt co-precipitates components that would remain in solution when heated without roast malt. The reason for this could be due to a sterically demanding and complex melanoidin structure of the proteins in the roasted malt, which might trap other, less modified, protein segments in solution during denaturation and causes them to precipitate.
Formation of hot trub in dependence of the EBC value referred to a wort with 16°P and a volume of 1 L. Total hot trub dry mass after precipitation and purification (A1 and A2). Raw protein contents of the analyzed hot trubs (B1 and B2). Percentage of precipitated protein calculated from the alanine content in wort and hot trub after acid hydrolysis (C1 and C2). Presented are the mean values and standard deviations from at least two independent wort boiling runs
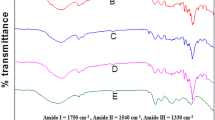
To better understand the structure of the roasted malt hot trub and the background of the precipitation process, the hot trub was structurally characterized. To obtain an overview of the functional groups of the hot trub, ATR-IR investigations were used. The ATR-IR spectrum (Fig. 3A) of the bright hot trub shows pronounced vibrations of the protein backbone [35]. Thus, the amide I, amide II and amide III bands at 1630 cm−1, 1520 cm−1 and 1230 cm−1 are distinct in the spectrum. This is in agreement with the high crude protein content determined. The ATR-IR spectrum of the hot trub derived from colored malt extract is clearly different from the spectrum of the light malt. The amide bands are only faintly discernible and a prominent band in the region of 1025 cm−1, which can be assigned to C–O–H stretching vibrations of carbohydrates, characterizes the spectrum [36]. The band of the OH stretching vibration in the range 3000–3600 cm−1 increases significantly in width with increasing color malt content, which is characteristic for melanoidins that have been subjected to intense heating of more than 150 °C [37, 38]. This is not unusual for the production of color malts, where kiln-drying temperatures of 180 °C and more are reached [8, 39, 40]. Further typical for melanoidins is the band at 1675 cm−1, which can be assigned to C=O stretching vibrations in different chemical environments [38]. Accordingly, the ATR-IR spectrum of the hot trub derived from colored malt indicates a melanoidin character that is characterized less by the protein structure and more by carbohydrates associated with a protein backbone [41]. The ATR-IR spectra of the hot trub, from the mixtures of the malt extracts (Fig. 3B), indicate a structure dominated by protein up to a proportion of 10% colored malt. Already from an addition of 15% colored malt, the structure of the resulting hot trub is dominated by functional groups that can be assigned to carbohydrates or melanoidins, respectively. In comparison, only slight differences can be seen in the ATR-IR spectra of the hot trub from unmixed malts in the EBC range from 16 to 130 (Fig. 3A), which is consistent with the observations from the crude protein contents. The biggest change is seen between the lowest and second lowest EBC value, where the intensity of the band at 1030 cm−1 increases. This band can be attributed to the C–O stretching vibrations, which indicates an increase in glycation of the protein backbone. The intensity of the band does not change up to an EBC value of 130.
ATR-IR spectra of hot trub from worts of different EBC values (A) and from blends between a light malt extract (16 EBC) and a roasted malt extract (551 EBC) with a proportion of the roasted malt of 5, 10, 15, 30 and 60% (B). Shown are the mean values and standard deviations from at least five separate measurements. The spectra were normalized to the band intensity of the C–H bending vibration at 1450 cm−1
The amino acid composition of the hot trub, the worts and the boiled worts were determined by amino acid analysis after acid hydrolysis. For the worts and the boiled worts, both the total amount and the amount of protein-bound amino acids after dialysis (MWCO 3.5 kDa) were determined. The percentage amino acid compositions of the five malt extracts of different EBC values (EBC 16–551) were considered together due to the small differences, although significant differences were observed in the absolute amino acid content between the different EBC values (data not shown). Hence, the absolute contents of native amino acids in the roasted malt are significantly lower than those of the moderately kilned malts, and as a measure of non-proteinogenic nitrogen (NPN), the proportion of amino acid nitrogen in the crude protein nitrogen decreases with increasing EBC value from over 96% (16 EBC) to 56% (551 EBC). This further underlines the melanoidin character and, associated with this, the presence of acid-stable amino acid modifications. The ratio of amino acid nitrogen to crude protein nitrogen in hot trub is significantly higher (mean 82%) than in wort and boiled wort (mean 60%). This indicates a high proportion of non-protein nitrogen, e.g., nucleic acids in the malt extracts and boiled wort [42].
There is no detectable difference in the relative amino acid composition between malt extract and boiled wort (Fig. S1), and both compositions agree well with the amino acid composition in barley [43]. Clear differences can be seen in the composition of the liquid components (wort/boiled wort and the high-molecular-weight fraction of the wort/boiled wort) and the hot trub precipitate. Thus, elevated levels of the hydrophobic amino acids leucine, isoleucine, valine and phenylalanine are present in the hot trub. This may be due to poorer hydration of proteins and peptides with high levels of hydrophobic amino acids, which, therefore, are less stable in solution, allowing them to coagulate more easily via hydrophobic interactions after denaturation [44, 45]. Also enriched in the hot trub are the amino acids methionine and tyrosine. In contrast, the more polar amino acids aspartic acid, lysine and glycine are underrepresented in the hot trub. The amino acid analysis also clarifies which amino acids are predominantly present in protein-bound or free form. Cysteine, threonine, serine and tyrosine are mainly present in protein-bound form, whereas proline and phenylalanine are present mainly as free amino acids [46]. Alanine is largely evenly distributed between liquid and solid components as well as free and protein-bound form.
Based on the high contents of NPN in wort and boiled wort, the crude protein content is not suitable for determining the protein proportions distributed from the wort to hot trub and boiled wort. A more straightforward method is to refer to a selected reference amino acid, which should be unaffected by chemical reactions and be present in similar proportions in wort, boiled wort and hot trub, as well be balanced between its free and protein-bound form. The reference amino acid leucine [14] used so far in the literature is not applicable because of its accumulation in the hot trub, which is why only alanine meets the set requirements. Neglecting the tryptophan content, one kilogram of protein contains an average of 744 mmol alanine (median 730 mmol, range 679–814 mmol). Based upon alanine as reference amino acid, a conversion from the alanine equivalents of each amino acid to the corresponding amino acid content per kilogram protein for wort, boiled wort and hot trub can be performed. The fraction of protein precipitation calculated as the transfer of alanine from the wort to the hot trub is shown in Fig. 2C. This plot compensates for different protein contents in the worts (malt extracts) caused by raw material variations and different mash parameters. For the EBC range from 16 to 130 EBC, between 3.6 and 6.5% of the dissolved protein present in the wort precipitate during wort boiling, which can be explained on the basis of a higher temperature resistance of more glycated proteins, which protects the proteins from coagulation even in the denatured state and stabilizes them in solution [33]. A considerable difference with over 30% of the protein precipitating during wort boiling is apparent when using the roasted malt alone. Similarly, in the range up to 130 EBC, the use of roasted malt leads not only to more hot trub, as already described, but also to an increased protein precipitation. This supports the assumption that proteins are co-precipitated by the roast malt melanoidins. The consequences of this increased protein precipitation on the turbidity and foam stability of the finished beer requires further studies.
Maillard reaction products in wort, boiled wort and hot trub
Since some Maillard products are acid labile, total enzymatic hydrolysis [14] was used for the analysis of CML, CEL, MG-H1, maltosine and pyrraline. The contents of pentosidine and furosine were measured after acid hydrolysis. To estimate the extent of the enzymatic hydrolysis, the determined content of unreactive amino acids after enzymatic hydrolysis is compared with their content after acid hydrolysis [14]. Assuming complete hydrolysis of the protein in the presence of hydrochloric acid, a relative yield of hydrolysis can be calculated for the enzymatic hydrolysates. Figure 4 shows the hydrolysis yield values for the selected unreactive amino acids leucine, isoleucine, valine, phenylalanine and alanine. Up to an EBC value of 130 EBC, the enzymatic hydrolysis yield of the poorly soluble hot trubs are within a range between 66 and 101% (Table S4), depending on the analyte, which is comparable to data from the literature [14, 47]. Slight variations in the hydrolysis yield are reflected equally in the five amino acids considered, meaning that no preference or discrimination of amino acids by the enzymes is apparent. When considering roast malt mixtures, a linear decrease (R2 = 0.85–0.89) of the hydrolysis yield with increasing content of roast malt can be observed. The reasons for the strong decrease can be attributed to the sterically demanding structure of the melanoidin-like roast malt hot trub, whose protein backbone is embedded in carbohydrate-like structures and, thus, makes hydrolysis by the proteases more difficult. To obtain information about the influence of wort boiling on the amounts of Maillard products and composition in the wort, knowledge of both the physicochemical influencing factors already considered (like the precipitation quantity) and the chemical influencing factors (MRP contents and composition) are necessary. In the following, the chemical changes will be discussed in detail. Since there is a deliberate reduction in volume during wort boiling, specifying analyte contents in relation to a volume or mass inevitably leads to inaccuracies, and the values obtained cannot be transferred to other systems due to different evaporation rates. The choice of a reasonable reference parameter is, therefore, essential to obtain reliable and comparable values. Because the sum of proteinogenic components (amino acids, peptides, proteins) in the entire system does not change during wort boiling and the proportion of alanine in the wort, boiled wort and hot trub protein fractions is virtually constant (see Fig. S1), the analyzed alanine content was chosen as the reference parameter. It should be noted that alanine is somewhat more strongly represented in free form in wort and boiled wort, which leads to a slight underestimation of the hot trub protein transfer.
Hydrolysis yields of enzymatic hydrolysis of hot trubs from worts of different EBC values. The degrees of hydrolysis were calculated by dividing the amino acid contents after enzymatic hydrolysis by those after acid hydrolysis of the same amino acid. Presented are the means and standard deviations of at least two separate hydrolysis runs
The analyte contents given in Table 1 are related to the respective alanine content of the sample and, thus, also take into account the incomplete release due to enzymatic hydrolysis. Based on the indicated alanine contents of the total protein, the content per protein or kilogram amino acids can easily be calculated. It should be noted that this is not an indication of the content of protein-bound MRPs, but rather the sum of protein-bound and free MRPs relative to the total amino acid content. The MRPs considered in this study (Fig. 1) were selected to represent a broad range of different compounds and to allow comparisons with other literature sources by choosing common representatives. Thus, in addition to a number of lysine modifications (e.g., CML, CEL, pyrraline, maltosine) with MG-H1, an important arginine modification is also included. Furosine is a representative for the MRP of the early phase of the Maillard reaction. Furthermore, some of the MRPs considered have specific dicarbonyl compounds as direct precursor molecules (CEL, MG-H1, pyrralin), whereas others are formed via more complex or various formation pathways (maltosine, CML). Pentosidine is representative of a gycation-induced cross-linking amino acid. Of the MRPs considered, the Amadori reaction products analyzed via furosine expressed as N-ε-fructosyllysine (FL; using the transfer factor 3.1) [48] have by far the highest content of the analyzed MRPs with up to 19.4–178.9 µmol/mmol alanine after conversion. The FL contents determined correspond to a lysine blockage of 11.7–29.8% (median 21.0%). Hellwig and Henle [14] found that FL followed by N-ε-maltulosyllysine (ML) are the two MRPs with the highest contents and determined in malt samples for FL and ML a maximum lysine modification of 15.9 and 4.9%, respectively. In beer, lysine modifications ranging from 14.7 to 20.3% could be determined [16]. Thereby, only 15–60% of the lysine blockage could be explained by the determined FL and ML contents. The remaining percent were attributed to ARPs with maltooligosaccharides such as maltotriose, maltotetraose, and maltopentaose. Only a small variation in furosine contents was found between the hot trubs of the different EBC values. In the ATR-IR spectra, the strong increase in the intensity of the band at 1030 cm−1, which can be attributed to C–O stretching vibrations, is accompanied only by a weak increase in the furosine content, so it cannot be ruled out that carbohydrate structures may also be bound to the protein backbone in other ways than via Amadori reaction products. The comparatively high values for ARP obtained in this study can be attributed to the use of spray-dried malt extracts. During the drying process, the increased formation of MRPs, especially of ARPs, is likely under the low water activity conditions [49]. The most abundant MRP following the ARP in terms of quantity is pyrraline, followed by MG-H1.
Overall, the MRP contents determined are in a comparable range to the contents determined by Hellwig and Henle in malt [14]. Based on the investigations, no correlations could be established between the EBC content of the malt extracts and the contents of the Maillard products analyzed (data not shown). The contents of MRPs in the EBC range from 16 to 130 were in a very similar range, only the extract of the roasted malt showed significantly lower contents of MRP. An exception is maltosine, which showed the highest content in the roasted malt. The other MRPs considered show the lowest values in the roasted malt, so that it can be assumed that these have already been degraded or incorporated into melanoidins by the strong heat effect during kilning.
Influence of wort boiling on MRPs
The Maillard reaction product contents and their changes during wort boiling must be evaluated in a differentiated manner. A distinction must be made between relative changes, i.e., changes in relation to a reference variable, for example the protein quantity or reference amino acids, and absolute changes, i.e., a change in the quantity in a part of the system, e.g., the liquid supernatant. Furthermore, it must be taken into account that the precipitation of the hot trub is not statistically uniform, but that some proteins and protein fragments and, thus, also MRPs preferentially precipitate or remain in solution, as was shown on the basis of the enrichment of hydrophobic amino acids. In the following, we took a closer look at the change in MRP contents and the distribution of MRPs between wort and hot trub. If an increase in an analyte content relative to the amount of protein is noted, it is obvious to explain this by means of a reformulation of this analyte. However, it is additionally necessary to examine the analyte in the hot trub since a reduced precipitation of the analyte can also lead to an increased relative content per protein in the boiled wort and vice versa. Of course, it must be noted that only about 3.6–6.5% of the protein ends up in the hot trub (with the exception of the roasted malt) and this effect, therefore, has its limitations. When considering MRP transfer from wort to boiled wort, the change in the relative contents in the liquid part of the system is critical. Figure 5A shows the change in the individual analytes. The calculation is made using alanine equivalents to compensate for differences in hydrolysis yields. For statements about the absolute changes, the precipitation behavior of each analyte, shown in Fig. 5B, must also be taken into account. The depicted enrichment factors are based on the comparison of the protein distribution between wort and hot trub (calculated on the basis of the alanine distribution) with the distribution of the respective analyte between wort and hot trub. If a statistically uniform precipitation occurs, the distribution of the analyte is equal to the distribution of alanine, resulting in the enrichment factor taking the value of 1. A value below 1 indicates less precipitation than would be expected, whereas a value above 1 indicates increased precipitation and therefore removal from the liquid phase. The MRPs studied show very individual behaviors, but these can be explained in terms of some patterns. It is noteworthy that the observed trends are largely independent of the EBC value, with the exception of the colored roast malt.
Percentage change in the examined MRPs related to the reference amino acid alanine calculated by the comparison before and after boiling (A). Enrichment factor of the MRPs analyzed in this study (B). The values were calculated by comparison the distribution of the MRPs with the distribution of the reference amino acid alanine between boiled wort and hot trub. Presented are the mean values and errors, calculated by error propagation, from at least two separate wort boiling runs for the five EBC values investigated
CML does not undergo any changes in concentration as a result of wort boiling. This observation is accompanied by uniform precipitation in the hot trub. The structurally related CEL also shows a uniform distribution between hot trub and boiled wort but increases significantly during wort boiling. MG-H1 undergoes the greatest neoformation and accumulates more in the hot trub. These observations underline the results from the literature that MG-H1 is the main compound resulting from the reaction between the guanidino group of arginine and methylglyoxal (MGO) in the aqueous environment [50]. The significantly greater increase of MG-H1 (plus 300%) compared to CEL (plus 50%) emphasizes the higher reactivity of MGO towards arginine compared to the ε-amino group of lysine [50]. Formation of MG-H1 from arginine changes the pKa values of the side chains from 12.5 for the guanidino group to about 4.8 for the hydroimidazolone function [51]. Accordingly, since the pKa value of the hydroimidazolones is below the wort pH, the protonation and, thus, the charge of the protein changes, possibly making the proteins more susceptible to coagulation. A similar effect can be used to explain the accumulation of pyrraline in hot trub. Modification of the lysine side chain changes the charge in the pH range between 5 and 6 from positive to neutral and, thus, the charge (zeta potential) of the molecule, favoring precipitation. However, since pyrraline is preferentially formed under dry conditions [52], there is little increase under wort boiling conditions. Likewise, hardly any increase is observed for maltosine. This contradicts somewhat the expectation, since the wort contains high levels of precursor molecules, such as maltooligosaccharides and maltose, and a strong increase in maltosine was recorded for pasta during cooking [30]. The low accumulation in the hot trub may be explained by the fact that the lysine modification leads only to a small change in the pKa value (pKa lysine = 10.8, pKa maltosine = 9.2), resulting in no change in charge under wort boiling conditions. However, due to the low concentration of maltosine, a real influence cannot be assumed. Rather, the reaction sites at which maltosine is formed could be an influence on precipitation. The reduced precipitation contributes to the increase of the relative content of maltosine in solution for the wort from the roasted malt (Fig. 5A).
The sum of ARPs, determined via furosine, decreases slightly over the course of wort boiling, which cannot be accounted for by increased precipitation (Fig. 5B). A possible cause for the decrease is the increased degradation of ARPs to advanced MRPs, such as the dicarbonyl compounds [53], at high water activity. Furthermore, glycation improves the solubility of the protein moieties, which leads to a deterioration of coagulability and, thus, to a decrease in precipitation. In this study, pentosidine serves as a proxy of other Maillard-induced cross-link compounds such as glyoxal-lysine dimer, methylglyoxal-lysine dimer, glyoxal-derived imidazoline cross-link and methylglyoxal-derived imidazoline cross-link. In the experiments performed, pentosidine could only be quantified in the hot trub. The values obtained are in the range for contents reported in the literature [27, 52]. No detection of pentosidine was possible in worts and boiled worts, traces could be detected in the high-molecular-weight (HMW) fractions. Statements about the formation during boiling are, therefore, not possible. However, these observations indicate at least a strong enrichment in the hot trub. It is, thus, suggested that proteins and protein fragments that are crosslinked during wort boiling are increasingly precipitated from the wort. The cross-linking of several protein segments results in a significant increase in molar mass and hydrodynamic radius, which makes the branched proteins susceptible to precipitation.
In summary, it was shown that the amino acids and Maillard products are affected differently by hot trub precipitation depending on their individual structure. Hydrophobic compounds and those that reduce the isoelectric point and therefore, the molecular charge are predominantly accumulating in the hot trub, whereas hydrophilic MRPs and amino acids are precipitated to a reduced extent.
In particular, the experiments showed a pronounced increase in the concentration of MG-H1. In general, MRPs with small dicarbonyls as precursor molecules showed the largest increase. No trend in the content of Maillard reaction products could be found for the different EBC values. The precipitation behavior and the associated change in amino acid composition were also largely unaffected by the color strength, with only the roasted color malt showing significant deviations in some cases. The addition of roasted malt to the malt mixture in the range of 5–15% leads to a significantly increased hot trub and protein precipitation. The resulting hot trub has a lower protein content and a melanoidin-like structure characterized by a high carbohydrate portion. The influence of this on beer characteristics, such as foam and storage stability, remains unclear. Furthermore, the influence of the upstream mashing step and the associated proteolytic activity of the enzymes on hot trub precipitation and Maillard product composition must be investigated.
Abbreviations
- AA:
-
Amino acid
- AGE:
-
Advanced glycation end-product
- ARP:
-
Amadori rearrangement product
- ATR-IR:
-
Attenuated total reflection infrared spectroscopy
- CEL:
-
N-ε-carboxyethyllysine
- CML:
-
N-ε-carboxmethyllysine
- kDa:
-
Kilo Dalton
- DAD:
-
Diode array detection
- EBC:
-
European brewery convention
- FL:
-
N-ε-fructosyllysine
- HFBA:
-
Heptafluorobutyric acid
- HMW:
-
High molecular weight
- HPLC:
-
High-pressure liquid chromatography
- MG-H1:
-
Methylglyoxal-derived hydroimidazolone 1
- MGO:
-
Methylglyoxal
- ML:
-
N-ε-maltulosyllysine
- MRM:
-
Multiple reaction monitoring
- MRP:
-
Maillard reaction product
- MS:
-
Mass spectrometry
- MWCO:
-
Molecular weight cut-off
- NFPA:
-
Nonafluoropentanoic acid
References
Narziß L, Back W (2009) Die Bierbrauerei Band 2 : Die Technologie der Würzebereitung, 8. Auflage. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim
Schisler DO, Ruocco JJ, Mabee MS (1982) Wort trub content and its effects on fermentation and beer flavor. J Am Soc Brew Chem 40:57–61. https://doi.org/10.1094/ASBCJ-40-0057
Kühbeck F, Schütz M, Thiele F et al (2006) Influence of lauter turbidity and hot trub on wort composition, fermentation, and beer quality. J Am Soc Brew Chem 64:16–28. https://doi.org/10.1094/ASBCJ-64-0016
Kühbeck F, Back W, Krottenthaler M (2006) Release of long-chain fatty acids and zinc from hot trub to wort. Monatsschrift für Brauwiss. https://doi.org/10.1063/1.1303546
Steiner E, Becker T, Gastl M (2010) Turbidity and haze formation in beer—insights and overview. J Inst Brew 116:360–368. https://doi.org/10.1002/j.2050-0416.2010.tb00787.x
Coghe S, Adriaenssens B, Leonard S, Delvaux FR (2004) Fractionation of colored maillard reaction products from dark specialty malts. J Am Soc Brew Chem 62:79–86. https://doi.org/10.1094/asbcj-62-0079
Coghe S, D’Hollander H, Verachtert H, Delvaux FR (2005) Impact of dark specialty malts on extract composition and wort fermentation. J Inst Brew 111:51–60. https://doi.org/10.1002/j.2050-0416.2005.tb00648.x
Coghe S, Gheeraert B, Michiels A, Delvaux FR (2006) Development of maillard reaction related characteristics during malt roasting. J Inst Brew 112:148–156. https://doi.org/10.1002/j.2050-0416.2006.tb00244.x
Kuntcheva MJ, Obretenov TD (1996) Isolation and characterization of melanoidins from beer. Eur Food Res Technol 202:238–243. https://doi.org/10.1007/BF01263547
Dack RE, Black GW, Koutsidis G, Usher SJ (2017) The effect of Maillard reaction products and yeast strain on the synthesis of key higher alcohols and esters in beer fermentations. Food Chem 232:595–601. https://doi.org/10.1016/j.foodchem.2017.04.043
Saison D, De Schutter DP, Uyttenhove B et al (2009) Contribution of staling compounds to the aged flavour of lager beer by studying their flavour thresholds. Food Chem 114:1206–1215. https://doi.org/10.1016/j.foodchem.2008.10.078
Hellwig M, Beer F, Witte S, Henle T (2018) Yeast metabolites of glycated amino acids in beer. J Agric Food Chem 66:7451–7460. https://doi.org/10.1021/acs.jafc.8b01329
Nobis A, Kunz OS, Gastl M et al (2021) Influence of 3-DG as a key precursor compound on aging of lager beers. J Agric Food Chem 69:3732–3740. https://doi.org/10.1021/acs.jafc.0c08003
Hellwig M, Henle T (2020) Maillard reaction products in different types of brewing malt. J Agric Food Chem 68:14274–14285. https://doi.org/10.1021/acs.jafc.0c06193
Hellwig M, Kiessling M, Rother S, Henle T (2016) Quantification of the glycation compound 6-(3-hydroxy-4-oxo-2-methyl-4(1H)-pyridin-1-yl)-l-norleucine (maltosine) in model systems and food samples. Eur Food Res Technol 242:547–557. https://doi.org/10.1007/s00217-015-2565-0
Hellwig M, Witte S, Henle T (2016) Free and protein-bound maillard reaction products in beer: method development and a survey of different beer types. J Agric Food Chem 64:7234–7243. https://doi.org/10.1021/acs.jafc.6b02649
Bremner TS (1963) Effect of boiling on the colour of laboratory malt worts. J Inst Brew 69:406–411. https://doi.org/10.1002/j.2050-0416.1963.tb01947.x
Siefker JA, Pollock GE (1956) Melanoidins in the brewing processes. I. Formation of aldehydes during wort boiling. Proc Annu Meet Am Soc Brew Chem 14:5–12. https://doi.org/10.1080/00960845.1956.12006472
Briggs DE, Boulton CA, Brookes PA, Stevens R (2004) Brewing: science and practice
dos Santos Mathias TR, Alexandre VMF, Cammarota MC et al (2015) Characterization and determination of brewer’s solid wastes composition. J Inst Brew 121:400–404. https://doi.org/10.1002/jib.229
Kühbeck F, Back W, Krottenthaler M (2006) Influence of lauter turbidity on wort composition, fermentation performance and beer quality—a review. J Inst Brew 112:215–221. https://doi.org/10.1002/j.2050-0416.2006.tb00716.x
Saraiva BR, Anjo FA, Vital ACP et al (2019) Waste from brewing (trub) as a source of protein for the food industry. Int J Food Sci Technol 54:1247–1255. https://doi.org/10.1111/ijfs.14101
Henle T, Bachmann A (1996) Synthesis of pyrraline reference material. Z Leb Forsch 202:72–74. https://doi.org/10.1007/BF01229689
Hellwig M, Geissler S, Peto A et al (2009) Transport of free and peptide-bound pyrraline at intestinal and renal epithelial cells. J Agric Food Chem 57:6474–6480. https://doi.org/10.1021/jf901224p
Hellwig M, Geissler S, Matthes R et al (2011) Transport of free and peptide-bound glycated amino acids: synthesis, transepithelial flux at caco-2 cell monolayers, and interaction with apical membrane transport proteins. ChemBioChem 12:1270–1279. https://doi.org/10.1002/cbic.201000759
Geissler S, Hellwig M, Markwardt F et al (2011) Synthesis and intestinal transport of the iron chelator maltosine in free and dipeptide form. Eur J Pharm Biopharm 78:75–82. https://doi.org/10.1016/j.ejpb.2010.12.032
Henle T, Schwarzenbolz U, Klostermeyer H (1997) Detection and quantification of pentosidine in foods. Z Leb Forsch 204:95–98. https://doi.org/10.1007/s002170050043
Müller J (2017) Dumas or Kjeldahl for reference analysis? Comparison and considerations for Nitrogen/Protein analysis of food and feed. Anal beyond Meas 1–5
Schwarzenbolz U, Hofmann T, Sparmann N, Henle T (2016) Free maillard reaction products in milk reflect nutritional intake of glycated proteins and can be used to distinguish “organic” and “conventionally” produced milk. J Agric Food Chem 64:5071–5078. https://doi.org/10.1021/acs.jafc.6b01375
Hellwig M, Kühn L, Henle T (2018) Individual Maillard reaction products as indicators of heat treatment of pasta—a survey of commercial products. J Food Compos Anal 72:83–92. https://doi.org/10.1016/j.jfca.2018.06.009
Förster A, Kühne Y, Henle T (2005) Studies on absorption and elimination of dietary Maillard reaction products. Ann N Y Acad Sci 1043:474–481. https://doi.org/10.1196/annals.1333.054
Watson B (2021) National Beer Sales & Production Data | Brewers Association. In: 2021. https://www.brewersassociation.org/statistics-and-data/national-beer-stats/. Accessed 12 Jul 2021
Perrocheau L, Rogniaux H, Boivin P, Marion D (2005) Probing heat-stable water-soluble proteins from barley to malt and beer. Proteomics 5:2849–2858. https://doi.org/10.1002/pmic.200401153
Weyermann H SINAMAR ® WEYERMANN ® MALZ. www.weyermann.de. Accessed 12 Jul 2021
Barth A (2007) Infrared spectroscopy of proteins. Biochim Biophys Acta Bioenerg 1767:1073–1101. https://doi.org/10.1016/j.bbabio.2007.06.004
Wiercigroch E, Szafraniec E, Czamara K et al (2017) Raman and infrared spectroscopy of carbohydrates: a review. Spectrochim Acta Part A Mol Biomol Spectrosc 185:317–335. https://doi.org/10.1016/j.saa.2017.05.045
Mohsin GF, Schmitt FJ, Kanzler C et al (2018) Structural characterization of melanoidin formed from D-glucose and L-alanine at different temperatures applying FTIR, NMR, EPR, and MALDI-ToF-MS. Food Chem 245:761–767. https://doi.org/10.1016/j.foodchem.2017.11.115
Mohsin GF, Schmitt FJ, Kanzler C et al (2019) PCA-based identification and differentiation of FTIR data from model melanoidins with specific molecular compositions. Food Chem 281:106–113. https://doi.org/10.1016/j.foodchem.2018.12.054
Parr H, Bolat I, Cook D (2021) Modelling flavour formation in roasted malt substrates under controlled conditions of time and temperature. Food Chem. https://doi.org/10.1016/j.foodchem.2020.127641
Samaras TS, Camburn PA, Chandra SX et al (2005) Antioxidant properties of kilned and roasted malts. J Agric Food Chem 53:8068–8074. https://doi.org/10.1021/jf051410f
De Marco LM, Fischer S, Henle T (2011) High molecular weight coffee melanoidins are inhibitors for matrix metalloproteases. J Agric Food Chem 59:11417–11423. https://doi.org/10.1021/jf202778w
Abernathy DG, Spedding G, Starcher B (2009) Analysis of protein and total usable nitrogen in beer and wine using a microwell ninhydrin assay. J Inst Brew 115:122–127. https://doi.org/10.1002/j.2050-0416.2009.tb00356.x
Newman RK, Newman CW (2008) Barley: genetics and nutrient composition. In: Barley for food and health. John Wiley & Sons, Ltd, pp 56–94
Stumpe MC, Grubmüller H (2007) Interaction of urea with amino acids: Implications for urea-induced protein denaturation. J Am Chem Soc 129:16126–16131. https://doi.org/10.1021/ja076216j
Neurath H, Greenstein JP, Putnam FW, Erickson JO (1944) The chemistry of protein denaturation. Chem Rev 34:157–265. https://doi.org/10.1021/cr60108a003
Kabelová I, Dvořáková M, Čížková H et al (2008) Determination of free amino acids in beers: a comparison of Czech and foreign brands. J Food Compos Anal 21:736–741. https://doi.org/10.1016/j.jfca.2008.06.007
Hellwig M, Rückriemen J, Sandner D, Henle T (2017) Unique pattern of protein-bound maillard reaction products in manuka (Leptospermum scoparium) honey. J Agric Food Chem 65:3532–3540. https://doi.org/10.1021/acs.jafc.7b00797
Krause R, Knoll K, Henle T (2003) Studies on the formation of furosine and pyridosine during acid hydrolysis of different Amadori products of lysine. Eur Food Res Technol 216:277–283. https://doi.org/10.1007/s00217-002-0649-0
Lee HM, Yang SY, Han J et al (2019) Optimization of spray drying parameters and food additives to reduce glycation using response surface methodology in powdered infant formulas. Food Sci Biotechnol 28:769–777. https://doi.org/10.1007/s10068-018-0524-9
Rabbani N, Thornalley PJ (2012) Methylglyoxal, glyoxalase 1 and the dicarbonyl proteome. Amino Acids 42:1133–1142. https://doi.org/10.1007/s00726-010-0783-0
Eisner C, Faulhaber-Walter R, Wang Y et al (2010) Major contribution of tubular secretion to creatinine clearance in mice. Kidney Int 77:519–526. https://doi.org/10.1038/KI.2009.501
Moeckel U, Duerasch A, Weiz A et al (2016) Glycation reactions of casein micelles. J Agric Food Chem 64:2953–2961. https://doi.org/10.1021/acs.jafc.6b00472
Pan GG, Melton LD (2007) Nonenzymatic browning of lactose and caseinate during dry heating at different relative humidities. J Agric Food Chem 55:10036–10042. https://doi.org/10.1021/jf072257n
Acknowledgements
We kindly thank Karla Schlosser and Dr. Anke Förster, Institute of Food Chemistry, TU Dresden, for performing the amino acid analysis.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contribution for the Special Issue: The chemistry behind malt and beer production—from raw material to product quality.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Böhm, W., Stegmann, R., Gulbis, O. et al. Amino acids and glycation compounds in hot trub formed during wort boiling. Eur Food Res Technol 249, 119–131 (2023). https://doi.org/10.1007/s00217-022-04138-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04138-0