Abstract
Application of an aroma extract dilution analysis (AEDA) to the volatiles isolated from raspberry fruits by solvent extraction and solvent-assisted flavour evaporation (SAFE) resulted in 40 odour-active compounds with flavour dilution (FD) factors between 1 and 4096. Among the most potent odorants were violet-like smelling β-ionone (FD factor 4096), fruity smelling methyl 3-methylbutanoate (1024), baked-apple-like smelling β-damascenone (1024), raspberry-like smelling raspberry ketone (128), and floral, raspberry-like smelling α-ionone (64). These five odorants were subsequently monitored during processing of raspberry fruits to freeze-dried fruit foam. Major losses occurred during separation of the pulp from the seeds and during the final freeze-drying step. It was shown that the pulp fraction directly adherent to the seeds contained higher odorant concentrations than the outer parts of the pulp, thus losses associated with the removal of the seeds can be minimised by increasing the efficiency of the separation. Losses associated with the freeze-drying process could be reduced using microwave-assisted freeze drying instead of conventional freeze drying. Higher amounts of potato protein and maltodextrin used as foaming agent and foam stabiliser, respectively, reduced the odorant recoveries in the dried foams. Only a small part of the odorants not recovered in the dried fruit foams was found in the condensate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A wide variety of commonly consumed foods feature foam structures, e.g. bread and other bakery products, breakfast cereals, whipped cream and ice cream. The incorporation of air bubbles into the food structure reduces the density of the product and changes its texture and rheology, resulting in a more pleasant mouthfeel and a more attractive visual appearance. In some cases, foam structure can also aid mastication and enhance flavour delivery [1, 2].
A special kind of foamed products are dried fruit foams. Due to their valuable nutritional properties, nutritionists recommend a higher intake of fruit products and particularly berry fruits show a high potential of beneficial effects on human health [3]. Therefore, dried fruit foams can be a healthy alternative to conventional snack products. Fruit foams have already been produced by foam-mat drying from an assortment of different fruits, including figs [4], blueberry [5], muskmelon [6], cantaloupe [7] and papaya [8]. The foam structure allows for an economic and fast drying process at relatively low temperatures resulting in high-quality products, which retain most of the fruits’ nutritional quality and are easy to rehydrate [9]. The accelerated drying of foamed foods can be explained by the rapid heat and mass transfer due to the larger inner surface area and the decreased resistance caused by the capillary effect of the lamellae in the foam [10, 11]. The drying process can be further accelerated by microwave heating, which overcomes some heat transfer limitations of convective and conductive heating approaches when applied in oxygen-free and low temperature conditions, as is the case with microwave-assisted freeze drying [11].
Ozcelik et al. applied microwave-assisted freeze drying to foams produced from raspberry (Rubus idaeus) fruit pulp and compared drying behaviour and product characteristics to the conventional freeze-drying process [12,13,14]. By incorporation of microwave energy to the freeze-drying process, the drying time could be drastically decreased. This led to improved efficiency and decreased energy consumption during the process [12]. The impact of microwave-assisted freeze drying on important quality parameters of the dried fruit foam like the retention of micronutrients, ascorbic acid and anthocyanins as well as texture and colour was shown to be comparable to conventional freeze drying [14].
An important aspect that has not been investigated is the effect of microwave-assisted freeze drying on the aroma of freeze-dried fruit foams. As the food’s sensory characteristics are the most important criteria consumers base their food selection on [15], elucidating the impact of microwave-assisted freeze drying on sensory properties is essential to fully evaluate this emerging technique.
Of all sensory perceptions, olfaction has the most substantial influence on our overall sensory impression during food consumption [16]. Olfactory sensations are caused by volatile compounds binding to and activating olfactory receptor proteins located in the olfactory epithelium. To be odour-active, volatiles must be present in the food in concentrations above their specific odour threshold. As a consequence, only a minority of the volatiles present in a specific food is odour-active [17]. To distinguish odour-active compounds from the bulk of odourless volatiles in a food, gas chromatography–olfactometry (GC–O) and aroma extract dilution analysis (AEDA) are valuable tools [16].
First investigations on odour-active compounds in raspberries were conducted in 1957 [18]. 4-(4-Hydroxyphenyl)butan-2-one was identified as character impact compound in raspberries and named raspberry ketone owing to its characteristic raspberry-like odour [19]. Since then, over 200 volatile compounds have been identified in raspberries. However, only few studies evaluated the influence of individual compounds on the overall aroma of raspberries [20]. Larsen and Poll [21] related the concentrations of selected odour-active compounds in ten different raspberry varieties to their respective odour thresholds. They found raspberry ketone, α-ionone and β-ionone to be the most important compounds in raspberry aroma. Geraniol and linalool were shown to be of additional relevance in certain varieties.
An activity-guided screening for odour-active compounds in raspberries was performed in two other studies. Roberts and Acree [22] applied a combined hedonic aroma response measurement (CHARM) analysis to raspberry headspace samples. They reported high CHARM values for β-damascenone, butane-2,3-dione, sotolon, 1-hexen-3-one, 1-nonen-3-one, 1-octen-3-one and (3Z)-hex-3-enal. Klesk et al. [23] applied an AEDA to a raspberry volatiles isolate obtained by solvent extraction and solvent-assisted flavour evaporation. High FD factors were reported for β-ionone, abhexone, 4-hydroxy-2,5-dimethylfuran-3(2H)-one, 2-ethyl-4-hydroxy-5-methylfuran-3(2H)-one, and β-damascenone, followed by (3Z)-hex-3-enal, acetic acid, ethyl 2-methylbutanoate and methyl hexanoate.
Aaby et al. [24] investigated eight genotypes of red raspberry in parallel by descriptive sensory analysis as well as by dynamic headspace GC–MS analysis. Statistical data evaluation revealed that C6-aldehydes and (3Z)-hex-3-en-1-ol positively correlated with green odour and α- and β-ionone positively correlated with flowery notes. However, no attempts were made to establish causalities.
Given the inconsistency of the data in the literature, the first aim of this study was to re-investigate the odour-active compounds in raspberries by application of an AEDA [25] to the volatiles isolated from the fruits by a mild and artefact-avoiding approach including solvent extraction and solvent-assisted flavour evaporation (SAFE) [26]. On the basis of the results, major odorants could be selected and their concentration changes during processing of the raspberries to dried fruit foam were studied in detail. In particular, the following questions were addressed: (1) does microwave-assisted freeze drying with its significantly shorter drying times better preserve the natural raspberry odorants? (2) do the concentrations of foaming agent and foam stabiliser influence the recovery of raspberry odorants in the dried fruit foam? and (3) can the condensate obtained as a side product after both, conventional and microwave-assisted freeze drying, be utilized as a source of odorants for the preparation of natural flavourings?
Materials and methods
Fruits and further foam ingredients
Frozen raspberry fruits of the variety “Willamette” were supplied by Mainfrucht GmbH & Co. KG (Gochsheim, Germany). The frozen material was stored at − 80 °C. Potato protein was purchased from Solanic300 (Veendam, the Netherlands), Maltodextrin DE 6 was from Nutricia (Erlangen, Germany). Highly (68%–76%) esterified citrus pectin was provided by Herbstreith & Fox (Neuenbürg/Württ, Germany).
Reference odorants
Reference odorants 1–4, 6–11, 13, 14, 17–23, 25–27, 29–31, 33, 36, 37, 39, and 40 were purchased from Merck (Darmstadt, Germany). Compounds 24, 35 and 38 were obtained from Carl Roth (Karlsruhe, Germany). Compounds 5 and 28 were purchased from Alfa Aesar (Karlsruhe, Germany). Reference compound 32 was a gift from Symrise (Holzminden, Germany). The following reference odorants were synthesised according to literature procedures: 12 [27], 16 [28].
Isotopically substituted odorants
(13C2)-40 [4-(4-hydroxyphenyl)(1,3-13C2)butan-2-one] was prepared by crossed aldol condensation of (1,3-13C2)acetone with 4-hydroxybenzaldehyde, followed by catalytic hydration using rhodium as catalyst as described previously for the isotopically unmodified compound [29]. The following compounds were prepared as described previously: (2H3)-38 [(3E)-4-[6,6-dimethyl-2-(2H3)methylcyclohex-1-en-1-yl]but-3-en-2-one] [30] and (2H6)-32 [(2E)-1-[6,6-(2H6)dimethyl-2-methylcyclohexa-1,3-dien-1-yl]but-2-en-1-one] [31]. (2H3)-5 [(2H3)methyl 3-methylbutanoate] was prepared from (2H4)methanol and 3-methylbutanoic acid using the approach previously described for the synthesis of (2H3)ethyl butanoate [32].
Miscellaneous chemicals
Dichloromethane was purchased from VWR (Darmstadt, Germany) and freshly distilled through a column (120 cm × 5 cm) packed with Raschig rings before use. (2H4)methanol, 3-methylbutanoic acid, 4-hydroxybenzaldehyde, (1,3-13C2)acetone and rhodium on alumina (0.5%) were purchased from Merck.
GC–O/FID system
A gas chromatograph 5160 (Carlo Erba, Hofheim, Germany) was equipped with a cold on-column injector, a flame ionisation detector (FID) and a sniffing port [28]. The fused silica column used was either a DB-FFAP, 30 m × 0.32 mm i.d., 0.25 µm film (Agilent, Waldbronn, Germany) or a ZB-5, 30 m × 0.25 mm i.d., 0.25 µm film (Phenomenex, Aschaffenburg, Germany). Helium was used as carrier gas at 70 kPa (DB-FFAP) and 95 kPa (ZB-5) constant pressure. The injection volume was 1 µl. The initial temperature of the oven was 40 °C (2 min). The temperature was raised with a gradient of 6 °C/min up to 230 °C (DB-FFAP) and 240 °C (ZB-5). After the main column, the effluent was divided by a deactivated Y-shaped glass splitter and transferred via deactivated fused silica capillaries (50 cm × 0.25 mm i.d.) to the FID (250 °C) and the sniffing port (230 °C). During GC–O analyses, a trained panellist placed the nose closely above the sniffing port to evaluate the effluent gas stream. Whenever an odour could be detected, the retention time was marked on the FID chromatogram printed by a recorder and the corresponding odour quality was noted. Retention indices (RI) were calculated for the odour-active compounds from their retention times and the retention times of adjacent n-alkanes by linear interpolation.
GC–sector field MS system
A HP 5890 Series II gas chromatograph (Hewlett-Packard, Heilbronn, Germany) was connected to a MAT 95 sector field mass spectrometer (Finnigan, Bremen, Germany). The fused silica column used was either a DB-FFAP, 30 m × 0.25 mm i.d., 0.25 µm film or a DB-5, 30 m × 0.25 mm i.d., 0.25 µm film (both Agilent). Helium was used as carrier gas at 1.9 mL/min constant flow. Further GC conditions were as described in the GC–O/FID section. Mass spectra were recorded either in EI mode (70 eV) with a scan range of m/z 35–300 or in CI mode (150 eV) with isobutane as reagent gas and a scan range of m/z 85–350. The evaluation was performed using Xcalibur software (Thermo Scientific, Dreieich, Germany).
GC–ion trap MS system
A gas chromatograph GC 431 (Varian, Darmstadt, Germany) was linked to an ion trap 220-MS mass spectrometer (Varian). The fused silica column used was either a DB-FFAP, 30 m × 0.25 mm i.d., 0.25 µm film or a DB-5, 30 m × 0.25 mm i.d., 0.25 µm film (both Agilent). Helium was used as carrier gas at 1 mL/min constant flow. Further GC conditions were as described in the GC–O/FID section. The mass spectrometer was operated in CI mode with methanol as reagent gas and a scan range of m/z 60–250.
Raspberry pulp and foam preparation
Raspberry pulp and foams were prepared as detailed previously [12,13,14, 33]. In brief, frozen raspberries were thawed at room temperature and seedless raspberry pulp puree was produced using a food mill (GEFU GmbH, Eslohe, Germany) with perforated disks and mesh sizes of 3, 2 and 1 mm. To the pulp, potato protein consisting of the low molecular weight fraction of the potato protease inhibitor was added as foaming agent as well as maltodextrin DE 6 and highly esterified citrus pectin as foam stabilisers. The pulp mixture was kept at 20 °C for 30 min and then whipped using an Artisan 5KSM150 mixer (KitchenAid, St. Joseph, MI, USA) at a maximum speed of 220 rpm for 10 min at 20 °C. For both, freeze drying and microwave-assisted freeze drying, portions of the pulp mixture (100 g) were placed into glass dishes (190 mm diameter) and the samples were frozen at − 80 °C for 24 h before drying.
Freeze drying
The freeze-drying process was carried out in a pilot-scale freeze dryer Model Gamma 1–20 (Christ, Osterode, Germany). The samples were heated via conductive heating on shelves at 30 °C. Chamber pressure was set to 10 Pa and the ice condenser temperature was set to − 50 °C. After completion of the drying process, the dryer was powered off and a 12-h dwelling period was commenced for thawing the accumulated ice and enabling maximum recovery of the evaporated water. The condensate was collected through a vane discharge pipe in ~ 96–98% yield.
Microwave-assisted freeze drying
A pilot-scale microwave freeze dryer Model µVac0150fd (Püschner Microwaves, Schwanewede, Germany) was used [12]. Microwave energy was produced by a 1000 W magnetron at a frequency of 2.45 GHz. The μWaveCAT dryer software (Püschner Microwaves) was used for operating the drying process automatically. The ice condenser unit contained two separate chillers (Dietz, Bremen, Germany). Vacuum was applied by a rotary vane pump (Pfeiffer, Asslar, Germany) and the condenser was run at − 50 °C. The chamber pressure and the maximum product temperature were set to 10 Pa and 30 °C, respectively, to match the parameters used in the freeze-drying approach. Collection of the condensate was performed as described for the conventional freeze-drying process.
Isolation and fractionation of raspberry volatiles
Raspberry fruits (10 g) and dichloromethane (40 mL) were homogenised without crushing of the seeds using a stainless steel blender. Under ice cooling, sodium sulphate (30 g) was added gradually under continuous blending until a homogeneous suspension was obtained. This suspension was stirred for 90 min and was subsequently filtered through defatted cotton wool and sea sand. The residue was rinsed with dichloromethane (2 × 20 mL), and the volatiles were isolated from the combined organic extracts by SAFE during 30 min at 40 °C. The distillate was concentrated to a final volume of 1 mL, first using a Vigreux column (50 × 1 cm) and subsequently a Bemelmans microdistillation device [34].
For fractionation, volatiles from 10 g raspberry fruit were isolated using the approach detailed above and the SAFE distillate was extracted with aqueous sodium hydrogen carbonate solution (0.5 mol/L; 1 × 100 mL, 2 × 50 mL). The combined aqueous extracts were washed with dichloromethane (50 mL), acidified (pH 2) with hydrochloric acid (16%) and re-extracted with dichloromethane (1 × 100 mL, 2 × 50 mL). The organic re-extracts were combined, dried over anhydrous sodium sulphate and concentrated (1 mL) to obtain the acidic volatiles fraction. The acid-free SAFE distillate representing the neutral and basic volatiles fraction was dried over anhydrous sodium sulphate and concentrated (1 mL).
Aroma extract dilution analysis (AEDA)
The concentrated raspberry volatiles isolate (1 mL) was subjected to stepwise 1:2 dilutions with dichloromethane to receive dilutions of 1:2, 1:4, 1:8, 1:16 etc. Each diluted sample was analysed by GC–O using the ZB-5 column. The dilution of the extract was continued until no odour-active compound was detected during GC–O analysis. Each odorant was assigned a FD factor defined as the dilution factor of the highest diluted sample in which the odorant was perceived during GC–O analysis [18].
Quantitation of raspberry odorants
Whole raspberry fruits were homogenised without crushing of the seeds using a stainless steel blender. Dried foam was homogenised to a powder using mortar and pestle. Fruit pulp, pulp mixture, fresh foam, frozen foam, seed fraction and washed seeds were used without further homogenisation. Isotopically substituted analogues of the target compounds (0.05 µg–1 mg) in dichloromethane (30–250 mL) were added as internal standards to the sample material (1–50 g). The mixture was blended with sodium sulphate (15–150 g) until a homogeneous suspension was obtained. This suspension was stirred for 90 min, filtered and the filtrate was subjected to SAFE. To condensate samples (15–30 mL), isotopically substituted analogues of the target compounds (0.1 µg) in dichloromethane (15–50 mL) were added as internal standards and the mixture was shaken in a separatory funnel. The aqueous phase was shaken with two more portions of dichloromethane (15–50 mL). The organic extracts were combined, dried over anhydrous sodium sulphate and subjected to SAFE. For quantitation of raspberry ketone, the SAFE distillate was shaken with aqueous sodium hydrogen carbonate solution (0.5 mol/L; 2 × 30 mL). The combined aqueous extracts were washed with dichloromethane (30 mL), acidified (pH 2) with hydrochloric acid (16%) and the target compound was re-extracted with dichloromethane (2 × 30 mL). The organic re-extracts were combined, dried over anhydrous sodium sulphate and concentrated (1 mL) to obtain the acidic volatiles fraction. For the quantitation of all other compounds, the SAFE distillate was concentrated (0.2–1 mL) without further separation.
Aliquots of the concentrated isolates were analysed by GC–MS using either the DB-5 column (raspberry ketone) or the DB-FFAP column (β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate). Peak areas corresponding to analyte and internal standard were obtained from the extracted ions chromatograms using the quantifier ions detailed in Online Resource 1, Table S1. The concentration of each target compound in the samples was then calculated from the area counts of the analyte peak, the area counts of the standard peak, the amount of sample material and the amount of standard added by employing a calibration line equation previously obtained from the analysis of analyte/standard mixtures in known concentrations. Calibration line equations are detailed in Online Resource 1, Table S1.
Results and discussion
Screening raspberry volatiles for odour-active compounds
Performing AEDA of the raspberry volatiles isolate obtained by solvent extraction and SAFE resulted in the detection of 40 odour-active compounds with FD factors between 1 and 4096. A comparison of the retention indices and odour qualities of the compounds with data from different databases [35, 36] was used as a first step towards structure elucidation. If a match with the database occurred, authentic reference compounds were analysed by GC–O in parallel to the raspberry volatiles isolate for confirmation. This was done on two separation systems of different polarity (DB-5, FFAP). Final structure verification was achieved by GC–MS analysis. To avoid coelution, the raspberry volatiles isolate was fractionated by acid–base extraction into an acidic volatiles fraction and a neutral–basic volatiles fraction. The raspberry odorants were localised in the fractions by GC–O and the fractions were then analysed by GC–MS in EI and CI mode on both separation systems and in parallel to the reference compounds. This approach allowed for the structural identification of 38 of the 40 odorants (Table 1).
High FD factors were obtained for floral, violet-like smelling β-ionone (38; FD factor 4096), fruity smelling methyl 3-methylbutanoate (5; 1024), baked apple-like smelling β-damascenone (32; 1024), clove-like smelling eugenol (31; 128), peach-like smelling δ-decalactone (39; 128), raspberry-like smelling raspberry ketone (40; 128), and floral, raspberry-like smelling α-ionone (35; 64). 3-(Methylsulfanyl)propanal, geraniol, and octanoic acid showed FD factors of 32 and ethyl 3-methylbutanoate, 4-hydroxy-2,5-dimethylfuran-3(2H)-one (HDMF), linalool, 2,3-diethyl-5-methylpyrazine and 3-phenylpropanoic acid showed FD factors of 16. For all other 25 compounds lower FD factors (1–8) were obtained.
In agreement with our results, high FD factors for β-damascenone (FD factor 512), α-ionone (2048) and β-ionone (2048) had also been reported in the study of Klesk et al. [23]. In addition, β-damascenone had shown the highest CHARM value in the research of Roberts and Acree [21]. By contrast, to raspberry ketone, although considered the character impact compound of raspberries [18, 19, 21], rather low FD factors/CHARM values had been attributed in previous activity-guided screenings [22, 23]. δ-Decalactone had also been detected as odour-active compound by Klesk et al. [23], but its FD factor was rather low. Despite its high FD factor of 1024 in the current study, methyl 3-methylbutanoate (5) has not been reported as raspberry volatile so far. Of all 38 compounds identified as raspberry odorants in our study, 23 have previously been reported as raspberry volatiles [21,22,23,24, 37,38,39,40,41,42,43,44,45], whereas 15 have yet been unknown in raspberries. Besides methyl 3-methylbutanoate, the latter included ethyl 3-methylbutanoate (8; FD 16), 2,3-diethyl-5-methylpyrazine (22; FD 16), 3-phenylpropanoic acid (30; FD 16), pentane-2,3-dione (3; FD 8) and further ten volatiles with rather low FD factors in the range of 1–4.
On the basis of the results obtained from the AEDA and the data previously published on odour-active volatiles in raspberries, the following five compounds were selected to study concentration changes during processing of the fruits to dried foam: raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate.
Odorant recoveries during processing of fruit to dried foam
Raspberry fruits were separated into a seed fraction and a seedless pulp. To the latter, 5% potato protein was added as foaming agent and 15% maltodextrin as well as 2.5% pectin were added as foam stabilisers. This recipe was chosen, because it had shown favourable technical characteristics in previous studies [12, 14]. The mixture was foamed, the foam was frozen and finally dried by microwave-assisted freeze drying. Raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate were quantitated in the whole raspberry fruits used as starting material, the intermediate products fruit pulp, pulp mixture, fresh foam, frozen foam, and the final product, the dried foam.
Figure 1 shows a clear decrease in all raspberry odorants during processing. A major decrease could be observed in the first processing step, the removal of the seeds. This decrease clearly exceeded the percentage of the seed fraction which was only 20% of the starting material by weight. The loss of odorants, however, ranged between 40% for β-damascenone and 77% for methyl 3-methylbutanoate. Potential reasons for these disproportionately high losses include (1) evaporation of odorants into the ambient air, (2) presence of a major fraction of the odorants in the seeds and not in the pulp, and (3) presence of a major fraction of the odorants in the part of the pulp directly adherent to the seeds. Hypothesis 1 was supported by the fact that the highest loss was found for methyl 3-methylbutanoate, the odorant with the lowest boiling point (~ 116 °C), but high losses were also observed for the other four odorants, all of which show rather high boiling points (β-damascenone: ~ 276 °C, β-ionone: ~ 269 °C, α-ionone: ~ 238 °C, raspberry ketone: ~ 292 °C). This suggested that the boiling point was at least not the only parameter contributing to the losses. Hypothesis 2 could be excluded, because the seeds do not exhibit the typical raspberry aroma and also because the seeds were not crushed during homogenisation of the fruits and the short contact time during solvent extraction did not suggest an efficient extraction of volatiles from the intact seeds. Thus, an unequal distribution between the outer parts of the pulp and the pulp fraction directly adherent to the seeds as depicted in hypothesis 3 might have been the crucial point.
Another processing step that consistently caused major losses in all five odorants was the drying of the frozen foam. During microwave-assisted freeze drying, the odorant concentrations decreased by 50–75%. Losses in this step were to be expected since freeze drying can be considered as a vacuum steam distillation process. This raised the question whether the odorants lost during microwave-assisted freeze drying could be recovered in the condensate and this fraction could be further used, e.g. as odorant source for the preparation of natural flavourings.
In summary, the losses during processing of raspberry fruits to dried fruit foam resulted in recoveries in the final product that ranged only between 10% (methyl 3-methylbutanoate) and 23% (β-damascenone).
Odorant losses during preparation of seedless pulp
To substantiate our hypothesis on odorant losses with the seed fraction, a separate experiment was conducted in which raspberry fruits were processed into a seed fraction and a seedless pulp. From a part of the seed fraction, the seeds were isolated by washing with water. Raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate were quantitated in the whole raspberry fruits used as starting material, in the pulp obtained in 80% yield, in the 20% fraction containing the seeds and the adherent pulp as well as in the seeds (8%) from which the adherent pulp (12%) was washed off.
Figure 2 shows total recoveries of the five odorants ranging between 73% for methyl 3-methylbutanoate and 105% for α-ionone. In the washed seeds, the recovery of all five odorants was < 1% and was thus not displayed in Fig. 2. Data confirmed a higher concentration of all five odorants in the seed fraction than in the seedless pulp. Despite the fact that the seed fraction amounted only to 20% of the whole fruits, it contained a percentage of 26–58% of the odorants, whereas the percentage of the odorants recovered in the 80% of the seedless pulp ranged only between 33% for methyl 3-methylbutanoate and 54% for β-ionone. Results thus confirmed that the important raspberry odorants raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate are enriched in the fruit pulp close to the seeds and depleted in the outer parts of the drupelets, explaining the high losses depicted in Fig. 1 for the first processing step. Results suggested that during industrial processing the efficient separation of the pulp from the seeds is crucial to obtain a highly flavoured pulp product.
Odorant recoveries in dried foam and condensate
To clarify whether the losses of odorants observed during the drying process (cf. Figure 1, last processing step) could be explained by a transfer to the condensate, a pulp mixture prepared as described above was processed to dried fruit foam. Raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate were quantitated in the pulp mixture used as starting material, in the dried fruit foam and in the condensate obtained during the drying process. To further evaluate the microwave-assisted freeze-drying approach, conventional freeze drying was applied to all samples in parallel as a reference method.
Figure 3 shows total recoveries of the five odorants after microwave-assisted freeze drying between 20% for raspberry ketone and 81% for α-ionone. Thus, a major fraction of the odorants is neither recovered in the dried foam nor in the condensate and is probably lost with the exhaust of the vacuum pump. After conventional freeze drying, most recoveries were lower than after microwave-assisted freeze drying and ranged between 5% for raspberry ketone and 48% for α-ionone. Recoveries of the five odorants in the dried fruit foam after microwave-assisted freeze drying in reference to the pulp mixture were in the same range as reported in Fig. 1, however with some deviations. In detail, recoveries were raspberry ketone 19% (Fig. 1: 13%), β-ionone 40% (29%), α-ionone 66% (38%), β-damascenone 22% (37%) and methyl 3-methylbutanoate 19% (35%). Recoveries of the five odorants in the dried fruit foam after conventional freeze drying were lower than after microwave-assisted freeze drying for raspberry ketone, β-ionone, α-ionone and β-damascenone, only for methyl 3-methylbutanoate results were reverse. Better recoveries after microwave-assisted freeze drying might be associated with the significantly higher drying rates and thus shorter drying times [12]. Independent of the applied drying method, only minor parts of the odorants were recovered in the condensate fractions. This percentage ranged from 1% (raspberry ketone) to 19% (β-damascenone after conventional freeze drying). To sum it up, (1) microwave-assisted freeze drying showed better recoveries in the dried fruit foam for the majority of odorants investigated than conventional freeze drying and (2) the recoveries in the condensate were too low to consider it as a source of odorants for the preparation of natural flavourings.
Influence of maltodextrin and potato protein content on odorant recoveries after freeze drying
It has been shown in previous studies that the concentrations of foaming agents and foam stabilisers have a vital impact on foam stability, bubble size distribution and overrun of fresh foams [33], on the drying time and drying rate during fruit foam drying [12], on the ascorbic acid and anthocyanin recovery in dried foams as well as on the structure of dried foams [14]. To study whether foaming agent and foam stabiliser concentration do also influence the recoveries of odorants after freeze drying, the recipe previously used in our study was varied using different amounts of maltodextrin and potato protein in the pulp mixture. As previous studies had shown that the combination of certain recipes with microwave-assisted freeze drying can result in the formation of hot spots [12], only conventional freeze drying was applied.
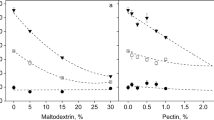
In a first set of experiments, maltodextrin concentrations of 5 and 30% were tested, while the potato protein concentration was kept at 5%. Raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate were quantitated in the pulp mixtures used as starting material, in the dried fruit foams obtained by conventional freeze drying and in the condensates obtained during the drying process. Results are depicted in Fig. 4. Data revealed a clear tendency towards lower recoveries with increasing maltodextrin concentration for the ionones and β-damascenone, whereas no clear trend could be observed in the case of raspberry ketone and methyl 3-methylbutanoate. Results further confirmed low recoveries in the condensates, particularly for raspberry ketone.
In the second set of experiments, potato protein concentrations of 2.5, 7.5 and 10% were additionally tested while the maltodextrin concentration was kept at 15%. Raspberry ketone, β-ionone, α-ionone, β-damascenone and methyl 3-methylbutanoate were quantitated in the pulp mixtures, in the dried fruit foams obtained by conventional freeze drying and in the condensates obtained during the drying process. Results are depicted in Fig. 5. Data revealed a tendency towards lower recoveries in the dried foam with increasing potato protein concentration for raspberry ketone, β-ionone, α-ionone and β-damascenone. However, the values obtained for β-ionone, α-ionone, β-damascenone and the sample with 10% potato protein did not fit into this scheme nor did the results obtained for methyl 3-methylbutanoate. Higher odorant recoveries in foams with lower potato protein concentration might be associated with the shorter drying times observed for these recipes [12]. An increased overrun in the fresh foams might also promote the odorant losses due to an increased inner surface area. Similar to the results of the first set of experiments with varied maltodextrin concentration, recoveries in the condensates were low and particularly raspberry ketone was virtually absent.
Conclusions
Processing of raspberries to freeze-dried fruit foam leads to major losses of important odorants. Critical processing steps are the separation of the seeds from the pulp and the drying process. As the pulp fraction directly adherent to the seeds contains higher odorant concentrations, efficient separation of pulp and seeds is crucial for good odorant recoveries. During foam drying, microwave-assisted freeze drying preserves the natural raspberry odorants better than conventional freeze drying. Higher amounts of maltodextrin and potato protein in the pulp mixture lead to lower odorant recoveries in the freeze-dried foams, thus their concentration should be limited to the technologically required minimum. The amounts of the odorants recovered in the condensate obtained as a side product after both microwave-assisted and conventional freeze drying processes were too low to consider the distillates a suitable source of natural flavourings.
Change history
18 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00217-021-03789-9
Abbreviations
- AEDA:
-
Aroma extract dilution analysis
- CHARM:
-
Combined hedonic aroma response measurement
- FD:
-
Flavour dilution
- FFAP:
-
Free fatty acid phase
- FID:
-
Flame ionisation detector
- GC–MS:
-
Gas chromatography–mass spectrometry
- GC–O:
-
Gas chromatography–olfactometry
- HDMF:
-
4-Hydroxy-2,5-dimethylfuran-3(2H)-one
- RI:
-
Retention index
- SAFE:
-
Solvent-assisted flavour evaporation
References
Campbell GM, Mougeot E (1999) Creation and characterisation of aerated food products. Trends Food Sci Technol 10:283–296
Niranjan K, Silva SFJ (2008) Bubbles in foods: creating structure out of thin air! In: Gutierrez-Lopez GF, Barbosa-Canovas GV, Welti-Chanes J, Parada-Arias E (eds) Food Engineering: Integrated Approaches. Springer, New York, pp 183–192
Cianciosi D, Simal-Gándara J, Forbes-Hernández TY (2019) The importance of berries in the human diet. Mediterranean J Nutr Metab 12:335–340
Varhan E, Elmas F, Koç M (2019) Foam mat drying of fig fruit: optimization of foam composition and physicochemical properties of fig powder. J Food Process Eng 42:e13022
Darniadi S, Ho P, Murray BS (2018) Comparison of blueberry powder produced via foam-mat freeze-drying versus spray-drying: evaluation of foam and powder properties. J Sci Food Agric 98:2002–2010
Asokapandian S, Venkatachalam S, Swamy GJ, Kuppusamy K (2016) Optimization of foaming properties and foam mat drying of muskmelon using soy protein. J Food Process Eng 39:692–701
Salahi MR, Mohebbi M, Taghizadeh M (2015) Foam-mat drying of cantaloupe (Cucumis melo): optimization of foaming parameters and investigating drying characteristics. J Food Process Preserv 39:1798–1808
Kandasamy P, Varadharaju N, Kalemullah S, Maladhi D (2014) Optimization of process parameters for foam-mat drying of papaya pulp. J Food Sci Technol 51:2526–2534
A S, Venkatachalam S, John SG, Kuppuswamy K, (2015) Foam mat drying of food materials: a review. J Food Process Preserv 39:3165–3174
Brygidyr AM, Rzepecka MA, McConnell MB (1977) Characterization and drying of tomato paste foam by hot air and microwave energy. Can Inst Food Sci Technol J 10:313–319
Ozcelik M, Püschner PA (2017) Microwave plant requirements and process control for advanced applications. In: Regier M, Knoerzer K, Schubert H (eds). The Microwave Processing of Foods, 2nd edn. Woodhead Publishing, Cambridge
Ozcelik M, Ambros S, Heigl A, Dachmann E, Kulozik U (2019) Impact of hydrocolloid addition and microwave processing condition on drying behavior of foamed raspberry puree. J Food Eng 240:83–91
Ozcelik M, Ambros S, Morais SIF, Kulozik U (2020) Storage stability of dried raspberry foam as a snack product: effect of foam structure and microwave-assisted freeze drying on the stability of plant bioactives and ascorbic acid. J Food Eng 270:109779
Ozcelik M, Heigl A, Kulozik U, Ambros S (2019) Effect of hydrocolloid addition and microwave-assisted freeze drying on the characteristics of foamed raspberry puree. Innovative Food Sci Emerging Technol 56:102183
International Food Information Council Foundation (2019) Food and Health Survey. https://www.foodinsight.org. Accessed 19 Apr 2020
Steinhaus M (2020) Gas chromatography–olfactometry: principles, practical aspects and applications in food analysis. In: Tranchida PQ (ed) Advanced Gas Chromatography in Food Analysis. The Royal Society of Chemistry, Cambridge, pp 337–399
Dunkel A, Steinhaus M, Kotthoff M, Nowak B, Krautwurst D, Schieberle P, Hofmann T (2014) Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew Chem Int Ed 53:7124–7143
Schinz H, Seidel CF (1957) Untersuchungen über Aromastoffe. 1. Mitteilung. Über das Himbeeraroma Helv Chim Acta 40:1839–1859
Firmenich C (1961) Nachtrag zu der Arbeit Nr. 194 von H. Schinz und C. F. Seidel in Helv. 40, 1839 (1957). Helv Chim Acta 44:278–278
Aprea E, Biasioli F, Gasperi F (2015) Volatile compounds of raspberry fruit: from analytical methods to biological role and sensory impact. Molecules 20:2445–2474
Larsen M, Poll L, Callesen O, Lewis M (1991) Relations between the content of aroma compounds and the sensory evaluation of 10 raspberry varieties (Rubus idaeus L). Acta Agric Scand 41:447–454
Roberts DD, Acree TE (1996) Effects of heating and cream addition on fresh raspberry aroma using a retronasal aroma simulator and gas chromatography olfactometry. J Agric Food Chem 44:3919–3925
Klesk K, Qian M, Martin RR (2004) Aroma extract dilution analysis of cv. Meeker (Rubus idaeus L.) red raspberries from Oregon and Washington. J Agric Food Chem 52:5155–5161
Aaby K, Skaret J, Røen D, Sønsteby A (2019) Sensory and instrumental analysis of eight genotypes of red raspberry (Rubus idaeus L.) fruits. J Berry Res 9:483–498
Schieberle P, Grosch W (1987) Evaluation of the flavour of wheat and rye bread crusts by aroma extract dilution analysis. Z Lebensm Unters Forsch 185:111–113
Engel W, Bahr W, Schieberle P (1999) Solvent assisted flavour evaporation – a new and versatile technique for the careful and direct isolation of aroma compounds from complex food matrices. Eur Food Res Technol 209:237–241
Buttery RG, Ling LC, Juliano BO, Turnbaugh JG (1983) Cooked rice aroma and 2-acetyl-1-pyrroline. J Agric Food Chem 31:823–826
Steinhaus M, Sinuco D, Polster J, Osorio C, Schieberle P (2008) Characterization of the aroma-active compounds in pink guava (Psidium guajava, L.) by application of the aroma extract dilution analysis. J Agric Food Chem 56:4120–4127
Smith LR (1996) Rheosmin (“raspberry ketone”) and zingerone, and their preparation by crossed aldol-catalytic hydrogenation sequences. Chem Educ 1:1–18
Greger V, Schieberle P (2007) Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J Agric Food Chem 55:5221–5228
Sen A, Laskawy G, Schieberle P, Grosch W (1991) Quantitative determination of beta-damascenone in foods using a stable isotope dilution assay. J Agric Food Chem 39:757–759
Steinhaus M, Sinuco D, Polster J, Osorio C, Schieberle P (2009) Characterization of the key aroma compounds in pink guava (Psidium guajava L.) by means of aroma re-engineering experiments and omission tests. J Agric Food Chem 57:2882–2888
Dachmann E, Hengst C, Ozcelik M, Kulozik U, Dombrowski J (2018) Impact of hydrocolloids and homogenization treatment on the foaming properties of raspberry fruit puree. Food Bioprocess Technol 11:2253–2264
Bemelmans JMH (1979) Review of isolation and concentration techniques. In: Land GG, Nursten HE (eds) Progress in Flavour Research. Applied Science Publishers, London, pp 79–98
Van Dongen WD, Donders JJH (2020) VCF Volatile Compounds in Food: database, Version 16.7. BeWiDo B.V., Reeuwijk. https://www.vcf-online.nl/. Accessed 19 Apr 2020
Kreissl J, Mall V, Steinhaus P, Steinhaus M (2019) Leibniz-LSB@TUM Odorant Database, Version 1.0. Leibniz-Institute for Food Systems Biology at the Technical University of Munich (Leibniz-LSB@TUM), Freising. https://www.leibniz-lsb.de/en/databases/leibniz-lsbtum-odorant-database. Accessed 19 Apr 2020
Aprea E, Biasioli F, Carlin S, Endrizzi I, Gasperi F (2009) Investigation of volatile compounds in two raspberry cultivars by two headspace techniques: solid-phase microextraction/gas chromatography−mass spectrometry (SPME/GC−MS) and proton-transfer reaction−mass spectrometry (PTR−MS). J Agric Food Chem 57:4011–4018
Giuggioli NR, Briano R, Baudino C, Peano C (2015) Effects of packaging and storage conditions on quality and volatile compounds of raspberry fruits. CyTA J Food 13:512–521
Honkanen E, Pyysalo T, Hirvi T (1980) The aroma of Finnish wild raspberries, Rubus idaeus, L. Z Lebensm Unters Forsch 171:180–182
Malowicki SMM, Martin R, Qian MC (2008) Volatile composition in raspberry cultivars grown in the pacific northwest determined by stir bar sorptive extraction−gas chromatography−mass spectrometry. J Agric Food Chem 56:4128–4133
Pabst A, Barron D, Etievant P, Schreier P (1991) Studies on the enzymic hydrolysis of bound aroma constituents from raspberry fruit pulp. J Agric Food Chem 39:173–175
Pyysalo T (1976) Identification of volatile compounds in hybrids between raspberry (Rubus idaeus, L.) and Arctic Bramble (Rubus arcticus, L.). Z Lebensm Unters Forsch 162:263–272
Robertson GW, Griffiths DW, Woodford JAT, Birch ANE (1995) Changes in the chemical composition of volatiles released by the flowers and fruits of the red raspberry (Rubus idaeus) cultivar Glen Prosen. Phytochemistry 38:1175–1179
Vrhovsek U, Lotti C, Masuero D, Carlin S, Weingart G, Mattivi F (2014) Quantitative metabolic profiling of grape, apple and raspberry volatile compounds (VOCs) using a GC/MS/MS method. J Chromatogr B 966:132–139
Winter M, Enggist P (1971) Recherches sur les arǒmes. 17e communication. Sur l'arǒme de framboise. IV Helv Chim Acta 54:1891–1898
Acknowledgments
This research was part of Project No. 19015 N of the FEI supported via AiF within the programme for promoting the Industrial Collective Research (IGF) of the German Ministry of Economic Affairs and Energy (BMWi), based on a resolution of the German Parliament. The authors thank Monika Riedmaier for excellent technical assistance, Püschner Microwaves (Schwanewede, Germany) for their technical support, Mainfrucht (Gochsheim, Germany) for supplying us with raspberry fruits and Herbstreith & Fox KG (Neuenbürg/Württ., Germany) for supplying pectin.
Funding
Open access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lang, S., Ozcelik, M., Kulozik, U. et al. Processing of raspberries to dried fruit foam: impact on major odorants. Eur Food Res Technol 246, 2537–2548 (2020). https://doi.org/10.1007/s00217-020-03595-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03595-9