Abstract
This study is based on 2-year experimental results aimed at evaluating the nutritional value and pomological characteristics of wild fruits and berries from the walnut-fruit forests of southern Kyrgyzstan including apple (Malus sieversii var. kirgizorum), pear (Pyrus korshinskyi Litv.), rosehip (Rosa canina), or barberry (Berberis oblonga). Wild pear, characterised by its high level of alimentary fibres (8.76 g/100 g), offers a promising potential for industrial pectin production. Barberry features higher radical scavenging activity (antioxidant activity) as compared to Iranian and Turkish ecotypes. Among the investigated fruits and berries, barberry and rosehip represent a good source of bioactive phytochemicals due to their high phenolic, anthocyanin, vitamin C and mineral contents. Regular consumption of such wild fruits can contribute between 26 and 100% to the recommended dietary allowance of selected mineral elements (Ca, Zn, Fe, Mn) helping to combat micro-nutrient deficiency in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Food compositional data such as data on mineral, vitamin, or antioxidant content are essential for many fields including clinical practice, nutrition policy, public health, education, or for the food-manufacturing industry; with such data being used in a variety of ways including national programs for the assessment of the nutritional status of the population, the development of therapeutic and institutional diets, or for nutrition labelling of foods [1]. Studies provided by The United Nations Children’s Fund (UNICEF) on the nutritional status of children and their mothers conducted in the Kyrgyz Republic show that stunting, low birth weight, or vitamin and mineral deficiencies are major issues affecting the health and well-being of children in Kyrgyzstan. Among mothers, the prevalence of folate deficiency lies at 49.3%. Iron deficiency anemia among children aged 5 years declined only slightly from 50% (1997) to 43% (2012) [2].
Fruits and berries are important sources of nutrients that should be taken into account to improve the nutritional status of a population. The unique walnut-fruit forests of Kyrgyzstan are the oldest of their kind in the world and are composed of natural stands of Persian walnut (Juglans regia L.) and a variety of wild fruits and berries. The species diversity and genetic polymorphism of the extensive walnut-fruit forest of Kyrgyzstan are substantial. For example, Rosaceous species of wild apple (M. sieversii var. kirgisorum), together with wild pear (Pyrus korshinskyi Litv.), wild cherry plum (Prunus divaricata Ledeb.), or rosehip (Rosa canina) are found in these forest ecosystems [3]. Malus sieversii var. kirgizorum is a widespread wild apple native to the walnut-fruit forests of Kyrgyzstan. Malus sieversii has been shown to be a wild relative of domesticated apple cultivars (Malus domestica) and, therefore, is a genetic resource of global importance [4].
Pyrus korshinski is the major pear rootstock for all central Asian countries. The tree can reach up to 10–12 m in height and features white flowers of 2–2.5 cm diameter with purple anthers. This wild pear is considered more drought tolerant and disease resistant than P. regelii. Rehd [5] and its fruits are characterized by their green–yellow colour and an astringent taste.
Rosehip (R. canina) pseudo fruits, often referred to as rosehips, grow naturally in the walnut-fruit forests. Rosehips have been used as a herbal medicine for more than 2000 years, yet research has only recently begun to outline specific mechanisms of how this plant product may affect human health. The fruits of R. canina L. (Rosaceae) are best known for their high content of vitamin C, flavonoids, carotenoids, and fatty acids and are, therefore, commonly used for the treatment of colds and influenza [6].
Barberry (Berberidaceae) is also used to treat various diseases including scurvy, hypertension, Alzheimer’s disease, depression, diarrhoea, diabetes, jaundice, kidney stones, gout, rheumatism, or skin diseases [7, 8]. The Barberry ecotype Berberis oblonga is common throughout the walnut-fruit forests of Kyrgyzstan.
However, despite their benefits, these fruits are not widely used in the Kyrgyz diet or Kyrgyz medicine. This study, therefore, attempts to contribute to improved sustainable dietary use of wild berries and fruits through scientific research, which ultimately will facilitate the sustainable management and use of forest resources. There is currently a lack of information on the phytochemical composition of fruits and berries from the Kyrgyz walnut-fruit forest in the scientific literature. Therefore, the aim of this study is to investigate and document the nutritional value of selected key wild fruits and berries from Kyrgyzstan using standard methods, and to compare them with values reported from other regions.
Materials and methods
Materials
Fully mature samples of apple (Malus sieversii var. kirgizorum), barberry (B. oblonga), rosehip (R. canina), and pear (Pyrus korshinskyi Litv.) were collected (5 kg per variety) during their respective harvesting seasons in 2017 and 2018 at two different locations in the natural walnut-fruit forests: in Arslanbap (A), located at N 41° 22′ 8.33″, E 72° 3′ 45.974″, at an altitude of 1300 m; and in Kyzyl-Unkur (KU), located at N 41° 18′ 20.903″, E 72° 57′ 48.209″, at an altitude of 1466 m (Table 1). The samples were transported in air-conditioned vehicles in portable fridges at 4–6 °C (for 9–12 h) to the laboratory in Bishkek, Kyrgyzstan and independently identified by two specialists from the Department of Botany of the Kyrgyz Academy of Sciences. All samples were stored in plastic bags at two different conditions: at 4–6 °C for analysis of physical properties and at − 23 °C for analysis of the chemical composition.
Determination of nutritional values and pomological characteristics
Moisture, invert sugar, titratable acidity, ascorbic acid, crude fibre, and ash contents were determined according to the AOAC method [9].
Pomological properties of fruits (including fruit weight, size, sphericity, average radius, aspect ratio, volume, and bulk density) involved the examination of 25 sample fruits in 3 replications according to Mohsenin [10], as described in Smanalieva et al. [11].
Determination of total polyphenol and total anthocyanin contents
For the polyphenolic analysis, sample extracts were prepared according to Kalt et al. [12]. Total polyphenols in the extracts were determined by the Folin–Ciocalteau micro-method according to Waterhouse [13] using a UV–Vis spectrophotometer (Specord 50, Analytic Jena, Germany) at 765 nm wavelength. Total polyphenolics were expressed as mg gallic acid equivalents (GAE) per kg of the fresh sample.
For the anthocyanin analysis, fruit extracts were prepared according to Tonutare et al. [14] using a solvent containing ethanol and 0.1 M HCl at 85:15% (v:v). Total anthocyanins were determined based on the pH differential spectroscopic method using two different buffers: 0.025 M potassium chloride (pH 1.0) and 0.4 M sodium acetate (pH 4.5), respectively. A UV–Vis spectrometer (Specord 50, Analytic Jena, Germany) was used to measure absorbance at 510 and 700 nm. Total anthocyanins were calculated as cyanidin-3-glucoside using the molar absorptivity coefficient (İ) value 26,900/M/cm, and the molecular weight 449. The results were calculated according to Giusti and Wrolstad [15] as follows:
The content of total anthocyanins (TA) was calculated as follows:
where DF is the dilution factor, E is the cuvette optical pathlength (1 cm), and m is the weight of the sample (g). The total anthocyanin content was expressed as mg anthocyanin in 100 g fresh weight (FW).
Determination of total antioxidant capacity
Both extraction and analysis of total antioxidants were performed according to Hangun-Balkir and McKenney [16] using a solution of 80% ethanol. Individual solutions of antioxidants were prepared at five concentrations (1, 2.5, 5, 7.5 and 10 µg/ml) in 80% ethanol. As a radical, solution of 0.01% DPPH (2,2 diphenyl-1-picrylhydrazyl) in 80% ethanol was used. The absorbance of the control and sample solutions was measured at 517 nm using a UV–Vis spectrophotometer (Specord 50, Analytic Jena, Germany). The values are expressed as IC50, the concentration of the samples that cause 50% scavenging of the DPPH radical.
Determination of mineral composition
To determine macro- and microelement concentrations (mg/100 g fresh weight), fresh fruit samples were washed according to the AOAC 942.05 method [9]. Subsequently, 0.15 g of sample ash was dissolved in 15 ml 10% HNO3 and shaken for 30 s and filtrated using a Whatman filter (No 42). The filtrates were analysed using Inductively Coupled Plasma-Optical Emission Spectrometry (ICP-OES, Optima 8000, PerkinElmer Waltham, USA) with the following working conditions: RF power − 1.0 to 1.4 kW (1.2–1.3 for axial); Plasma gas flow rate (Argon) –10–13 l/min; Quality control-10 mg/l; 1 mg/l; Plasma-13 l/min; Auxiliary gas flow rate − 0.6 l/min; Nebulizer-0.6 l/min.
Statistical analyses
All analyses were carried out in triplicates. The results are expressed as mean values with standard deviation (SD). Data were analysed by t test (p < 0.05) using the SPSS software, Version 13 to examine pairwise comparisons between different harvesting years and locations (SPSS Inc., Chicago, IL, USA).
Results and discussion
Pomological and nutritional composition of wild fruits
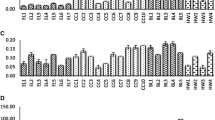
No significant differences in chemical or physical properties among fruits harvested in 2017 and 2018 could be observed; therefore, the mean values of the 2 years are shown in Table 2. The Dmax of wild apple is in the range of 31.6 and 33.4 mm with a weight between 11.6 and 15.2 g (insignificant differences between the two locations Arslanbap and Kyzyl-Unkur). For comparison, the cultivated apple variety Smith (Malus domectica) has a significantly larger fruit diameter of 71–81 mm at the ripening stage and weighs approx. 201.8 g [17]. The diameter of Gloster apples can be even larger ranging from 63.3 to 88.6 mm with the weight of these apples ranging from 101 to 256 g, respectively [18]. The wild pear fruit on average features 20.4 mm in diameter and 6.5 g in weight. In comparison, cultivated pear (Pyrus communis L.) features a weight ranging from 10 to 140 g [19, 20]. The shape of the analysed wild apples and pears was found to be almost round. In general, the wild fruits assessed in this study feature lower weights and sizes than those reported in the studies of cultivars mentioned above, most likely due to genetic variability.
Analysed rosehip (R. canina) fruits feature a red colour with their weight ranging from 3.12 to 3.53 g per fruit. The fruit weight of rosehip genotypes from the province Adıyaman, Turkey ranges from 1.29 to 2.72 g [21] and from 2.0 to 2.8 g in south Sweden and Denmark [22]. With regard to other physical characteristics, length ranged from 15.45 to 17.01 mm, width from 13.64 to 14.48 mm, and sphericity ranged from 0.81 to 0.86. Rosehip fruits from Turkey feature an average weight of 2.35 g, a width of 13.5 mm and a length of 20.70 mm [23]. The average values of rosehip fruits from Bosnia ranged from 14.73 to 20.69 mm for length, from 9.45 to 10.88 mm for fruit width, and from 1.31 to 1.48 g for fruit weight. Thus, compared to these values, Kyrgyz rosehip fruits can be considered large.
The investigated wild barberry fruits featured a weight between 0.16 and 0.22 g, a purplish-black colour and contained between one and four stones. The length, width and average radius of barberry fruits ranged from 9.19 to 10.22 mm, 5.21 to 5.27 mm, and 3.21 to 3.45 mm, respectively. The sphericity ranged from 0.63 to 0.68. Compared to barberry fruits from Iran, being 7.40–10.84 mm in berry length, 4.39–8.05 mm in berry width and 0.11–0.43 g in berry weight [24] or barberry fruits from Turkey being on average 15.95–20.77 mm in length, 10.91–16.40 mm in width and 12.57–17.69 mm in diameter with a sphericity ranging from 0.745 to 0.85 [25], fruits from the Kyrgyz walnut-fruit forests are considerably smaller than cultivated barberry fruits from Turkey. Overall, it appears that the physical properties of the investigated wild fruit samples, particularly concerning their smaller size and lower weight, represent a considerable limitation for their commercialisation, an exception being rosehip fruits. As demonstrated in Table 2, significant differences between the two locations can be observed for sphericity, aspect ratio and bulk density of rosehips and in volume and aspect ratio of barberries.
The moisture content of analysed wild apples and pears, with values between 70.18 and 82.48 g/100 g (Table 3) were lower than values reported in the literature for comparable domesticated fruits. For example, Red Delicious, Golden Delicious, Gala, Granny Smith, and Fuji apple varieties feature a moisture content of 85.56 g/100 g [26]. Barberry samples are characterized by an average moisture content of 58.86 g/100 g, which is also lower than the moisture content of barberry fruits from Turkey (71.42 g/100 g) [25]. The moisture content of investigated rosehip samples ranged from 66 to 70%, whereas rosehip species from Turkey varied from 60% (Rosa dumalis subsp. boissieri) to 66% (Rosa villosa) [27].
The highest invert sugar content was found in rosehip and pear fruits (9.30 and 10.45%, respectively), whereas barberry fruits showed the lowest invert sugars content with values between 6.42 and 7.62 g/100 g. Therefore, results for barberry are in agreement with findings from Akbulut et al. [25] (6.52%), but lower than findings of Awan et al. [28] who measured invert sugar content ranging from 7.72 to 9.00 g/100 g in B. orthobotrys and B. calliobotrys from Pakistan, respectively.
The pH value of evaluated rosehip fruits being in the range of 4.27–4.36 is similar to the results reported for Turkish rosehip (4.27) [23]. Barberry fruits featured pH values of 3.07–3.25 and values from 2.03 to 2.56% for total acidity (as malic acid). Such values are comparable to results obtained by Awan et al. [28], who reported pH values of 3.33–3.91 and 1.36–2.26% titratable acidity in barberry fruits from Pakistan.
Ascorbic acid is an antioxidant protecting plants from oxidative harm derived from either internal factors, such as respiration and photosynthetic metabolism, or external factors, such as environmental pollution [29]. In the present study, the highest ascorbic acid contents were identified in rosehip (467–491 mg/100 g) and in barberry (488–491 mg/100 g). Values for rosehip fruits corresponded to ascorbic acid contents reported in other studies, ranging from 391.7 mg/100 g [30] to 727 mg/100 g FW for R. villosa and reaching 943 mg/100 g FW for R. dumalis subsp. boissieri [27], respectively. In wild apple samples, high content of vitamin C was found ranging from 11.48 to 13.60 mg/100 g; however, ascorbic acid content levels of domesticated apples ranging from 19.38 mg/100 g (‘Well Spur’) and 32.08 mg/100 g (‘Starkrimson’) [18]. The lowest ascorbic acid contents were detected in wild pear fruits. Ascorbic acid content from ten varieties of common pear (P. communis) in Turkey varies between 4.4 and 10.2 mg/100 g [20], which is in the range of or slightly higher than the values found for wild pears from Kyrgyz walnut-fruit forests.
Ash and crude fibre content of the investigated wild fruits ranged from 0.31 to 6.62 g/100 g and 1.77 to 8.76 g/100 g, respectively. With 3.98 g/100 g, ash content in rosehip was highest for all investigated fruit samples. The ash content of barberry fruits was determined at 1.86%, whereas lower values ranging from 0.79 to 1.13% were presented by Awan et al. [28] and values of 0.73 and 1.12% by Ardestani et al. [31] and Akbulut et al. [25], respectively. Such variations in nutritional values are likely to be influenced by genetic differences of the investigated genotypes as well as environmental conditions, such as temperature (average, maximum and minimum), water availability (e.g. precipitation patterns and total amount), soil conditions (e.g. nutrient content, soil depth), radiation (e.g. intensity, quality) and abiotic and biotic stressors, all affecting the content of minerals, and primary and secondary metabolites of wild fruits and berries.
Total phenolic, anthocyanin content and antioxidant activity by DPPH
The highest amount of total phenolic content in this study was found for rosehip from Arslanbap (RCA) with 907 mg GAE/100 g (Table 3), which is very high compared to values reported in the literature. For example, Roman et al. [32], reported 326.3–575.1 mg GAE/100 g total polyphenols of rosehip pulp in Transylvania, Fattahi et al. [33] obtained an average of 197 mg GAE/100 g in rosehip of Turkey and Yoo et al. [34], reported 818 mg GAE/100 g fresh rosehip fruit of China.
The lowest amount of total phenolic content was found in pear fruits (PKA) with 403 mg GAE/100 g. Abacı et al. [20] detected values from 300.1 to 687.2 mg GAE/100 g in the peel of different pear genotypes and values from 112.6 to 300 mg GAE/100 g in the flesh. Thus, the amount of total phenolic content of wild pears in our study can be considered in the range of cultivated pear genotypes. Total phenolic content of investigated wild apple samples ranged from 439 to 476 mg GAE/100 g, which is comparable to total phenolic content of apple (M. domestica) from West Himalaya with values between 94 and 700 mg GAE/100 g [35]. According to Francini and Sebastiani [36], the highest total phenolic concentration of apple (M. domestica Borkh.) was found in the peel with 500.2—588.9 mg GAE/100 g, whereas it was 75.7–93.0 mg of GAE/100 g—and, therefore, substantially lower—in the flesh. On the other hand, Navarro et al. [37] determined 619.6 mg GAE/100 g in the peel and 576.2 0 mg GAE/100 g in the flesh of apples from Costa Rica. It has been reported that polyphenolic contents of fruits can strongly differ, depending on environmental conditions during fruit development, with abiotic and biotic stress often increasing polyphenolic concentrations [38, 39]. However, when comparing fruits of different plant species from the same environment, it clearly shows a strong influence of the genetically determined potential, in this case, demonstrated by the highest values in rosehip and barberry and lowest in apples and pears.
All the investigated samples from Kyzyl-Unkur (KU) featured higher antioxidant activity (radical scavenging activity) as compared to samples from Arslanbap (A). Inhibitory concentrations (IC50) of barberry (BO) were 1.0 µg/ml in Kyzyl-Unkur (BOKU) and 1.7 µg/ml in Arslanbap (BOA). The IC50 of rosehip samples was 1.3 µg/ml (RCKU) and 1.4 µg/ml (RCA). In comparison, the IC50 of three Iranian barberry species ranged from 58.4 to 221.1 μg/ml [40], which is a significantly lower antioxidant capacity than wild barberry from Kyrgyz walnut-fruit forests. Wild apples from Kyzyl-Unkur (MSKU, 10.0 µg/ml) also showed higher antioxidant activity than wild apples from Arslanbap (MSA, 11.2 µg/ml). However, Navarro et al. [37] reported higher antioxidant capacity, i.e. lower IC50 of apple (M. domestica) from Costa Rica as 4.5 (skin) and 6.6 µg/ml (flesh). The antioxidant capacity of phenolic compounds is mainly due to hydroxyl groups and their redox properties, which allow them to act as reducing agents, hydrogen donors, singlet oxygen quenchers or metal chelators. Phenolics are presumed to be the major phytochemicals responsible for the antioxidant activity of plant material [41]. In fact, a sound linear correlation (R2 = 0.73) between total phenolic content and antioxidant activity by DPPH of wild fruits and berries can be observed (Fig. 1a), reflecting the species-specific composition patterns. The correlation between the antioxidant activity and the total polyphenol content as observed by other researches was R2 = 0.71 for rosehip [32], 0.72 for apple [42], and 0.98 for wild cherry plum [43]. The high free radical scavenging capacity of wild fruits may also be attributed to the presence of other bioactive components, such as vitamin C, tocopherols, pigments, as well as the interaction of these compounds [44]. In our study, the correlation of vitamin C with overall antioxidant capacity was 0.86 (Fig. 1b), which is in agreement with Roman et al. [32]. However, we have to admit that the relationship between antioxidant capacity and total phenolic content or vitamin C, respectively, is caused by the large difference between the content of the individual species’ fruits.
The amount of total anthocyanin content (TAC) was higher in barberry fruits from Arslanbap (1010 mg/kg) as compared to samples from Kyzyl-Unkur (980 mg/kg FW). The difference in TAC of fruit and berry samples from the two locations in our study may be due to differences in environmental conditions such as altitude and light intensity. According to Guerrero-Chavez et al. [45], TAC of strawberries correlates negatively with increasing altitude. With regard to light intensity, total anthocyanin concentration increases, whereas the trend is inversed in case of medium light intensity [39]. However, values from both locations were slightly higher than total anthocyanins in fruits from Turkey as reported by Akbulut et al. [25] and Özgen et al. [46]. Akbulut et al. [25] determined total anthocyanins in barberry (Berberis vulgaris L.) with values of 931 mg/kg FW, while Özgen et al. [46] found values in the range of 506 to 803 mg/l in fruit juice (Fig. 2). Thus, wild barberry from Kyrgyz walnut-fruit forests can be considered rich in anthocyanins. In the other investigated fruit samples, the anthocyanin content was below detection limit.
Mineral composition of wild fruits
The results of the mineral composition analysis using ICP-OES showed that the maximum concentration of sodium (Na) was found in barberry from Kyzyl-Unkur (BOKU) and from Arslanbap (BOA) with a value of 45.46 and 33.25 mg/100 g, respectively (Table 4). These results are higher than literature values for wild barberry from Argentina (29.33 mg/kg) [47]. The recommended dietary allowance (RDA) value for Na is 2400 mg [48]. Therefore, 100 g fresh BOKU can contribute 1.8% of Na to RDA. Potassium (K) content was lowest in wild apple from Kyzyl-Unkur (MSKU) with 149.4 mg/100 g, whilst the highest content was found in barberry from Kyzyl-Unkur (BOKU, 466.35 mg/100 g). This value, however, is substantially lower than the 1,530 mg/100 g reported in Rahimi-Madiseh, (2016) [40]. The RDA for K is 3500 mg for adults [47]. Therefore, 100 g fresh BOKU can contribute 13.32% of potassium to RDA. The highest values for calcium (Ca), magnesium (Mg) and manganese (Mn) were found in rosehip from Arslanbap (RCA) with 213.32, 58.01 and 6.42 mg/100 g, respectively. It should be noted that the values of Ca (213.32 mg/100 g) and Mn (6.42 mg/100 g) in the RCA sample are higher than literature values for wild rosehips from the northern plains in India, with values of 169 mg Ca/100 g FW and 1.02 mg Mn/100 g being reported in the food composition database of USDA [26]. The RDA for Ca is 800 mg, therefore, 100 g RCA can contribute 26% of Ca to RDA. The Mn content of the RCA sample was higher than reported for rosehip from Turkey, with 32.0 mg Mn/100 g [49]. The RDA for Mn is 5 mg for adults [48]. Therefore, 100 g fresh RCA can contribute more than 100% of Mn to RDA. Phosphorus (P), zinc (Zn), and copper (Cu) concentrations were highest in barberry from Arslanbap (BOA) with 83.60 mg P/100 g, 8.61 mg Zn/100 g, and 4.43 mg Cu/100 g. Zn content was significantly higher than literature values for barberry (Berberis microphylla) from Argentina (20.53 mg Zn/kg) [47]. The RDA for Zn is 15 mg and for Cu 2 mg for adults. Therefore, 100 g fresh BOA can contribute 57.4% of Zn and two times more than RDA of Cu. The concentration of iron (Fe), copper (Cu), zinc (Zn), and phosphorus (P) were also high in barberry from Kyzyl-Unkur (BOKU), which were 11.62, 3.11, 5.44 and 52.52 mg/100 g, respectively. The RDA for Fe is 15 mg for adults. Therefore, 100 g of fresh BOKU can contribute 77% of Fe to RDA, which can help to combat iron deficiency. Molybdenum (Mo) content was 0.1 mg/100 g in all analysed samples and the RDA for Mo is 75 µg. Thus, 100 g of fresh BOKU can contribute more than 100% of Mo to RDA. Overall, samples from Arslanbap and Kyzyl-Unkur showed insignificant differences in terms of macro- and micro-elements, except for wild apple, which may be caused by differences in available nutrients in the soil. Regular consumption of such wild fruits can contribute between 26 and 100% to the recommended dietary allowance of selected micronutrients, helping to combat nutrient deficiency in humans.
Conclusions
The comparison of various physical and chemical properties of wild fruits and berries with the respective cultivated varieties as documented in scientific literature demonstrated that wild apples (Malus sieversii var. kirgizorum) and pears (Pyrus korshinskyi Litv.) cannot compete with cultivated forms. The size, weight, and high acidity of the wild fruits are a considerable limitation for fresh consumption. However, wild pear, characterized by its high levels of alimentary fibres, offers a promising potential for industrial pectin production. The high total phenolics, vitamin C, and mineral content (K, Ca, Fe, P) in barberry (B. oblonga) and rosehip (R. canina), make these species useful alimentary sources against vitamin and mineral deficiencies in human diets. Barberry was also found to be rich in anthocyanins, which could be used for the production of natural colourants. The mineral composition analysis of wild fruits displayed higher concentrations of P from Kyzyl-Unkur compared to Arslanbap, highlighting the variability of chemical fruit properties, potentially caused by differences in environmental conditions. Consequently, future research needs to address this phenomenon. The results of our study lay the foundation for the establishment of raw material standards for wild forest fruits, which is required for further promoting their sustainable use as a source of natural food additives such as fibre, colourants and other substances in the context of bio-economy strategies in the national food industry.
Change history
28 June 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00217-021-03799-7
References
Church S (2009) Food composition explained. EuroFIR synthesis report no. 7. Nutr Bull 34:250–272. https://doi.org/10.1111/j.1467-3010.2009.01775.x
UNICEF (2013) National survey of the nutritional status of children 6–59 months of age and their mothers the Kyrgyz Republic, The United Nations Children’s Fund. https://www.unicef.org/kyrgyzstan/reports/national-survey-nutritional-status. Accessed 4 Nov 2019
Orozumbekov A, Cantarello E, Newton AC (2015) Status, distribution and use of threatened tree species in the walnut-fruit forests of Kyrgyzstan. For Trees Livelihoods 24(1):1–17. https://doi.org/10.1080/14728028.2014.928604
Lamboy WF, Yu J, Forsline P, Weeden NF (1996) Partitioning of allozyme diversity in wild populations of Malus sieversii L. and implications for germplasm collection. J Am Soc Hortic Sci 121:982–987
USDA (2019) Germplasm Resources Information Network (GRIN). https://npgsweb.ars-grin.gov/gringlobal/search.aspx. Accessed 4 Nov 2019
Winther K, Campbell-Tofte J, Vinther Hansen AS (2016) Bioactive ingredients of rosehips (Rosa canina L.) with special reference to antioxidative and anti-inflammatory properties: in vitro studies. Botanics 6:11. https://doi.org/10.2147/BTAT.S91385
Yin J, Hu R, Chen M, Tang J, Li F, Yang Y, Chen J (2002) Effects of berberine on glucose metabolism in vitro. Metab Clin Exp 51(11):1439–1443. https://doi.org/10.1053/meta.2002.34715
Retnakaran R, Austin P, Shah B (2011) Effect of subsequent pregnancies on the risk of developing diabetes following a first pregnancy complicated by gestational diabetes: a population-based study. Diabet Med 28(3):287–292. https://doi.org/10.1111/j.1464-5491.2010.03179.x
AOAC (2011). In: Horwitz W, Latimer GW Jr (eds) Official methods of analysis of AOAC International, 18th edn. Revision 4. AOAC International. Gaithersburg, Maryland, USA
Mohsenin NN (1986) Physical properties of plant and animal materials: structure, physical characteristics, and mechanical properties. Gordon and Breach Science Publisher, New York. https://doi.org/10.1002/food.19870310724
Smanalieva J, Iskakova J, Oskonbaeva Zh, Wichern F, Darr D (2019) Determination of physicochemical parameters, phenolic content, and antioxidant capacity of wild cherry plum (Prunus divaricata Ledeb.) from the walnut-fruit forests of Kyrgyzstan. Eur Food Res Technol 245:2293–2301. https://doi.org/10.1007/s00217-019-03335-8
Kalt W, Forney CF, Martin A, Prior RL (1999) Antioxidant capacity, vitamin C, phenolics and anthocyanins after fresh storage of small fruits. J Agric Food Chem 47:4638–4644. https://doi.org/10.1021/jf990266t
Waterhouse L (2001) Determination of total phenolic. Handbook of food analytical chemistry. Wiley, New Jersey
Tonutare T, Moor U, Szajdak L (2014) Strawberry anthocyanin determination by pH differential spectroscopic method—how to get true results? Acta Scientiarum Polonorum Hortorum Cultus 13(3):35–47
Giusti MM, Wrolstad RE (2001) In: Wrolstad RE (ed) Anthocyanins. Characterization and measurement with UV–visible spectroscopy. Wiley, New York. https://doi.org/10.1002/0471142913.faf0102s00
Hangun-Balkir Y, McKenney ML (2012) Determination of antioxidant activities of berries and resveratrol. Green Chem Lett Rev 5(2):147–153. https://doi.org/10.1080/17518253.2011.603756
Miletić N, Popović B, Mitrović O, Kandić M (2012) Phenolic content and antioxidant capacity of fruits of plum cv. ‘Stanley’ (Prunus domestica L.) as influenced by maturity stage and on-tree ripening. Aust J Crop Sci 6(4):681–687
Kumar P, Sethi S, Sharma RR, Singh S, Saha S, Sharma VK, Verma MK, Sharma SK (2018) Nutritional characterization of apple as a function of genotype. J Food Sci Technol 55:2729–2738. https://doi.org/10.1007/s13197-018-3195-x
Kalkisim O, Okcu Z, Karabulut B, Ozdes D, Duran C (2018) Evaluation of pomological and morphological characteristics and chemical compositions of local pear varieties (Pyrus communis L.) grown in Gumushane Turkey. Erwerbs-Obstbau 60(2):173–181. https://doi.org/10.1007/s10341-017-0354-6
Abacı ZT, Sevindik E, Ayvaz M (2016) Comparative study of bioactive components in pear genotypes from Ardahan/Turkey. Biotechnol Biotechnol Equip 30(1):36–43. https://doi.org/10.1080/13102818.2015.1095654
Eroğul D, Oğuz Hİ (2018) Determining the physico-chemical characteristics of the rosehip genotypes grown naturally in Adiyaman province. Erwerbs-Obstbau 60(3):195–201. https://doi.org/10.1007/s10341-017-0358-2
Uggla M, Gao X, Werlemark G (2003) Variation among and withind dogrose taxa (Rosa sect. caninae) in Fruit weight, percentages of fruit flesh and dry matter, and vitamin C content. Acta Agric Scand Sect B Plant Soil Sci 53(3):147–155. https://doi.org/10.1080/09064710310011746
Dogan A, Kazankaya A (2006) Fruit properties of rosehip species grown in Lake Van Basin (Eastern Anatolia Region). Asian J Plant Sci 5:120–122. https://doi.org/10.3923/ajps.2006.120.122
Goodarzi S, Khadivi A, Abbasifar A, Akramian M (2018) Phenotypic, pomological and chemical variations of the seedless barberry (Berberis vulgaris L. var. asperma). Sci Hortic 238:38–50. https://doi.org/10.1016/j.scienta.2018.04.040
Akbulut M, Calisir S, Marakoglu T, Çoklar H (2009) Some physicomechanical and nutritional properties of barberry (Berberis vulgaris L.) fruits. J Food Process Eng 32:497–511. https://doi.org/10.1111/j.1745-4530.2007.00229.x
USDA (2019a) US Department of Agriculture, Agricultural Research Service. Nutrient Database for Standard Reference, Release 1. https://www.nal.usda.gov/fnic/foodcomp/search/. Accessed 4 Nov 2019
Ercisli S (2007) Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem 104:1379–1384. https://doi.org/10.1016/j.foodchem.2007.01.053
Awan MS, Ali S, Ali A, Hussain A, Qazalbash AM (2014) A comparative study of barberry fruits in terms of its nutritive and medicinal contents from CKNP region, Gilgit-Baltistan. Pak J Biol Environ Sci 5(2):9–17
Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Ann Bot 78:661–669. https://doi.org/10.1006/anbo.1996.0175
Tumbas VT, Čanadanović-Brunet JM, Četojević-Simin DD, Ćetković GS, Ðilas SM, Gille L (2012) Effect of rosehip (Rosa canina L.) phytochemicals on stable free radicals and human cancer cells. J Sci Food Agric 92:1273–1281. https://doi.org/10.10002/jsfa.4695
Ardestani SB, Sahari MA, Barzegar M, Abbasi S (2013) Some physicochemical properties of Iranian native barberry fruits (Abi and Poloei): Berberis integerrima and Berberis vulgaris. J Food Pharm Sci 1:67–74. https://doi.org/10.14499/jfps
Roman I, Stanila A, Stanila S (2013) Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem Cent J 7(73):1–10. https://doi.org/10.1186/1752-153X-7-73
Fattahi S, Jamei R, Hosseini Sarghein S (2012) Antioxidant and antiradicalic activity of Rosa canina and Rosa pimpinellifolia fruits from West Azerbaijan. Iran J Plant Physiol 2(4):523–529
Yoo KM, Lee CH, Lee H, Moon B, Lee CY (2008) Relative antioxidant and cytoprotective activities of common herbs. Food Chem 106:929–936. https://doi.org/10.1016/j.foodchem.2007.07.006
Bahukhandi A, Dhyani P, Bhatt I, Rawal R (2018) Variation in polyphenolics and antioxidant activity of traditional apple cultivars from West Himalaya. Uttarakhand Hortic Plant J 4(4):151–157. https://doi.org/10.1016/j.hpj.2018.05.001
Francini A, Sebastiani L (2013) Phenolic compounds in apple (Malus x domestica Borkh.): compounds characterization and stability during postharvest and after processing antioxidants. Antioxid Basel 2:181–193. https://doi.org/10.3390/antiox2030181
Navarro M, Moreira I, Arnaez E, Quesada S, Azofeifa G, Vargas F, Alvarado D, Chen P (2018) Polyphenolic characterization and antioxidant activity of Malus domestica and Prunus domestica cultivars from costa rica. Foods 7(15):1–19. https://doi.org/10.3390/foods7020015
Hakulinen J, Julkunen-Titto R, Tahvanainen J (1995) Does nitrogen fertilization have an impact on the trade-off between willow growth and defensive secondary metabolism? Trees Struct Funct 9:235–240. https://doi.org/10.1007/BF00195278
Arena ME, Postemsky PD, Curvetto NR (2017) Changes in the phenolic compounds and antioxidant capacity of Berberis microphylla G. Forst. berries in relation to light intensity and fertilization. Sci Hortic 218:63–71. https://doi.org/10.1016/j.scienta.2017.02.004
Rahimi-Madiseh M, Gholami-Arjenaki M, Bahmani M, Mardani G, Farzan M, Rafieian-Kopaei M (2016) Evaluation of minerals, phenolics and anti-radical activity of three species of Iranian berberis fruit. Der Pharma Chemica 8(2):191–197
Balasundram N, Sundram K, Sammar S (2006) Phenolic compounds in plants and agrindustrial by-products: antioxidant activity. Occur Potential Uses Food Chem 99(1):191–203. https://doi.org/10.1016/j.foodchem.2005.07.042
Vieira FGK, Borges GDC, Copetti C, Amboni RDMC, Denardi F, Fett R (2009) Physico-chemical and antioxidant properties of six apple cultivars grown in southern Brazil. Sci Hort 122:421–425. https://doi.org/10.1016/j.scienta.2009.06.012
Wang Y, Chen X, Zhang Y, Chen X (2012) Antioxidant activities and major anthocyanins of myrobalan plum (Prunus cerasifera Ehrh.). J Food Sci 77(4):388–393. https://doi.org/10.1111/j.17503841.2012.02624.x
Barros L, Carvalho AM, Morais JS, Ferreira ICFR (2010) Strawberry-tree, blackthorn and rose fruits: detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem 120:247–254
Guerrero-Chavez G, Scampicchio M, Andreotti C (2015) Influence of the site altitude on strawberry phenolic composition and quality. Sci Hortic 192:21–28. https://doi.org/10.1016/j.scienta.2015.05.017
Özgen M, Saraçoğlu O, Gezer EN (2012) Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits. Hortic Environ Biotechnol 53(6):447–451. https://doi.org/10.1007/s13580-012-0711-1
Damascos MA, Arribere M, Svriz M, Bran D (2008) Fruit mineral contents of six wild species of the North Andean Patagonia, Argentina. Biol Trace Elem Res 125(1):72–80. https://doi.org/10.1007/s12011-008-8159-y
NRC (1989) National Research Council. Recommended daily allowance. National Academy Press, Washington
Kazaz S, Baydar H, Erbas S (2009) Variation in chemical compositions of Rosa damascene Mill. and Rosa canina L. fruits. Czech J Food Sci 27(3):178–184. https://doi.org/10.17221/5/2009-CJFS
Acknowledgements
We thank Nuraika Nazarova, Tattybubu Madomorova, Lia Moreno Codinachs and Conor Watson for technical support and Kathrin Meinhold for proofreading of the manuscript. The project is financially supported by the German Federal Ministry of Education and Research (BMBF) [Grant number 01DK17016], which we gratefully acknowledge.
Funding
Open access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirements
This paper does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Smanalieva, J., Iskakova, J., Oskonbaeva, Z. et al. Investigation of nutritional characteristics and free radical scavenging activity of wild apple, pear, rosehip, and barberry from the walnut-fruit forests of Kyrgyzstan. Eur Food Res Technol 246, 1095–1104 (2020). https://doi.org/10.1007/s00217-020-03476-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-020-03476-1