Abstract
In grape berries (Vitis vinifera L.), sesquiterpenes are mainly accumulated as hydrocarbons in the epicuticular wax layer of grapes, whereas monoterpenes, which are predominantly present as alcohols, are glycosylated and are stored as glycosides in the vacuoles of grape berry cells. In this study, extensive analysis of grape berry hydrolysates by means of comprehensive two-dimensional gas chromatography–time-of-flight–mass spectrometry demonstrated that glycosylated sesquiterpene alcohols show very little structural diversity when compared to the sesquiterpene hydrocarbon fraction in the cuticle and are glycosylated to a rather low extent when compared to monoterpenols. Twenty-four enzymatically released terpenols were found in hydrolysates of the aromatic white wine variety Gewürztraminer (V. vinifera subsp. vinifera) after previous solid-phase extraction and headspace solid-phase microextraction. The detection of only three sesquiterpene alcohols, namely farnesol, nerolidol and drimenol, shows that most sesquiterpene hydrocarbons do not have a related hydroxylated structure in grapes. Nevertheless, the presence of the acyclic aglycone farnesol and nerolidol may be of importance for the wine aroma, since these structural isomers can be converted into numerous sesquiterpenes by nonenzymatic acid-catalyzed reactions during wine production. Grape-derived glycosidically bound sesquiterpene alcohols, therefore, represent, in addition to free sesquiterpene hydrocarbons, another pool of compounds that may influence the aroma profile of wines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Terpenes play a key role as plant secondary metabolites for the aroma of grapes (Vitis vinifera L.) and wines [1]. Both mono- (C10) and sesquiterpenes (C15), which are formed via the cytosolic mevalonate-dependent metabolic pathway (MVA) and the mevalonate-independent 1-deoxy-d-xylulose 5-phosphate/2-C-methyl-d-erythritol 4-phosphate biosynthesis pathway (DOXP/MEP) occurring in plastids [2, 3], are of central importance for the aroma [4, 5]. According to current knowledge, monoterpenes are stored predominantly as sugar bound alcohols in the vacuole whereas sesquiterpenes are accumulated as hydrocarbons in the nonpolar epicuticular wax layer of grape berries [6]. While free and volatile terpenes contribute directly to the aroma, a much larger fraction of glycosidically bound, non-volatile and thus aroma-inactive terpenoids is referred to as “hidden aromatic potential” in ripe grapes [7,8,9]. In 1974, the first evidence of glycosidically bound terpenes in grape berries was published by Cordonnier and Bayonove [10]. In the following decade, Williams et al. confirmed the presence of monoterpene glycosides by hydrolytic cleavage of the glycosidic bond, identifying both acyclic monoterpene alcohols such as linalool, nerol and geraniol as well as monocyclic terpenols such as α-terpineol as released aglycones [11,12,13]. 6-O-α-l-Rhamnopyranosyl-β-d-glucopyranosides, 6-O-α-l-arabinofuranosyl-β-d-glucopyranosides, 6-O-β-d-apiofuranosyl-β-d-glucopyranosides and β-d-glucopyranosides could be determined as sugar residues of monoterpene glycosides [11, 14]. While monoterpene glycosides in grapes have been extensively investigated in recent years [15,16,17,18], glycosidically bound sesquiterpenes in V. vinifera are still largely unknown. The first sesquiterpene glycosides in grapes were recently tentatively identified using ultra-high-performance liquid chromatography (UHPLC) quadrupole time-of-flight (qTOF) mass spectrometry (MS), but without further characterization of the aglycones [19]. Since glycosidically bound terpene alcohols are partly released both enzymatically and acid catalytically during winemaking [20, 21], grape-derived sesquiterpene glycosides represent an unexplored aroma potential, in addition to monoterpene glycosides. The potential importance of sesquiterpene alcohols for the wine aroma has recently been demonstrated by microvinification experiments. The yeast-derived, acyclic sesquiterpene alcohols nerolidol and farnesol can be converted into numerous other sesquiterpenes by acid-catalyzed reactions during the vinification process, thus significantly altering the sesquiterpene profile of wines [22]. In the presented study, the terpene alcohols released from grape-derived terpene glycosides were analyzed using the Gewürztraminer variety. The glycosidically bound terpenes from isolated exocarp of grapes were separated and concentrated using solid-phase extraction (SPE) and subsequently hydrolyzed enzymatically. The hydrolysates were analyzed by comprehensive two-dimensional gas chromatography–time-of-flight–mass spectrometry (GC × GC–TOF–MS) after previous headspace solid-phase microextraction (HS-SPME).
Materials and methods
Chemicals
Milli-Q water, ethanol (purity: ≥ 99.8%) and zinc sulfate (purity: 99.9%) were purchased from VWR International (Darmstadt, HE, Germany). The solvents dichloromethane (purity: 99.99%) and methanol (purity: ≥ 99.9%) were acquired from Fisher Scientific UK Limited (Loughborough, Leicestershire, UK) and Honeywell (Seelze, NI, Germany). Di-sodium hydrogen phosphate (purity: ≥ 99%) and sodium dihydrogen phosphate monohydrate (purity: ≥ 99%) were obtained from Sigma-Aldrich Chemie GmbH (Taufkirchen, BY, Germany). Potassium ferrocyanide trihydrate (purity: ≥ 99%) was acquired from Acros Organics (Geel, Antwerp Province, Belgium). Citric acid monohydrate (purity: ≥ 99.5%) and geraniol (purity: ≥ 90%) were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, BW, Germany) and the enzyme preparation (β-glucosidase, polygalacturonase) from Oenobrands SAS (Montpellier, Occitanie, France). The compounds citronellol (purity: ≥ 95%), nerol (purity: 98.7%), carvacrol (purity: 99.4%), thymol (purity: > 99.9%), menthol (purity: 99.3%), dihydrocitronellol (purity: 98.3%) and a C7–C30 saturated alkane standard solution (certified reference material) were obtained from Sigma-Aldrich (Steinheim, BW, Germany). Nerolidol (cis + trans, purity: 97.5%), farnesol (mixture of isomers, purity: 98.0%), α-terpineol (purity: 96%) and linalool (purity: 98.5%) were purchased from Alfa Aesar (Ward Hill, MA, USA).
Sample material
The grape berries were sampled on two dates, September 15, 2016 and October 4, 2016 from two aromatic clones (11 Gm and FR 46-106) of Gewürztraminer at the Hochschule Geisenheim University (Geisenheim, HE, Germany; GPS coordinates: 49.98505, 7.94582; altitude: 96 m a.s.l). The stage of maturation of the grapes was described on the basis of refractometrically determined soluble solids (September 15, 2016: 11 Gm, 19.0°Bx; FR 46-106, 19.5°Bx and October 4, 2016: 11 Gm, 21.0°Bx; FR 46-106, 23.0°Bx).
Sample preparation
Tissue extraction
The isolation of the terpene glycosides from the skin of grape berries was carried out based on a previous paper [23]. As described, the skin of the grapes was peeled off and adherent pulp was removed. For the terpene analysis, 20 g (fresh weight) of the grape berry skin was grounded in liquid nitrogen. The material was extracted under nitrogen and exclusion of light in a phosphate buffer (0.1 M; Na2HPO4/NaH2PO4; pH 7) and 13% (v/v) ethanol for 24 h. The extracts were clarified with Carrez reagents and then centrifuged at 24,000 × g, 5 °C for 20 min. The supernatant was purified and concentrated by solid-phase extraction.
Solid-phase extraction (SPE)
To separate the free terpenes from the glycosidically bound terpenes a 500 mg Lichrolut EN column from Merck KGaA (Darmstadt, HE, Germany) was conditioned as stated previously [24]. The free terpenes were eluted with dichloromethane and the glycosidically bound terpenes with methanol. The fraction containing the separated glycosidically bound terpenes was concentrated under reduced pressure until dryness.
Hydrolysis
The residue was dissolved in 20 mL citrate-HCl-buffer (0.1 M; pH 4) and 100 mg of the enzyme preparation was added. All samples were stored at − 80 °C until analysis. Sample preparation was performed three times, resulting in three biological replicates.
Analysis of the hydrolysates
The hydrolysates of the grape berries were thawed and homogenized before analysis. A Combi PAL-xt autosampler with a heatable agitator and fiber conditioning station from CTC Analytics (Zwingen, BL, Switzerland) was used. The terpene alcohols were extracted from headspace using a mixed SPME fiber for volatile and semivolatile compounds (C3–C20) from Supelco (Bellefonte, PA, USA), based on a method described by Welke et al. [25]. After each analysis, the SPME fiber was baked-out to avoid sample carryover. In addition, empty vials were measured as control. The injection was carried out into a 7890B gas chromatograph from Agilent Technologies (Bellefonte, PA, USA) equipped with a ZX2 GC × GC cryogenic modulator from Zoex Corp (Houston, TX, USA). Gas chromatographic separation was performed by combining a highly polar capillary column from Agilent Technologies (Bellefonte, PA, USA) with a medium polar GC column from MEGA s.n.c. (Legnano, MI, Italy), a combination that was used in a previous work for the analysis of sesquiterpene hydrocarbons in grape berry exocarp [26]. The GC × GC separation was followed by a mass spectrometric analysis using a BenchTOF-Select time-of-flight–mass spectrometer from Markes International Limited (Llantrisant, Wales, UK). The method parameters are listed in Table 1. Available mono- and sesquiterpene standards were diluted in ethanol and the resulting 1 mg/kg solutions were injected with a liquid syringe (1 μL injection volume, split mode).
Data analysis
Data were collected in ProtoTof (version 2.0) as *. LSC files and processed using the software platform ChromSpace (version 1.5.1). Both programs are from Markes International Limited (Llantrisant, Wales, UK). The data files were subjected to a dynamic background compensation (dbc) with a peak width of 100 ms. Data analysis was carried out using filtering methods (extracted-ion chromatogram, parametric filtering settings) and integration algorithms (persistence, deconvolution). Compound identification was performed using mass spectra (MS), retention indices (RI) and authentic standards (STD). Recorded mass spectra were compared with library spectra from the National Institute of Standards and Technology (NIST) database of the mass spectral search program (NIST, Gaithersburg, MD, USA; version 2.2). The similarity of the mass spectra was indicated by match factors (MF) and reverse match factors (RMF). After liquid injection of a saturated n-alkane standard solution (C7–C30), the retention indices (RIexp) were calculated using the method of van Den Dool and Kratz for temperature-programmed gas chromatography [27]. The mono- and sesquiterpene alcohols were subsequently identified by comparing the experimentally determined retention indices with literature values (RIlit). The sesquiterpene alcohols (E)-nerolidol and farnesol as well as the monoterpene alcohols linalool, menthol, dihydrocitronellol, α-terpineol, β-citronellol, nerol, geraniol, thymol and carvacrol were additionally identified by commercially available standard compounds. To determine the ratio of sesquiterpene aglycones to monoterpene aglycones, the mean values of the peak areas of all terpenols were summed and the percentage of each analyte calculated.
Results and discussion
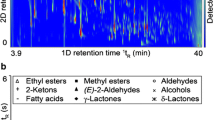
The gas chromatographic separation of terpene aglycones from grapes (V. vinifera L.) requires prior cleavage of the glycosidic bond by means of enzymes or acids. By combining suitable extraction methods (SPE–HS-SPME) with comprehensive two-dimensional gas chromatography–time-of-flight–mass spectrometry (GC × GC–TOF–MS), a total of 24 terpene aglycones were found in hydrolysates of grape berry exocarp. Figure 1 shows the two-dimensional separation of the released terpene alcohols.
GC × GC–TOF–MS total ion chromatogram (TIC) of a hydrolysate from grape berries of the white wine variety Gewürztraminer (Vitis vinifera subsp. vinifera). The x axis represents the primary column retention time (1tR) and the y axis corresponds to the retention time on the secondary column (2tR). The peak intensity, which is proportional to analyte concentration, is represented by color-coding from low (violet) to high (red) on a white background. Note that a logarithmic color gradient has been selected, as numerous trace volatile compounds would otherwise not be visible in a single contour plot due to some very dominant peaks. Peak numbers of mono- and sesquiterpene aglycones released by enzymatic hydrolysis refer to those of Fig. 2 and Table 2
Structural formulas of the enzymatically released terpene alcohols from grape-derived terpene glycosides, grouped by sesquiterpene and monoterpene aglycones. 1–2 acyclic sesquiterpene alcohols, 3 bicyclic sesquiterpene alcohol, 4–11 acyclic monoterpene alcohols, 12–23 monocyclic monoterpene alcohols, 24 bicyclic monoterpene alcohol
Twenty-one monoterpene alcohols were identified in the hydrolysates of grape berries of the terpene-rich white wine variety Gewürztraminer (V. vinifera subsp. vinifera, clones 11 Gm and FR 46-106). Among these, the structural diversity of the p-menthane-related monoterpenols is remarkable. The detection of menthol is particularly interesting because its precursor piperitone contributes to the positive mint aroma of red Bordeaux wines, as described by Picard et al. [28]. The large number of monoterpenols found in the hydrolysates is in marked contrast to the few free monoterpene alcohols found in the volatile profile of ripe grape berries (Gewürztraminer cultivar) identified by May [29]. The GC × GC–TOF–MS measurements thus confirm the assumption that monoterpenols are predominantly glycosidically bound in grapes [6].
Although more than 80% of the peak area of all terpene aglycones detected can be attributed to only six monoterpenols, nerol, α-terpineol, linalool, geraniol, β-citronellol and dihydrocarveol, the first detection of grape-derived sesquiterpene aglycones in the Gewürztraminer variety, especially (E)-nerolidol and farnesol [30, 31], is noteworthy. While previous studies showed that free mono- and sesquiterpene hydrocarbons as well as monoterpene alcohols mainly characterize the wine aroma, the detection of grape-derived sesquiterpene aglycones reveals an undiscovered pool of potential aroma components in the Gewürztraminer variety.
It is known that wine yeasts are able to form the sesquiterpene alcohols farnesol and nerolidol [32]. Recently, deuterium-labeling experiments showed that yeast-derived farnesol and nerolidol significantly alter the terpene profile of wines during the fermentation process [22]. The sesquiterpene alcohols act as precursors for the formation of numerous other sesquiterpenes. Due to the slightly acidic pH during vinification, many sesquiterpenes can already be formed by acid catalysis from nerolidol and farnesol without the presence of corresponding sesquiterpene cyclases [33].
In addition to the acyclic sesquiterpene alcohols nerolidol and farnesol, the first tentative and indirect detection of glycosidically bound drimenol in grape berry exocarp was obtained by GC × GC–TOF–MS analysis. The bicyclic drimenol has a drimane skeleton that has not been found in V. vinifera L. so far. The detection of drimenol is particularly interesting due to the antifungal activity against Botrytis cinerea [34], a pathogen that affects numerous plants including grapes [35]. At this point, it should be mentioned that drimenol can also be formed by rearrangement from farnesol [36].
The fact that only three sesquiterpene alcohols were found in the hydrolysates after extensive GC × GC analysis shows that sesquiterpene alcohols are rather by-products of sesquiterpene hydrocarbon synthases that are responsible for the biosynthesis of structurally diverse carbon skeletons. Glycosylated sesquiterpene alcohols are probably stored in the vacuole of the grape berry cell as water-soluble flavor precursors, similar to glycosylated monoterpene alcohols [37].
It should also be noted that both, numerous monoterpene hydrocarbons and numerous monoterpene alcohols are present in grape berries. In the case of sesquiterpenes, hydrocarbons dominate the volatile profile without the corresponding sesquiterpene alcohol analogues. However, these grape-derived glycosidically bound sesquiterpene alcohols, in particular farnesol and nerolidol, represent another important source of aroma precursors that may influence the wine aroma.
References
Mele MA, Kang H-M, Lee Y-T, Islam MZ (2020) Grape terpenoids: flavor importance, genetic regulation, and future potential. Crit Rev Food Sci Nutr 1–19
Luan F, Wüst M (2002) Differential incorporation of 1-deoxy-d-xylulose into (3S)-linalool and geraniol in grape berry exocarp and mesocarp. Phytochemistry 60:451–459
May B, Lange BM, Wüst M (2013) Biosynthesis of sesquiterpenes in grape berry exocarp of Vitis vinifera L.: evidence for a transport of farnesyl diphosphate precursors from plastids to the cytosol. Phytochemistry 95:135–144
Li Z, Howell K, Fang Z, Zhang P (2020) Sesquiterpenes in grapes and wines: occurrence, biosynthesis, functionality, and influence of winemaking processes. Compr Rev Food Sci Food Saf 19:247–281
Mateo JJ, Jiménez M (2000) Monoterpenes in grape juice and wines. J Chromatogr A 881:557–567
Schwab W, Wüst M (2015) Understanding the constitutive and induced biosynthesis of mono- and sesquiterpenes in grapes (Vitis vinifera): a key to unlocking the biochemical secrets of unique grape aroma profiles. J Agric Food Chem 63:10591–10603
Günata YZ, Bayonove CL, Baumes RL, Cordonnier RE (1985) The aroma of grapes I. Extraction and determination of free and glycosidically bound fractions of some grape aroma components. J Chromatogr A 331:83–90
Stahl-Biskup E, Intert F, Holthuijzen J, Stengele M, Schulz G (1993) Glycosidically bound volatiles—a review 1986–1991. Flavour Fragr J 8:61–80
Lamorte SA, Gambuti A, Genovese A, Selicato S, Moio L (2008) Free and glycoconjugated volatiles of V. vinifera grape ‘Falanghina.’ Vitis 47:241–243
Cordonnier R, Bayonove C (1974) Mise en évidence dans la baie de raisin, variété Muscat d’Alexandrie, de monoterpènes liés révélables par une ou plusieurs enzymes du fruit. CR Acad Sci Paris 278:3387–3390
Williams PJ, Strauss CR, Wilson B, Massy-Westropp RA (1982) Novel monoterpene disaccharide glycosides of Vitis vinifera grapes and wines. Phytochemistry 21:2013–2020
Williams PJ, Strauss CR, Wilson B, Massy-Westropp RA (1982) Studies on the hydrolysis of Vitis vinifera monoterpene precursor compounds and model monoterpene β-d-glucosides rationalizing the monoterpene composition of grapes. J Agric Food Chem 30:1219–1223
Williams PJ, Strauss CR, Wilson B, Massy-Westropp RA (1982) Use of C18 reversed-phase liquid chromatography for the isolation of monoterpene glycosides and nor-isoprenoid precursors from grape juice and wines. J Chromatogr A 235:471–480
Günata YZ, Dugelay I, Sapis J-C, Baumes R, Bayonove C (1994) Role of Enzymes in the Use of the Flavour Potential from Grape Glycosides in Winemaking. In: Schreier P, Winterhalter P (eds) Progress in flavour precursor studies. Proceedings of the international conference. Allured Publishing Corporation, Carol Stream, pp 219–245
Flamini R, De Rosso M, Panighel A, Dalla Vedova A, De Marchi F, Bavaresco L (2014) Profiling of grape monoterpene glycosides (aroma precursors) by ultra-high performance-liquid chromatography-high resolution mass spectrometry (UHPLC/QTOF). J Mass Spectrom 49:1214–1222
Stanitzek S (2014) Monoterpenylglucosyltransferasen aus Vitis vinifera: Funktionelle Charakterisierung und Analytik der Produkte mittels LC-MS/MS. Ph.D. thesis, University of Bonn
Hjelmeland AK, Ebeler SE (2015) Glycosidically bound volatile aroma compounds in grapes and wine: a review. Am J Enol Vitic 66:1–11
Wang X-j, Song H-c, Yang Y, Tao Y-s (2020) Chemical profile of terpene glycosides from Meili grape detected by GC–MS and UPLC–Q-TOF-MS. Eur Food Res Technol 246:2323–2333
Caffrey AJ, Lerno LA, Zweigenbaum J, Ebeler SE (2020) Direct analysis of glycosidic aroma precursors containing multiple aglycone classes in Vitis vinifera berries. J Agric Food Chem 68:3817–3833
Carrau FM, Boido E, Dellacassa E (2008) Terpenoids in grapes and wines: origin and micrometabolism during the vinification process. Nat Prod Commun 3:577–592
Sarry J-E, Günata YZ (2004) Plant and microbial glycoside hydrolases: volatile release from glycosidic aroma precursors. Food Chem 87:509–521
Könen PP, Wüst M (2020) Dissecting sesquiterpene profiles of Lemberger red wines using ex vivo tissue deuterium-labeling and comprehensive two-dimensional gas chromatography–time-of-flight–mass spectrometry. J Agric Food Chem 68:8936–8941
Bönisch F, Frotscher J, Stanitzek S, Rühl E, Wüst M, Bitz O, Schwab W (2014) A UDP-glucose: monoterpenol glucosyltransferase adds to the chemical diversity of the grapevine metabolome. Plant Physiol 165:561–581
Piñeiro Z, Palma M, Barroso CG (2004) Determination of terpenoids in wines by solid phase extraction and gas chromatography. Anal Chim Acta 513:209–214
Welke JE, Manfroi V, Zanus M, Lazarotto M, Alcaraz Zini C (2012) Characterization of the volatile profile of Brazilian Merlot wines through comprehensive two dimensional gas chromatography time-of-flight mass spectrometric detection. J Chromatogr A 1226:124–139
Könen PP, Wüst M (2019) Analysis of sesquiterpene hydrocarbons in grape berry exocarp (Vitis vinifera L.) using in vivo-labeling and comprehensive two-dimensional gas chromatography–mass spectrometry (GC × GC–MS). Beilstein J Org Chem 15:1945–1961
Van Den Dool H, Kratz PD (1963) A generalization of the retention index system including linear temperature programmed gas–liquid partition chromatography. J Chromatogr A 11:463–471
Picard M, Lytra G, Tempere S, Barbe J-C, De Revel G, Marchand S (2016) Identification of piperitone as an aroma compound contributing to the positive mint nuances perceived in aged Red Bordeaux wines. J Agric Food Chem 64:451–460
May B (2015) Biosynthese und Analytik von Sesquiterpenen in Weinbeeren (Vitis vinifera). Ph.D. thesis, University of Bonn
López R, Ezpeleta E, Sánchez I, Cacho J, Ferreira V (2004) Analysis of the aroma intensities of volatile compounds released from mild acid hydrolysates of odourless precursors extracted from Tempranillo and Grenache grapes using gas chromatography–olfactometry. Food Chem 88:95–103
Pedroza MA, Zalacain A, Lara JF, Rosario Salinas M (2010) Global grape aroma potential and its individual analysis by SBSE–GC–MS. Food Res Int 43:1003–1008
Fagan GL, Kepner RE, Webb AD (1981) Production of linalool, cis- and trans-nerolidol, and trans, trans-farnesol by Saccharomyces fermentati growing as a film on simulated wine. Vitis 20:36–42
Gutsche CD, Maycock JR, Chang CT (1968) Acid-catalyzed cyclization of farnesol and nerolidol. Tetrahedron 24:859–876
Robles-Kelly C, Rubio J, Thomas M, Sedán C, Martinez R, Olea AF, Carrasco H, Taborga L, Silva-Moreno E (2017) Effect of drimenol and synthetic derivatives on growth and germination of Botrytis cinerea: evaluation of possible mechanism of action. Pestic Biochem Physiol 141:50–56
Williamson B, Tudzynski B, Tudzynski P, van Kan JAL (2007) Botrytis cinerea: the cause of grey mould disease. Mol Plant Pathol 8:561–580
Kwon M, Cochrane SA, Vederas JC, Ro D-K (2014) Molecular cloning and characterization of drimenol synthase from valerian plant (Valeriana officinalis). FEBS Lett 588:4597–4603
Fontes N, Gerós H, Delrot S (2011) Grape berry vacuole: a complex and heterogeneous membrane system specialized in the accumulation of solutes. Am J Enol Vitic 62:270–278
Brophy JJ, Forster PI, Goldsack RJ (1998) Essential oils of some Australian monimiaceae. Flavour Fragr J 13:273–276
Ledauphin J, Saint-Clair J-F, Lablanquie O, Guichard H, Founier N, Guichard E, Barillier D (2004) Identification of trace volatile compounds in freshly distilled calvados and cognac using preparative separations coupled with gas chromatography–mass spectrometry. J Agric Food Chem 52:5124–5134
Pannequin A, Tintaru A, Desjobert J-M, Costa J, Muselli A (2017) New advances in the volatile metabolites of Frullania tamarisci. Flavour Fragr J 32:409–418
Pino JA, Almora K, Marbot R (2003) Volatile components of papaya (Carica papaya L., Maradol variety) fruit. Flavour Fragr J 18:492–496
Cho IH, Choi H-K, Kim Y-S (2006) Difference in the volatile composition of pine-mushrooms (Tricholoma matsutake Sing.) according to their grades. J Agric Food Chem 54:4820–4825
Paolini J, Costa J, Bernardini A-F (2005) Analysis of the essential oil from aerial parts of Eupatorium cannabinum subsp. corsicum (L.) by gas chromatography with electron impact and chemical ionization mass spectrometry. J Chromatogr A 1076:170–178
Shimoda M, Wu Y, Osajima Y (1996) Aroma compounds from aqueous solution of haze (Rhus succedanea) honey determined by adsorptive column chromatography. J Agric Food Chem 44:3913–3918
Chung HY, Cadwallader KR (1993) Volatile components in Blue Crab (Callinectes sapidus) meat and processing by-product. J Food Sci 58:1203–1207
Brophy JJ, Goldsack RJ, O’Sullivan W (2004) Chemistry of the Australian gymnosperms Part VII. The leaf oils of the genus Actinostrobus. Biochem Syst Ecol 32:867–873
Chang LP, Sheng LS, Yang MZ, An DK (1989) Retention index of essential oil in temperature-programmed capillary column gas chromatography. Acta Pharm Sin 24:847–852
Castilho P, Liu K, Rodrigues AI, Feio S, Tomi F, Casanova J (2007) Composition and antimicrobial activity of the essential oil of Clinopodium ascendens (Jordan) Sampaio from Madeira. Flavour Fragr J 22:139–144
Verzera A, Trozzi A, Zappalá M, Condurso C, Cotroneo A (2005) Essential oil composition of Citrus meyerii Y. Tan. and Citrus medica L. cv. diamante and their lemon hybrids. J Agric Food Chem 53:4890–4894
Choi H-S, Lee Kim M-S, Sawamura M (2002) Constituents of the essential oil of cnidium officinale Makino, a Korean medicinal plant. Flavour Fragr J 17:49–53
Sing ASC, Smadja J, Brevard H, Maignial L, Chaintreau A, Marion J-P (1992) Volatile constituents of Faham (Jumellea fragrans (Thou.) Schltr.). J Agric Food Chem 40:642–646
Howard KL, Mike JH, Riesen R (2005) Validation of a solid-phase microextraction method for headspace analysis of wine aroma components. Am J Enol Vitic 56:37–45
Verzera A, Campisi S, Zappalà M, Bonaccorsi I (2001) SPME-GC-MS analysis of honey volatile components for the characterization of different floral origin. Am Lab 33:18–21
Chung TY, Eiserich JP, Shibamoto T (1993) Volatile compounds isolated from edible Korean Chamchwi (Aster scaber Thunb). J Agric Food Chem 41:1693–1697
Riu-Aumatell M, López-Tamames E, Buxaderas S (2005) Assessment of the volatile composition of juices of apricot, peach, and pear according to two pectolytic treatments. J Agric Food Chem 53:7837–7843
Njoroge SM, Koaze H, Karanja PN, Sawamura M (2005) Volatile constituents of Redblush grapefruit (Citrus paradisi) and Pummelo (Citrus grandis) peel essential oils from Kenya. J Agric Food Chem 53:9790–9794
Bousmaha L, Boti JB, Bekkara FA, Castola V, Casanova J (2006) Infraspecific chemical variability of the essential oil of Lavandula dentata L. from Algeria. Flavour Fragr J 21:368–372
Babushok VI, Linstrom PJ, Zenkevich IG (2011) Retention indices for frequently reported compounds of plant essential oils. J Phys Chem Ref Data 40:043101–1-043101–47
Boti JB, Koukoua G, N’Guessan TY, Muselli A, Bernardini A-F, Casanova J (2005) Composition of the leaf, stem bark and root bark oils of Isolona cooperi investigated by GC (retention index), GC-MS and 13C-NMR spectroscopy. Phytochem Anal 16:357–363
Pinto E, Pina-Vaz C, Salgueiro L, Gonçalves MJ, Costa-de-Oliveira S, Cavaleiro C, Palmeira A, Rodrigues A, Martinez-de-Oliveira J (2006) Antifungal activity of the essential oil of Thymus pulegioides on Candida, Aspergillus and dermatophyte species. J Med Microbiol 55:1367–1373
Acknowledgements
We thank the DFG for financial support (WU 322/6-1 and INST 217/783-1 FUGG). The grapes of the white wine variety Gewürztraminer were harvested at the Hochschule Geisenheim University. We would like to thank the Department of Grapevine Breeding for providing the sample material.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Research Foundation (DFG, WU 322/6-1 and INST 217/783-1 FUGG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Könen, P.P., Stötzel, I., Schwab, W. et al. Qualitative profiling of mono- and sesquiterpenols in aglycon libraries from Vitis vinifera L. Gewürztraminer using multidimensional gas chromatography–mass spectrometry. Eur Food Res Technol 247, 1117–1124 (2021). https://doi.org/10.1007/s00217-021-03692-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-021-03692-3