Abstract
Extra virgin olive oil-in-water nanoemulsions stabilised with synthetic or clean label surfactants (Tween 20 or soy lecithin) was prepared using high-pressure homogenisation (HPH). The effect of HPH pressure and the number of cycles were assessed through response surface methodology to optimise homogenisation processing parameter. Mean droplet diameter (MDD), polydispersity index (PDI), thermal stability and oxidation stability of the resulting emulsions were evaluated. The results showed that the formation and stability of nanoemulsions can be affected by the homogenisation processing parameters (pressure and cycles) and the properties of surfactants (interfacial tension, viscoelasticity and molecule structure). Although MDD and PDI of Tween 20 stabilised nanoemulsions were influenced by homogenisation pressure and cycles, there was not a significant effect on lecithin-stabilised nanoemulsions. A homogenisation pressure of at least 400 bars produced Tween 20 stabilised nanoemulsion (MDD < 200 nm), whereas lecithin-stabilised nanoemulsion were obtained after high-speed homogenisation without using HPH. HPH at 400 bars for 1 cycle produced nanoemulsions with greater physical stability when using either Tween 20 or lecithin. Tween 20 stabilised nanoemulsion showed significantly higher (p < 0.05) thermal stability and lipid oxidative stability than lecithin-stabilised nanoemulsion. Following an optimisation study using regression modelling, the optimal homogenisation parameter for MDD of Tween 20 stabilised emulsion was found at pressure of 764 bars with 1 cycle, while lecithin-stabilised emulsion was found at pressure of 3 bars with 2 cycles. Overall, this study has important implications for optimising nanoemulsion production for potential application in the food industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nanoemulsions with oil droplets size of less than 200 nm [1] are increasingly used in the food industry because of their unique properties and potential applications such as improving physicochemical stability of functional compounds, texture modification, and nutrient enrichment [1, 2]. Previous studies have established that the characteristics and stability of nanoemulsions depend on several factors such as the composition and processing conditions [3,4,5]. Extra virgin olive oil contains high level of monounsaturated fatty acids (oleic acid) [6] and minor bioactive compounds such as phenolics and tocopherols that could scavenge the free radicals and contribute to the antioxidant activity [6, 7]. There has been a positive correlation between the phenolic and tocopherol content and radical scavenging activity [8, 9].
Polyoxyethylene sorbitan esters (polysorbates) are small molecular and non-ionic surfactants that are of interest in this study; they have been reported as effective surfactants to formulate stable nanoemulsions [4, 10, 11] due to their ability to rapidly absorb to the droplet surfaces and reduce interfacial tension [12, 13]. However, polysorbates can give a bitter taste when used at high concentrations [14], limiting their food applications. On the other hand, consumers are increasingly demanding clean labels on food and beverage products; therefore, there is an increasing interest in using surfactants that are obtained from natural sources [15] that could be effective in creating stable nanoemulsions with minimal impact on their organoleptic properties. Soy lecithin has been widely used as emulsifying agent in the food industry [16]; it is commonly obtained from soybeans [15]. Soy lecithin is an amphiphile molecule, which is derived from sn-glycero-3 phosphate. The lipophilic groups are fatty acids that attached to carbon atom position 1 and 2, while the hydrophilic groups generally are zwitterionic of amino and anionic of polyvalent alcohol [17, 18].
High-energy mechanical processes are performed at industrial scale due to an easy control of the homogenization machine [19]. High-pressure homogenisation (HPH) is a high-energy method used to produce nanoemulsions [15]; a coarse emulsion passes through a narrow channel of the high-pressure valve homogenizer [19, 20]. Several studies have focused on the effect of high-pressure homogenisation processing parameters including pressure and number of cycles on nanoemulsion’s particle size, droplet size distribution, and physical stability [3,4,5, 21, 22]. In general, when the pressure and number of homogenisation cycles increases, the droplets size and particle-size distribution decreases [3, 22,23,24], and it has been reported that the physical stability of a nanoemulsion increases initially when the pressure or number of cycles are increased [4, 23]. However, there is limited research on the effect of high-pressure homogenisation parameters on nanoemulsion stabilised by surfactants from natural sources such as soy lecithin in comparison to synthetic surfactants such as Tween 20. Kim, Ji, Lee, and Hong [25] indicated that the droplet size of nanoemulsion decreased when partially replacing Tween 20 with soy lecithin or sorbitan monooleate (prepared at 1034 bars), which was attributed to a considerable improvement of Ostwald ripening stability of curcumin-loaded medium-chain triglycerides (MCT) nanoemulsion. Mehmood [4] studied the optimisation of canola oil-based vitamin E nanoemulsions when using mixed surfactants (Tween 80 and soy lecithin), and the results on particle size and emulsion stability showed that the optimum processing parameters were homogenisation pressure (135 MPa), oil concentration (6.18%), surfactant concentration (6.39%), and vitamin E acetate contents (1%). Therefore, not only processing parameters but also the type of surfactant could influence the physicochemical properties of nanoemulsions.
Nanoemulsion droplet size could promote lipid oxidation by accelerating reactions at the surface of the droplets; a high rate of lipid oxidation is attributed to a high droplet surface area as the droplet size decreases [20, 26]. Surfactant type also played a major role in lipid oxidation, particularly when prooxidants such as transition metals (Fe, Cu) are present into the aqueous phase; therefore, the emulsion droplet interface can influence the interaction between prooxidant and lipids [27,28,29]. When formulating nanoemulsions, lipid oxidation is a parameter to control, because it can lead to an undesirable flavour including off-flavours and off-odours [18]. The objective of the present study was therefore to evaluate the effect of homogenisation processing conditions and surfactant type (soy lecithin or Tween 20) on physical stability and lipid oxidation of nanoemulsions. This research work initially focused on the characterisation of surfactants (Tween 20 and soy lecithin) by measuring interfacial tension and viscoelastic properties. Additionally, the influence of homogenisation conditions (pressure and number of cycles) on physicochemical properties (mean droplet diameter and polydispersity index) and stability of emulsions (thermal stability and lipid oxidation) were also assessed. The results of this study will provide useful information about the emulsifying capacities soy lecithin and the design of an optimum HPH process to produce clean label and stable nanoemulsions for application in food and beverage products.
Materials and methods
Materials
Nanoemulsions were elaborated with extra virgin olive oil (EVOO) (14.26% of saturated fat, 77.69% of monosaturated fat and 8.04% of polyunsaturated fat (Napolina brand, UK retail market) and two types of emulsifiers: Tween 20 (polyoxyethylene) with hydrophilic-lipophilic balance (HLB) value of 16.7 was used as synthetic non-ionic surfactant (Sigma-Aldrich Co., Ltd, UK) or soy lecithin (HLB value ranges between 2 and 8) was used as zwitterionic surfactant (Louis Francois Co, France). The molecular weight of soy lecithin and Tween 20 are 643.9 and 522.g/mol, respectively [30, 31]. Trichloroacetic acid, thiobarbituric acid, and malondialdehyde (MDA) were purchased from Sigma-Aldrich Co., Ltd (UK), and hydrochloric acid purchased from Fisher Scientific Co., Ltd (UK). High-purity water was used for the preparation and dilution of reagents.
Nanoemulsion preparation
The emulsion preparation procedure was based on the methods described by Arancibia, Navarro-Lisboa, Zúñiga and Matiacevich [32] and Taha, Hu, Hu, Zhang, Bakry, Khalifa, and Pan [33] with some modifications. Emulsions were prepared in three steps. First, a magnetic stirrer (Model SS3H, ChemLab, UK) was used to prepare the aqueous phase dispersing Tween 20 and soy lecithin (5% w/w) in water (85% w/w) at 200 rpm for 30 min at ambient temperature to ensure complete dispersion. Then, the oil was added (10% w/w) to the aqueous phase during continuous stirring. Second, the emulsions were homogenised with a high-speed homogenizer (Model L4RT, Silverson, Chesham, UK) at 10,000 rpm for 10 min. Third, the coarse emulsions were passed through a high-pressure homogenizer (8.30H, Rannie APV, Denmark). For the evaluation of the effect of the homogenisation pressure on emulsion properties, the coarse emulsions were homogenised at 1, 200, 400, 600 and 800 bars for 1 cycle. For the study of the effect of cycle number on emulsion properties, the coarse emulsions were passed through the high-pressure homogeniser for 1, 2, and 3 cycles at different homogenisation pressures (200 and 400 bars). Then, the emulsions were left to cool down for 2 h at ambient temperature before measurements. All emulsions were prepared in triplicates.
Interfacial tension and critical micelle concentration of surfactants
The interfacial tension was determined according to the method of Bai, Huan, Gu, and McClements [34] and Luo, Zhou, Bai, Liu, Zhang, Zhang, Zheng, Deng, and McClements [35]. The interfacial tension between oil–water interface with different emulsifiers (Tween 20 at 5% w/w or soy lecithin at 5% w/w) was determined using a pendant drop analyser (DS4270, Krüss GmbH, Hamburg, Germany) at 20 °C. An axisymmetric drop (20 µL) of surfactant dispersion was delivered and allowed to stand at the tip of the needle inside a quartz container of extra virgin olive oil (9 mL) for 15 min, to achieve emulsifier adsorption at oil–water interface. Three analytical repetitions of each measurement were done for each emulsion batch.
The critical micelle concentration (CMC) of each emulsifier was determined according to the method of Mukherjee, Moulik and Rakshit [36] and El-Sukkary, Syed, Aiad, and El-Azab [37]. Several concentrations of Tween 20 and soy lecithin were prepared: 0.00, 0.0001, 0.001, 0.01, 0.1, 1.0, 5.0, and 10.0%w/w. CMC was determined at the intersection points of the interfacial tension values versus the surfactant concentration (logarithm) plot. Three analytical repetitions of each measurement were done for each emulsion batch.
Physical and chemical properties of nanoemulsions
Rheological properties of emulsions
The viscoelastic behaviour of surfactant solutions was determined using a rheometer (Anton Paar MCR 302, Anton Paar, Graz, Austria) equipped a Peltier temperature control device. A serrated parallel plate geometry was used; the diameter of lower stationary plate (PPTD 200/56/1) and superior plate (PP50/ P2) was 50 mm and the gap between the plates was 1 mm. The samples were allowed to rest in the measurement position for 5 min for relaxation and temperature equilibration (20 °C). Strain sweeps were carried out at strain amplitude range of 0.001 to 1000% at a constant frequency of 1 Hz to determine the linear viscoelasticity region (LVR). The LVR was identified where the storage modulus (G′) and loss modulus (G″) were not influenced by applied strain from this region, and constant strain amplitude of 10% was selected. Frequency sweeps between 0.1 and 100 Hz were performed at constant stress amplitude (10%). The G′ and G″ moduli values were recorded; G′ characterises of the elastic nature or solid-like behaviour of a substance, while G″ is indicative of viscous nature or liquid-like behaviour of a substance. Measurements were performed in duplicate in two emulsion batches.
Measurement of emulsion mean droplet diameter (MDD) and polydispersity index (PDI)
Particle size and polydispersity index of emulsions were determined in a dynamic light scattering (DLS) instrument (Zetasizer Nano ZS, Malvern Instruments Ltd., Worcestershire, UK) following the method of Guerra-Rosas, Morales-Castro, Ochoa-Martínez, Salvia-Trujillo and Martín-Belloso [10] and Sharif, Goff, Majeed, Liu, Nsor-Atindana, Haider, Liang, and Zhong [11]. Emulsions were diluted 100-fold with deionized water and agitated to avoid multiple light scattering effects. The dispersion was decanted into polystyrene cuvettes for measuring MDD and PDI at wavelength of 633 nm at 25 °C. Three analytical repetitions of each measurement were done for each emulsion batch.
Thermal stability (TS)
Emulsion stability at high temperature was determined as described by [38]. Each emulsion (10 mL) was heated in a water bath at 80 °C for 30 min followed by centrifugation at 1200 g for 10 min. The height (mm) of initial emulsion, cream layer, and sedimentation phase were measured with a Digital Vernier Caliper. Emulsion thermal stability was calculated according to Eq. 1
where \(HE\) was the height of initial emulsion (mm), \(HS\) was the height of sedimentation phase (mm), and \(HC\) was the height of cream layer (mm).
Determination of thiobarbituric acid reactive substances (TBARS)
TBARS were determined according to the method of [11, 39] with some modifications. Briefly, 1 ml of the emulsions was added to 5 ml of thiobarbituric acid (TBA) solution, which was prepared by mixing 15 g of trichloroacetic acid (TCA), 0.375 g of TBA and 2.1 g hydrochloric acid (37% w/w). Samples were heated in a water bath at 95 °C for 10 min, and then, the samples were allowed to cool down to room temperature for 10 min, followed by centrifugation (Heraeus Multifuge 3SR Plus Centrifuge, Thermo Scientific Ltd., UK) at 10,000 g for 15 min. The absorbance of the supernatant was measured at 532 nm using a UV spectrophotometer (CECIL CE 1021 1000 Series, Cecil Instruments Ltd., UK). The absorbance of the samples was measured against a blank solution (7.5% w/v TCA). The concentrations of TBARS values were determined using a standard curve prepared using malondialdehyde MDA standard (4.17 M). A concentration of MDA standards between 0.02 and 0.10 mM was prepared where linear response was observed coefficient correlation (R2) = 0.9987. Three analytical repetitions of each measurement were done for each emulsion batch.
Statistical analysis
Statistical analysis of the data was performed using IBM SPSS 25 (Armonk, NY: IBM Corp, USA). To assess the effect of the pressure (1, 200, 400, 600, and 800 bars) on the properties of nanoemulsions one-way analysis of variance (ANOVA) and Tukey’s HSD test were used to evaluate the mean values’ difference (p < 0.05). Then, a two-way ANOVA was conducted to assess the effect of the processing conditions: pressure (200 bars and 400 bars) and number of cycles (1, 2, 3 cycles)). Furthermore, a two-tailed paired t test was used to compare the effect of the two-surfactant studied (tween 20 and soy lecithin) on emulsion properties. The experimental data were analysed and reported as means and standard deviations.
The regression ANOVA was applied to determine the best HPH conditions to produce stable nanoemulsions. This regression ANOVA was employed using the Statistical Analysis System (SAS) software (SAS Institute Inc., USA). The regression model of the influence of high-pressure homogenisation processing parameters were analysed; homogenisation pressure at 1, 200, 400, 600, and 800 bars and the cycle number of 1, 2, and 3 were optimised to minimise the response variables under study of MDD, PDI, thermal stability (TS), and TBARS, and to maximise the response variables under study of TS using response surface regression. The empirical second-order polynomial model used to fit the measured responses was according to Eq. 2
where \(y\) is the predicted response, \({\beta }_{0}\) is the model constant\(,{\beta }_{1}\) and \({\beta }_{2}\) are the linear coefficient, \({\beta }_{11}\) and \({\beta }_{22}\) are the quadratic coefficient, \({\beta }_{12}\) is the coefficient for the interaction effect, and \({x}_{1}\) and \({x}_{2}\) are independent variables. The goodness-of-fit model was evaluated by the lack-of-fit test, the determination coefficient (\({R}^{2}\)), and the analysis of variance (ANOVA) using the Response Surface Regression (RSREG) procedure of SAS. Statistical fit of the model was determined by Fisher’s statistical test. The robustness of the model was assessed by the determination coefficient (\({R}^{2}\)) and Fisher’s \(F\) test at 95% confidence level.
Results and discussion
Effect of HPH pressure on the physical and chemical properties of nanoemulsions
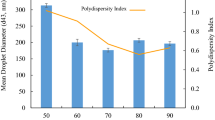
The effect of homogenisation pressure on MDD and PDI of emulsions formulated with Tween 20 or soy lecithin is shown in Table 1. Tween 20 stabilised emulsions showed a significant decreased (p < 0.05) in droplet diameter and polydispersity values when the homogenisation pressure increased up to 400 bars; above this pressure, there was no significant decrease in both parameters. The decrease in droplet size and polydispersity index of emulsions with increasing pressure can be attributed to an increase in shear forces and cavitation during HPH, which resulted in reduction of particle size and a more homogeneous particle-size distribution [5, 40]. The decrease in droplet size in the Tween 20 stabilised emulsions correlated to significantly higher (p < 0.05) lipid oxidation values in these emulsions (Table 1). An increase in droplet surface area as the droplet size decreases has been related to higher lipid oxidation rates [26, 41]. In the lecithin-stabilised emulsion, the homogenisation pressure did not have a significant (p > 0.05) effect on MDD, but PDI values significantly decreased (p < 0.05) with increasing homogenisation pressure up to 400 bars; higher pressures did not have a significant effect on emulsions’ PDI (p ≥ 0.05). The limited effect of HPH on the MDD values of lecithin emulsions could be attributed to its molecular weight (lecithin, 643.9 g/mol; Tween 20, 522 g/mol); larger molecules need more time to be absorbed at interfaces [12, 42]; thus, time during homogenization did not allow sufficient time to the surfactant to relocate at the interface. Moreover, as no further effects on nanoemulsions’ droplet size and polydispersity were observed above 400 bar, 200 and 400 bars were selected to study the effect of the two different surfactants and the interaction between pressure and number of cycles on nanoemulsion properties.
Effect of surfactant type on the physical and chemical properties of nanoemulsions
The interfacial activity of surfactants plays an important role providing emulsification and reducing the oil–water interfacial tension, protecting droplets against coalescence, and improving emulsion stability [43]. In this section, the effect of a non-ionic synthetic surfactant (Tween 20) and a zwitterionic surfactant extracted from a natural source (soy lecithin) on physicochemical properties and stability of nanoemulsions produced using high-pressure homogenisation at 400 bars and 1 cycle was investigated (Table 2). The HPH process was selected as the shortest and most efficient process that can produce stable nanoemulsions as shown in previous section about the effect of HPH pressure on the physical and chemical properties of nanoemulsions. The results of the interfacial tension measurements (Table 3) show that Tween 20 and lecithin reduced the interfacial tension between different phases, water and extra virgin olive oil. Tween 20 presented a lower interfacial tension value compared to lecithin. This result is in agreement with previous studies [29, 44], indicating that the interfacial tension value of lecithin was higher than Tween 20 and Tween 80.
Regarding the particle size and polydispersity index values (Table 2), lecithin gave place to nanoemulsions with significantly (p < 0.05) smaller droplet size than Tween 20. The effect of surfactant on particle size can be attributed to its concentration [29, 34] and viscoelastic properties [44]. When the viscoelasticity of the surfactant solutions was evaluated, both samples showed higher G' than G" in the whole frequency range studied, which indicated solid-like viscoelastic structure (Fig. 1). The lecithin solution presented greater G' and G" moduli values than Tween 20 solution. The molecules of lecithin have ability to form a thick viscoelastic film, which is strengthened by hydrogen bonding between phosphate groups on neighbouring molecules; whereas Tween 20 molecules form a weaker network with neighbouring molecules, as they are non-ionic surfactants [44, 45]. Similar results were observed when evaluating the viscoelasticity of oil-in-water interfaces when using lecithin or Tween 20 as surfactants at their minimum concentration to form nanoemulsions (Nash and Erk [44].
In terms of the thermal stability, soy lecithin nanoemulsions showed a significantly lower (p < 0.05) stability than Tween 20 nanoemulsion. It has been reported that lecithin molecules can transit from a solid-like to a liquid-like structure when increasing temperature [46]. This change in the viscoelastic properties of lecithin could lead to oil droplet aggregation and lower emulsion stability during storage and temperature changes. Soy lecithin nanoemulsion presented a significantly higher (p < 0.05) TBARS value than Tween 20 nanoemulsions. These results could be explained by two mechanisms; the first one relates to the structure of lecithin. The hydrophobic groups of soy lecithin are fatty acids [17, 18]; the double bonds of these fatty acids play an important role in the initiation step of lipid oxidation, because the hydrogen atom attached to the carbon between double bond is easily removed and provides alkyl radicals [47]. For this reason, soy lecithin-stabilised nanoemulsion could experience higher lipid oxidation rates compared to Tween 20 samples. The second mechanism is related to the rate of lipid oxidation at the surface of the oil droplets; a high rate of lipid oxidation is attributed to an increased surface area as the droplet size decreases [20, 26]. Therefore, the smaller droplet size of soy lecithin-stabilised nanoemulsion may promote a higher lipid oxidation rate than in Tween 20 nanoemulsion. These results are in agreement with previous studies in which it was found that emulsions stabilised by xanthan gum with smaller droplets (3.4 µm) showed higher lipid oxidation values than emulsions with larger droplets (6.4 µm) [41]; lipid oxidation of nanoemulsions (66 nm) stabilised by whey protein isolate was higher than in emulsions (325 nm) [48], indicating that emulsions with smaller droplet size were contributed to higher values of lipid oxidation.
Effect of HPH pressure and number of cycles’ interaction on the physical and chemical properties of nanoemulsions stabilised with Tween 20 and lecithin
The interaction between HPH pressure and cycle number on nanoemulsion properties was assessed. The pressures applied were 200 and 400 bars, because at these pressures, the MDD values of both emulsions were around 200 nm, as it has been shown in Table 1. The number of cycles selected was 1, 2, and 3 to produce nanoemulsions with oil droplets’ particle size of less than 200 nm. There was a significant interaction (p < 0.05) between homogenisation pressure and number of cycles for the MDD of nanoemulsions formulated with Tween 20 (Fig. 2A). At both homogenisation pressures (200 and 400 bars), when the number of cycles increased, the droplet size significantly decreased (p < 0.05). However, there was no significant interaction between factors (p > 0.05) for the PDI of nanoemulsions formulated with Tween 20. The PDI significantly decreased (p < 0.05) when the homogenisation pressure and number of cycles increased (Fig. 2 C). When the number of cycles increased, there were more events prompting to oil droplet break-up [49] due to the following cycles of homogenization allowed an increase in energy input for emulsification [40], and more time was available for the surfactant to be absorbed on the oil-in-water interface [12, 42]. Thus, there was more efficient surfactant absorption onto the new oil–water interface after several cycles of HPH. These results were in agreement with previous studies, in which increasing the number of homogenisation cycles resulted in a significant reduction in the droplet size and size distribution in o/w emulsions stabilised with Tweens surfactants [21, 23]. Interestingly, in terms of nanoemulsions formulated with soy lecithin, there was no significant interaction between homogenisation pressure and number of cycles (p > 0.05) for the MDD and PDI (Fig. 2B and 2D, respectively). In general, the increase in pressure or cycle number did not have a significant effect on the lecithin emulsions’ properties. This result could be due to the viscoelastic property of lecithin at the emulsion interface that formed a thick viscoelastic film stabilising the oil/water interface [44, 45], but the forces generated in the HPH and time timescale were not enough to promote a significant change in droplet particle size.
Interaction plots (mean ± 95% confidence interval). A and B Interactions between the pressure and number of cycles for the MDD of nanoemulsions formulated with Tween 20 and lecithin, respectively. C and D Interactions between the pressure and number of cycles for the PDI of nanoemulsions formulated with Tween 20 and lecithin, respectively. E and F Interactions between the pressure and number of cycles for the thermal stability of nanoemulsions formulated with Tween 20 and lecithin, respectively. Different capital or lower case letters above bars indicate significant differences between samples (p < 0.05)
Regarding the thermal stability, there was a significant interaction (p < 0.05) between homogenisation pressure and number of cycles for the thermal stability of both Tween 20 and lecithin-stabilised nanoemulsions (Fig. 2E and 2F), and an increase in the homogenisation pressure and number of cycles resulted in significantly (p < 0.05) higher stability. When applying 200 bars, the effect of the number of cycles was greater than at 400 bars. When evaluating the implications of scaling up energy intensive processes, such as HPH other factors, a part of product properties should be taking into account, such as time and costs of the process. For nanoemulsions formulated with Tween 20, the shorter and less-intensive homogenisation process that gave the emulsions with the greatest stability was 1 cycle at 400 bars (Fig. 2E), due to the smaller droplets size and narrower size distribution values than when processed at 200 bars (Fig. 2A and C). A decrease in particle droplet size gives place to a decreases of the attractive forces between the droplets [5, 50]; smaller droplets have better stability against droplet coalescence and flocculation because of the reduction in Brownian motion and gravitation forces [50], allowing nanoemulsions to be protected against flocculation phenomena. Regarding the nanoemulsions formulated with soy lecithin, the shorter and less-intensive homogenisation process was 1 cycle at 400 bars (Fig. 2F).
Optimisation of high-pressure homogenisation processing parameters
Model fitting
MDD, PDI, TS, and TBARS are most important properties of a nanoemulsion used to optimise homogenisation processing parameters [51,52,53]. The experimental conditions including homogenisation pressure and number of cycles with their corresponding response values were subjected to regression analysis. The regression coefficients for the second-order polynomial equations and results for the linear, quadratic, and interaction terms as well as the coefficient of determination (\({R}^{2}\)) are presented in Tables 4 and 5.
The P values were used as a tool to check the significance of the interactions among the variables. According to the ANOVA of the regression models (Table 4), there was a high coefficient of determination (\({R}^{2})\) obtained for dependent variables such as MDD and PDI without significant lack of fit, indicating a satisfactory adjustment of the polynomial model to the experimental data. The polynomial model of MDD and PDI was calculated to be between 0.737 and 0.982, indicating that 73.7% and 98.2% of the variability in the response could be explained by the second-order polynomial prediction equation. However, there was a low \({R}^{2}\) for TBARS and a significant lack-of-fit test for TS of Tween 20 stabilised emulsion, indicating that TS and TBARS were not a good fit for the model. Therefore, the models of MDD and PDI can be used to describe the optimisation of homogenisation parameters.
The regression coefficients along with corresponding P values for the model of MDD and PDI are shown in Table 5. Analysis of significance of each term of polynomial model obtained for MDD of Tween 20 stabilised emulsions indicated that linear terms of pressure (\({x}_{1}\)) and cycle (\({x}_{2}\)) and the quadratic term of pressure (\({x}_{2}^{2}\)) resulted significant for the response, whereas the polynomial model for MDD of soy lecithin-stabilised emulsions indicated that linear term of cycle (\({x}_{2}\)) and quadratic term of pressure (\({x}_{2}^{2}\)) resulted significant for the response. The second-order polynomial prediction model of MDD can be expressed as follows:
where \(y\) is the predicted response of MDD, and \({x}_{1}\) and \({x}_{2}\) are homogenisation pressure (bars) and number of cycles, respectively.
Analysis of significance of each term of polynomial model obtained for PDI of Tween 20 stabilised emulsions indicated that linear terms of pressure (\({x}_{1}\)) and cycle (\({x}_{2}\)), and the quadratic term of pressure (\({x}_{2}^{2}\)) resulted significant for the response; the PDI of soy lecithin-stabilised emulsions indicated that all terms of linear, quadratic, and the interaction resulted non-significant for the response. The second-order polynomial prediction model of PDI can be expressed as follows:
where \(y\) is the predicted response of PDI, and \({x}_{1}\) and \({x}_{2}\) are homogenisation pressure and number of cycles, respectively.
The \({R}^{2}\) of the quadratic model remained very high as seen in the Table 4, which indicates a very good statistical fit. This suggests that we can use the quadratic models to navigate the response space.
Canonical and ridge analysis
The canonical analysis of the response surface was performed to determine the shape of the fitted response and the estimated stationary point. The critical value of the canonical analysis represents the value of the factors (processing) with the highest sensitivity to the measured emulsion parameters with sensitivity. Regarding the variables of pressure and number of cycles, please note that both variables can be outside the design space in the canonical analysis output. For example, the number of cycles cannot be lower than 1 cycle and must be an integer. Therefore, the column next to calculated critical value for cycle represents the more meaningful output. As shown in Table 6, the canonical and stationary point analysis of MDD and PDI of Tween 20 indicated that the stationary point was a minimum point. Therefore, the estimated surface of Tween 20 stabilised emulsions has a unique optimum, and a ridge analysis was performed to determine the optimum. On the other hand, the stationary point of MDD and PDI of soy lecithin was saddle point (mixed signs of all eigenvalues), suggesting that movement away from these points would cause an increased or decreased response, depending upon direction of movement.
Optimal homogenisation parameters were determined by ridge minimum analysis. The method of ridge minimum analysis computes the estimated ridge of the minimum response by decreasing radius from the centre of the original design. The results of ridge analysis (Table 7 and 8) indicated that homogenisation pressure and cycle were positively related to the response. Due to the fact that both variables of pressure and number of cycles must be an integer, the ridge minimum analysis of Tween 20 stabilised emulsion indicated that the minimum value of MDD was 174.64 nm at the optimal pressure at 764 bars and 1 cycle, and the minimum value of PDI was 0.23 at the optimal pressure of 465 bars and 3 cycles. In terms of the soy lecithin-stabilised emulsions, the minimum value of MDD was 180.80 nm at the optimal pressure of 3 bars and 2 cycles, and the minimum value of PDI was 0.23 at optimal pressure of 223 bars and 3 cycles.
To visualise the impact of the processing parameters to the properties of the emulsions, two-dimensional contour plot between homogenisation pressure and cycle were constructed, as shown in Fig. 3 a–d. The prominent interaction effect is shown in Fig. 3 A and B. The significant effect of pressure and cycle could be observed in the graphs, which indicated that MDD and PDI of Tween 20 stabilised emulsions decreased when increasing the pressure and cycle of homogenisation. Regressing analysis (Table 5) also confirmed that MDD and PDI of Tween 20 stabilised emulsions were significantly (p < 0.05) affected by pressure and cycle, which is also in agreement with the classical statistical analysis (Table 2 and Fig. 2). On the other hand, the number of cycles was a main variable affecting on MDD of soy lecithin-stabilised emulsion, which MDD decreased when increasing cycle number. As a result of optimisation analysis and model fitting, although there is a good fit with the model for the MDD and PDI of both nanoemulsions, the experimental validation of the predicted model should be carried out as further work. Moreover, this optimisation analysis would be a more effective result if there was a larger size of samples for a more robust calibration.
Conclusions
This study has demonstrated the effect of high-pressure homogenisation processing parameters including pressure and number of cycles on the formation and physicochemical stability of extra virgin olive oil-in-water nanoemulsions stabilised by Tween 20 or soy lecithin. Although the particle size and size distribution of Tween 20 nanoemulsions were influenced by homogenisation pressure and cycles, there was not an effect on soy lecithin nanoemulsions. This showed that the molecular weight and viscoelastic properties of Tween 20 and soy lecithin play a major role in the formation and properties of emulsion. In addition, high-pressure homogenisation at pressure at least 400 bars and 1 cycle showed the better results in terms of nanoemulsion stability. Although Tween 20 nanoemulsion showed significantly higher values (p < 0.05) of thermal stability and lipid oxidative stability than soy lecithin nanoemulion, soy lecithin is an interesting surfactant for application in food industry as it is obtained from natural sources and provides more desirable taste compared to Tween 20. The optimal homogenisation parameters for both emulsions were successfully modelled by RSM and provided information to support the production of nanoemulsions at industrial scale. Overall, HPH proved to be an effective process to obtain nanoemulsions stabilised by soy lecithin for application in food and beverage products and the data of optimisation of processing parameters. For more complete the results, future work could include the validation of the model.
Data availability and materials
The data presented in this study are openly available in the University of Reading Research Data Archive at https://doi.org/10.17864/1947.000368. (15/03/2022).
References
Komaiko JS, McClements DJ (2016) Formation of food-grade nanoemulsions using low-energy preparation methods: a review of available methods. Compr Rev Food Sci Food Safe 15:331–352. https://doi.org/10.1111/1541-4337.12189
Pathakoti K, Manubolu M, Hwang H-M (2017) Nanostructures: current uses and future applications in food science. J Food Drug Anal 25:245–253. https://doi.org/10.1016/j.jfda.2017.02.004
Calligaris S, Plazzotta S, Bot F, Grasselli S, Malchiodi A, Anese M (2016) Nanoemulsion preparation by combining high pressure homogenization and high power ultrasound at low energy densities. Food Res Int 83:25–30. https://doi.org/10.1016/j.foodres.2016.01.033
Mehmood T (2015) Optimization of the canola oil based vitamin E nanoemulsions stabilized by food grade mixed surfactants using response surface methodology. Food Chem 183:1–7. https://doi.org/10.1016/j.foodchem.2015.03.021
Qian C, McClements DJ (2011) Formation of nanoemulsions stabilized by model food-grade emulsifiers using high-pressure homogenization: factors affecting particle size. Food Hydrocoll 25:1000–1008. https://doi.org/10.1016/j.foodhyd.2010.09.017
Szydłowska-Czerniak A, Karlovits G, Dianoczki C, Recseg K, Szłyk E (2008) Comparison of two analytical methods for assessing antioxidant capacity of rapeseed and olive oils. J Am Oil Chem Soc 85:141–149
Poyato C, Ansorena D, Navarro-Blasco I, Astiasarán I (2014) A novel approach to monitor the oxidation process of different types of heated oils by using chemometric tools. Food Res Intl 57:152–161. https://doi.org/10.1016/j.foodres.2014.01.033
Tuberoso CIG, Kowalczyk A, Sarritzu E, Cabras P (2007) Determination of antioxidant compounds and antioxidant activity in commercial oilseeds for food use. Food Chem 103:1494–1501. https://doi.org/10.1016/j.foodchem.2006.08.014
Bhatnagar AS, Prasanth Kumar PK, Hemavathy J, Gopala Krishna AG (2009) Fatty acid composition, oxidative stability, and radical scavenging activity of vegetable oil blends with coconut oil. J Am Oil Chem Soc 86:991–999. https://doi.org/10.1007/s11746-009-1435-y
Guerra-Rosas MI, Morales-Castro J, Ochoa-Martínez LA, Salvia-Trujillo L, Martín-Belloso O (2016) Long-term stability of food-grade nanoemulsions from high methoxyl pectin containing essential oils. Food Hydrocoll 52:438–446. https://doi.org/10.1016/j.foodhyd.2015.07.017
Sharif HR, Goff HD, Majeed H, Liu F, Nsor-Atindana J, Haider J, Liang R, Zhong F (2017) Physicochemical stability of β-carotene and α-tocopherol enriched nanoemulsions: Influence of carrier oil, emulsifier and antioxidant. Collo Surf Physicochem Eng Asp 529:550–559. https://doi.org/10.1016/j.colsurfa.2017.05.076
Karbstein H, Schubert H (1995) Developments in the continuous mechanical production of oil-in-water macro-emulsions. Chem Eng Process Process Intensif 34:205–211. https://doi.org/10.1016/0255-2701(94)04005-2
Donsì F, Sessa M, Ferrari G (2012) Effect of emulsifier type and disruption chamber geometry on the fabrication of food nanoemulsions by high pressure homogenization. Ind Eng Chem Res 51:7606–7618. https://doi.org/10.1021/ie2017898
Cottrell T, Jv P (2014) Sorbitan Esters and Polysorbates. In. John Wiley & Sons Ltd Chichester, UK, pp 271–296
Goindi S, Kaur A, Kaur R, Kalra A, Chauhan P (2016) 19-Nanoemulsions: an emerging technology in the foodindustry. Nanotechnology in the Agri-Food Industry, Emulsions. Academic press, pp 651–688. https://doi.org/10.1016/B978-0-12-804306-6.00019-2
Klang V, Valenta C (2011) Lecithin-based nanoemulsions. J Drug Deliv Sci Technol 21:55–76. https://doi.org/10.1016/S1773-2247(11)50006-1
Bueschelberger HG, Tirok S, Stoffels I, Shoeppe A (2014) Lecithins. In: Norn Viggo (Ed) Emulsifiers in food technology, 2nd edn. Wiley-Blackwell. https://doi.org/10.1002/9781118921265.ch2
McClements DJ (2016) Emulsion ingredients. In: McClements DJ (ed) Food emulsions: principles, practices, and techniques, 3rd edn. Taylor & Francis Group
Öztürk B (2017) Nanoemulsions for food fortification with lipophilic vitamins: Production challenges, stability, and bioavailability: nanoemulsion delivery systems for lipophilic vitamins. Eur J Lipid Sci Technol 119:1500539. https://doi.org/10.1002/ejlt.201500539
McClements DJ (2011) Edible nanoemulsions: fabrication, properties, and functional performance. Soft Matter 7:2297–2316. https://doi.org/10.1039/c0sm00549e
Galvão KCS, Vicente AA, Sobral PJA (2018) Development, characterization, and stability of o/w pepper nanoemulsions produced by high-pressure homogenization. Food Bioprocess Technol 11:355–367. https://doi.org/10.1007/s11947-017-2016-y
El Kinawy OS, Petersen S, Ulrich J (2012) Technological aspects of nanoemulsion formation of low-fat foods enriched with vitamin E by high-pressure homogenization. Chem Eng Technol 35:937–940. https://doi.org/10.1002/ceat.201100608
Yuan Y, Gao Y, Zhao J, Mao L (2008) Characterization and stability evaluation of β-carotene nanoemulsions prepared by high pressure homogenization under various emulsifying conditions. Food Res Int 41:61–68. https://doi.org/10.1016/j.foodres.2007.09.006
Qian C, Decker EA, Xiao H, McClements DJ (2012) Physical and chemical stability of β-carotene-enriched nanoemulsions: influence of pH, ionic strength, temperature, and emulsifier type. Food Chem 132:1221–1229. https://doi.org/10.1016/j.foodchem.2011.11.091
Kim S-H, Ji Y-S, Lee E-S, Hong S-T (2016) Ostwald ripening stability of curcumin-loaded MCT nanoemulsion: influence of various emulsifiers. Prev Nutr Food Sci 21:289–295. https://doi.org/10.3746/pnf.2016.21.3.289
McClements DJ, Decker EA (2000) Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci 65:1270–1282. https://doi.org/10.1111/j.1365-2621.2000.tb10596.x
Fomuso LB, Corredig M, Akoh CC (2002) Effect of emulsifier on oxidation properties of fish oil-based structured lipid emulsions. J Agric Food Chem 50:2957–2961. https://doi.org/10.1021/jf011229g
Uluata S, McClements DJ, Decker EA (2015) Physical stability, autoxidation, and photosensitized oxidation of ω-3 oils in nanoemulsions prepared with natural and synthetic surfactants. J Agric Food Chem 63:9333–9340. https://doi.org/10.1021/acs.jafc.5b03572
Arancibia C, Riquelme N, Zúñiga R, Matiacevich S (2017) Comparing the effectiveness of natural and synthetic emulsifiers on oxidative and physical stability of avocado oil-based nanoemulsions. Innov Food Sci Emerg Technol 44:159–166. https://doi.org/10.1016/j.ifset.2017.06.009
National Center for Biotechnology Information. PubChem Compound Summary for CID 443314, Polysorbate 20. https://pubchem.ncbi.nlm.nih.gov/compound/Tween-20. Accessed 23 Nov 2021
National Center for Biotechnology Information. PubChem Compound Summary for CID 57369748, Lecithin from Soybean. https://pubchem.ncbi.nlm.nih.gov/compound/Lecithin-from-Soybean. Accessed 23 Nov 2021
Arancibia C, Navarro-Lisboa R, Zúñiga RN, Matiacevich S (2016) Application of CMC as thickener on nanoemulsions based on olive oil: physical properties and stability. Int J Polym Sci 2016:1–10. https://doi.org/10.1155/2016/6280581
Taha A, Hu T, Hu H, Zhang Z, Bakry AM, Khalifa I, Pan S (2018) Effect of different oils and ultrasound emulsification conditions on the physicochemical properties of emulsions stabilized by soy protein isolate. Ultrason Sonochem 49:283–293. https://doi.org/10.1016/j.ultsonch.2018.08.020
Bai L, Huan S, Gu J, McClements DJ (2016) Fabrication of oil-in-water nanoemulsions by dual-channel microfluidization using natural emulsifiers: Saponins, phospholipids, proteins, and polysaccharides. Food Hydrocoll 61:703–711. https://doi.org/10.1016/j.foodhyd.2016.06.035
Luo X, Zhou Y, Bai L, Liu F, Zhang Z, Zhang R, Zheng B, Deng Y, McClements DJ (2017) Production of highly concentrated oil-in-water emulsions using dual-channel microfluidization: use of individual and mixed natural emulsifiers (saponin and lecithin). Food Res Int 96:103–112. https://doi.org/10.1016/j.foodres.2017.03.013
Mukherjee I, Moulik SP, Rakshit AK (2013) Tensiometric determination of Gibbs surface excess and micelle point: a critical revisit. J Colloid Interface Sci 394:329–336. https://doi.org/10.1016/j.jcis.2012.12.004
El-Sukkary MMA, Syed NA, Aiad I, El-Azab WIM (2008) Synthesis and characterization of some alkyl polyglycosides surfactants. J Surfactant Deterg 11:129–137. https://doi.org/10.1007/s11743-008-1063-9
Sahafi SM, Goli SAH, Kadivar M, Varshosaz J (2018) Preparation and characterization of bioactive oils nanoemulsions: effect of oil unsaturation degree, emulsifier type and concentration. J Dispers Sci Technol 39:676–686. https://doi.org/10.1080/01932691.2017.1381919
Qiu C, Zhao M, Decker EA, McClements DJ (2015) Influence of protein type on oxidation and digestibility of fish oil-in-water emulsions: gliadin, caseinate, and whey protein. Food Chem 175:249–257. https://doi.org/10.1016/j.foodchem.2014.11.112
Floury J, Desrumaux A, Lardières J (2000) Effect of high-pressure homogenization on droplet size distributions and rheological properties of model oil-in-water emulsions. Innov Food Sci Emerg Technol 1:127–134. https://doi.org/10.1016/S1466-8564(00)00012-6
Gohtani S, Sirendi M, Yamamoto N, Kajikawa K, Yamano Y (1999) Effect of droplet size on oxidation of docosahexaenoic acid in emulsion system. J Dispers Sci Technol 20:1319–1325. https://doi.org/10.1080/01932699908943855
Jafari SM, Assadpoor E, He Y, Bhandari B (2008) Re-coalescence of emulsion droplets during high-energy emulsification. Food Hydrocoll 22:1191–1202. https://doi.org/10.1016/j.foodhyd.2007.09.006
Kronberg B, Holmberg K, Lindman B (2014) Surface chemistry of surfactants and polymers. John Wiley & Sons
Nash JJ, Erk KA (2017) Stability and interfacial viscoelasticity of oil-water nanoemulsions stabilized by soy lecithin and Tween 20 for the encapsulation of bioactive carvacrol. Colloid Surf Physicochem Eng Asp 517:1–11. https://doi.org/10.1016/j.colsurfa.2016.12.056
Shchipunov YA (2001) Lecithin organogel: a micellar system with unique properties. Colloid Surf Physicochem Eng Asp 183:541–554. https://doi.org/10.1016/S0927-7757(01)00511-8
Pichot R, Watson RL, Norton IT (2013) Phospholipids at the interface: current trends and challenges. Intl J Mol Sci 14:11767–11794. https://doi.org/10.3390/ijms140611767
Choe E, Min DB (2006) Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci Food Saftey 5:169–186
Lee SJ, Choi SJ, Li Y, Decker EA, McClements DJ (2011) Protein-stabilized nanoemulsions and emulsions: comparison of physicochemical stability, lipid oxidation, and lipase digestibility. J Agr Food Chem 59:415–427. https://doi.org/10.1021/jf103511v
Lee L, Hancocks R, Noble I, Norton IT (2014) Production of water-in-oil nanoemulsions using high pressure homogenisation: a study on droplet break-up. J Food Eng 131:33–37. https://doi.org/10.1016/j.jfoodeng.2014.01.024
Tadros T, Izquierdo P, Esquena J, Solans C (2004) Formation and stability of nano-emulsions. Adv Colloid Interface Sci 108–109:303–318. https://doi.org/10.1016/j.cis.2003.10.023
Jafari SM, He Y, Bhandari B (2006) Optimization of nano-emulsions production by microfluidization. Eur Food Res Technol 225:733–741. https://doi.org/10.1007/s00217-006-0476-9
Ruiz-Montañez G, Ragazzo-Sanchez JA, Picart-Palmade L, Calderón-Santoyo M, Chevalier-Lucia D (2017) Optimization of nanoemulsions processed by high-pressure homogenization to protect a bioactive extract of jackfruit (Artocarpus heterophyllus Lam). Innov Food Sci Emerg Technol 40:35–41. https://doi.org/10.1016/j.ifset.2016.10.020
Sadeghpour Galooyak S, Sadeghpour Galooyak S, Dabir B, Dabir B (2015) Three-factor response surface optimization of nano-emulsion formation using a microfluidizer. J Food Sci Technol 52:2558–2571. https://doi.org/10.1007/s13197-014-1363-1
Acknowledgements
The authors are grateful to The Royal Thai Government for financing the Ph.D. scholarship of author Jansuda Kampa.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Conceptualisation, J.K, A.K., R.F., and J.R.-G.; methodology, J.K., A.K., S.K., and J.R.-G.; formal analysis, J.K. and S.K.; writing—original draft preparation, J.K. and J.R.-G.; writing—review and editing, A.K., J.R.-G., and R.F.; supervision, J.R.-G. and R.F. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Compliance with ethics requirements
This study does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kampa, J., Koidis, A., Ghawi, S.K. et al. Optimisation of the physicochemical stability of extra virgin olive oil-in-water nanoemulsion: processing parameters and stabiliser type. Eur Food Res Technol 248, 2765–2777 (2022). https://doi.org/10.1007/s00217-022-04088-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-022-04088-7