Abstract
Growing social interest in foods with high biological quality results in the need to look for possibilities of increasing the biological quality of food products. The aim of this research was an attempt to estimate the increase of polyphenolic compounds (by UPLC-PDA-MS/MS) in the flesh and skin of pear under the influence of stress elicitors such as UVC radiation, L-EMF, H-EMF and US with various exposure times. The applied stress factors differentiated the content of phenolic acids, flavan-3-ols and flavonols both in flesh and in skin. In all cases, pear skin demonstrated a decrease in the sum of polyphenolic compounds; however, when it comes to flavan-3-ols and procyanidins, the concentration of the compounds increased in two cases: after a 30-min exposure to L-EMF (+ 18%) and after a 5-min exposure to H-EMF (+ 20%). Following a 30-min flesh exposure to US, the determined sum of polyphenolic compounds was + 28% higher than in the control sample. It was observed that the level of flavan-3-ols and procyanidins in the flesh increased after a 60-min exposure to UVC radiation and low-frequency and high-frequency electromagnetic fields, and after a 20-min and 30-min exposure to ultrasounds. The presence of flavonols was only observed in pear skin. The research results show that it is possible to produce juice with a higher content of polyphenolic compounds, because juice is obtained mainly from the flesh; however, there is a need for further research to confirm the observed tendencies in the changes of polyphenolic compounds in fractions of pears.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The health and nutritional properties of pears have been known for ages. The fruit originated in Asia, where it was used in Chinese folk medicine already 3000 years ago [1]. Two commonly available pear species are European pear (Pyrus communis) and Asian pear (Pyrus pyrifolia). The People’s Republic of China is the global pear potentate. The differences between European and Asian pear include the form and texture, with the former being sweeter thanks to a higher content of fructose. The fruit is very nutritional and rich in glucose and fructose. In the case of European pear, the ratio of glucose to fructose is 0.12:0.52, whereas in the case of Asian pear, 0.39:0.59 [2]. Pears can be one of the major ingredients of a well-balanced diet. Their nutritional and health properties are mainly due to the result of antioxidant ingredients, which are scarce in the diet of the societies in developed countries. Therefore, it is important to search for possibilities to boost the antioxidant properties of the fruit and at the same time preserve the taste and nutritional value. According to a study conducted by Cebulak et al. [3] on chokeberries, it was observed that one of such factors can be exposure to particular stress elicitors.
Source literature shows that pears are particularly valued for their health benefits. They are rich in minerals: 100 g of dry mass contains 91.7 mg of potassium, 14.9 mg of calcium and 10.06 mg of magnesium, as well as 2.8 mg of fibre. Moreover, pear protein is rich in leucine and lysine [4]. The fruit also contains high amounts of biologically active ingredients, dominated by polyphenols, which determine the anti-diabetic, anti-inflammatory, antibacterial, anti-cancer properties and have a positive influence on the cardiovascular system [5, 6]. In research Simirgiotis et al. [7], and Wang et al. [8] conducted on diabetic mice [7, 8] observed that a daily intake of 500 mg of dried pear per kg b.w. for a period of 2 weeks had a significant hypoglycaemic effect. Pear skin contains a large amount of flavan-3-ols, polymeric procyanidins, flavonols and triterpenoids, whose content is positively correlated with the increased content of wax covering the skin [9]. The literature data indicate [10, 11] that the content of polyphenolic compounds in the local varieties of pear was higher than in commercial varieties, whereas significantly higher content was determined in the skins than in the flesh. However, the level of secondary metabolites depended on the degree of maturity, climatic factors, variety and cultivation technology applied.
Interest in the role of bioactive compounds in human health has impacted research on content of these compounds impact in food and opportunities maintaining their content and even improving [12]. Recently shown the use of many treatments as high or low temperature treatments, altered gas composition, ultraviolet and gamma irradiation, can enhance polyphenolic compounds, and thus antioxidant activity [13]. UV-C irradiation has been tested as a treatment to increase ascorbic acid and total phenolic contents in plants [14]. According to Yu et al. [15], the UV irradiation and heat stress lead to changes in polyphenolic compounds content, the range of which depends on time and/or the power of use abiotic stress factors. Effective use of these factors could increase the content of bioactive compounds in finished products. That is why it is so important to check under optimal conditions their impact on the health values of plants and their increase. Moreover, to our knowledge there are no reports on effect abiotic stress factors to phenolic compounds in pear fractions. Therefore, the objective of this study was an attempt to estimate the increase of polyphenolic compound contents in the flesh and skin of pear under the influence of stress elicitors such as UVC radiation, low-frequency electromagnetic field (L-EMF), high-frequency electromagnetic field (H-EMF) and ultrasounds (US) with various exposure times.
Materials and methods
Reagents and standards
Methanol and formic acid were from Sigma-Aldrich (Steinheim, Germany). Acetonitrile was from Merck (Darmstadt, Germany). Caffeic acid was purchased from Sigma-Aldrich (Steinheim, Germany). Kaempferol 3-O-glucoside, quercetin 3-O-glucoside, isorhamnetin 3-O-glucoside, cyanidin 3-O-glucoside, apigenin 7-O-glucoside, quinic, p-coumaric and ferulic acids, (+)-catechin and procyanidin B2 were purchased from Extrasynthese (Lyon, France).
The experimental material
The study was based on the fruit of the ‘Conference’ pear cultivar. It was acquired from a commercial orchard located in Dwikozy near Sandomierz (N 50°71′19.9060′′, E 21°77′74.7726′′). The fruit was harvested at the stage of technological ripeness in September 2017. Following initial processing involving washing and drying, the fruit was divided into 3-kg portions that were exposed to abiotic stress factors varying in terms of intensity and exposure time. The collected fruits were then subjected to abiotic stress factors: UV-C radiation using the NBV 30N lamp (UltraViol, Zgierz, Poland) with a radiation intensity of 2.3 W/m2; electromagnetic field (150 μT, 100 Hz); microwaves (100 W and 180 W) and ultrasound (800 W, 40 Hz). Different times of action were used for these stress factors. Exemplary codes with the appropriate stress factor and its duration of action are given below. (1) UVC UVC radiation for 20, 40 and 60 min; (2) L-EMF low-frequency electromagnetic radiation (150 μT) for 30 and 60 min; (3) H-EMF high-frequency electromagnetic radiation (40 μT) for 0.5, 2 and 5 min; (4) US ultrasounds for 20, 30 and 40 min. After the end of the elicitation process, the fruit was left to rest for 3 h. Then, the fruit was peeled and divided into smaller fragments, which were then frozen in liquid nitrogen. After freezing, the fruit fragments were ground in a lab grinder (IKA 11A) and lyophilised (Christ Alpha 1–4 LSC). Prior to the analysis, the lyophilisate was stored at − 80 °C.
Extraction
Polyphenolic compounds were isolated by means of extraction supported by ultrasounds. First, 1 g of the finely ground material was placed in a Falcon centrifuge tube (50 mL), to which 50% aqueous methanol containing formic acid (1%) was poured. The extraction was performed twice by incubation for 20 min under sonication (Sonic 6D, Polsonic, Warsaw, Poland) and with occasional shaking. Next, the slurry was centrifuged at 19,000g for 10 min and the supernatant was filtered through a Hydrophilic PTFE 0.20 μm membrane (Millex Samplicity Filter, Merck, Darmstadt, Germany) and used for analysis. The content of polyphenols in individual extracts was determined by means of the UPLC-PDA-MS/MS method [16]. All extractions were carried out in triplicate.
Identification and quantification of polyphenols by the UPLC-PDA-MS method
Qualitative and quantitative analyses of polyphenolic compounds were carried out with the use of ultra-performance liquid chromatography with a photodiode array detector (PDA) and a tandem mass detector measuring the quadrupole-time of flight (Q-TOF), based on a procedure described by Cebulak et al. [3]. Polyphenols were analysed in the negative-ion mode, while the remaining in their identification was done by comparing spectra of maximum UV-radiation absorption, molecular weight determined as the mass/charge ratio, retention times, as well as fragmentation spectra, with the available literature data (Table 1) [17, 18].
Phenolic compounds were identified by ultra-performance liquid chromatograph equipped with a binary solvent manager (BSM), a sample manager (SM) coupled with a PDA detector and a quadrupole-time-of-flight (Q–TOF) tandem mass detector (Waters, Manchester, UK). Separations were carried out on a 2.1 × 100 mm UPLC BEH C18 column containing 1.7 µm particles. Isocratic gradient elution was chosen as the elution mode, in which were used: 2% aqueous formic acid (A) and acetonitrile (B), at the flow of mobile phase velocity of 0.45 mL/min. Elution was initiated at 99% A for 1 min, then a linear gradient was applied to 75% B in 12 min. The column temperature was 30 °C and the volume of injections 5 µL. Operating parameters of the mass detector were as follows: capillary voltage of 2.5 kV and the sampling cone voltage of 30 V. Temperatures of the ion source and desolvation were 130 °C and 350 °C, respectively. Nitrogen, at a flow rate of 300 L/h, was used as carrier gas. Analyses were carried out in a full scan mode within the range 100–1500 m/z, upon the tolerance of 0.001 Da and resolution of 5000. The internal reference standards, leucine and enkephalin, were introduced continuously through lockspray reference channel. The chromatograms were analysed employing the base peak (BPI) calibrated to 12,400 cps (100%). Data were collected and analysed using MassLynx v4.1 software (Waters).
Statistical analysis
Statistical calculations were based on a significance test for means and cluster analyses, procedures available in the statistical analytics software Statistica 12 (Kraków, Poland). The significance for means was evaluated by a Student’s t test of independent samples with the intensity level of α = 0.05. Cluster analysis provided an image of interfactorial relations of the influence of abiotic stress factors on the differentiation of polyphenol content in the skin and flesh of pear.
Results
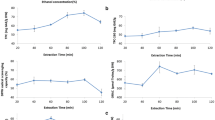
The influence of abiotic stress factors: UV-C radiation, ultrasounds [US], low-frequency electromagnetic radiation 150 µT [L-EMF] and high-frequency electromagnetic radiation of 40 mT [H-EMF], on the level of polyphenolic compounds in the flesh and skin of pear is presented in Tables 2, 3, 4, 5. The identified polyphenolic compounds in pear fruits subjected to abiotic stress factors are given in Table 1. Interrelations between the abiotic stress factor and the changes of polyphenol content in the skin and flesh of pear are presented in Figs. 1, 2, 3, 4, 5. The concentration of phenolic acids, flavan-3-ols, procyanidins and flavonols was calculated on the basis of standardised benchmark substances.
The influence of abiotic stress factors (UV-C, H-EMF, L-EMF, US) on the content of flavan-3-ols and procyanidins in flesh of pear fruits (1—cluster area statistically insignificant, 2—cluster area − 23% to control sample, 3—cluster area + 20% to control sample, 4—cluster area + 48% to control sample)
The highest concentration of polyphenolic compounds in the analysed skins was found in the control sample (3.19 mg/100 g dry matter) (Table 2), and the use of stress elicitors decreased the sum of polyphenolic compounds in all skin samples, with a slight increase in the flesh. The determined level of the sum of polyphenolic compounds in the flesh was significantly lower than in the skin (Tables 2, 3, 4, 5). The observed decrease of polyphenolic compound concentration in the skin ranged from − 3% (3.09 mg/100 g dry matter) after exposure to H-EMF for 5 min, to 2.37 mg/100 g dry matter in the skin exposed to H-EMF for 2 min, which corresponds to a decrease of − 25% of polyphenolic compounds. The concentration range of polyphenolic compounds in the flesh subject to stress factors ranged from 98.20 mg/100 g (− 24%) in the case of H-EMF-2, to 165.30 mg/100 g DM (+ 28%) in the case of US-30, with a concentration level of 129.20 g/100 g dry matter of the control sample.
Phenolic acid
Cluster analysis determined the intergroup relations between the abiotic stress factors and the concentration of phenolic acids in the skin and flesh of pear (Figs. 1, and 2). In the case of pear skin, the factors grouped in cluster area 1 (H-EMF-2, UV-C-60, H-EMF-0.5, L-EMF-60, L-EMF-30) on average decreased the concentration of phenolic acids by − 20.7%; cluster area 2 (H-EMF-5, US-30, US-20) included stress elicitors that resulted in an average decrease of phenolic acid concentration by − 13.8%, whereas cluster area 4 contained the factors that decreased the content of phenolic acids by − 6.2% on average.
Cluster area 3 involved the relation between the control sample and factor US-40, which did not result in a statistically significant change in the concentration of phenolic acids. In the case of flesh (Fig. 2), exposure to the same abiotic stress elicitors as in the skin contributed to the formation of two areas: the first one including stress elicitors (US-20, US-30, UV-C-40, H-EMF-5, H-EMF-0.5) related to the control sample, which caused changes in the concentration of phenolic acid that were not statistically significant . Cluster area 2 consisted of stress factors (UV-C-20, H-EMF-2, UV-C-60, L-EMF-30, L-EMF-60, US-40) that resulted in an average decrease of the phenolic acid level in the flesh by − 20%.
Flavan-3-ols and procyanidins
Changes in the concentration of flavan-3-ols and procyanidins in the skin and flesh of pear under the influence of stress elicitors are presented in Tables 2, 3, 4 and illustrated in Figs. 3, 4. The span of the determined flavan-3-ols and procyanidins in the skin ranged from 701 mg/100 g dry matter, when using H-EMF for 2 min, to 1181.00 mg/100 g dry matter for the same factor used for 5 min, which corresponded to concentration changes from − 29% to + 20% as compared to the control sample.
Statistical processing of data spaces with the use of cluster analysis determined three groups of abiotic stress factors that influenced the variability of the content of flavan-3-ols and procyanidins in the skin of the fruit in question (Fig. 3). Cluster area 1 included abiotic stress factors (US-20, UV-C-40, H-EMF-2) and the control sample whose result differences were statistically insignificant. The aggregation of stress elicitors in cluster area 2 (UV-C-20, L-EMF-60, UV-C-60, US-30, H-EMF-0.5, US-40) resulted in an average decrease of the analysed compounds by − 8% in comparison to the control sample, whereas the stress factors grouped in cluster area 3 (L-EMF-30 and H-EMF-5) increased the concentration of the analysed compound groups of flavan-3-ols and procyanidins by + 19% on average.
The concentration of flavan-3-ols and procyanidins in pear flesh under the influence of abiotic stress factors is presented in Fig. 4. The stress elicitors caused a significant variability of the concentration of flavan-3-ols and procyanidins in the flesh of the fruit in question. The use of cluster analysis as a tool ordering the data area enabled us to separate four groups differing in terms of the influence of stress elicitors on the content of flavan-3-ols and procyanidins in the flesh of pears. The first area comprised of stress factors (UV-C-60, H-EMF-5, UV-C-20, L-EMF-2) that resulted in changes in the content of the analysed compounds those were statistically insignificant as compared to the control sample. Group 2 included factors (UV-C-40, H-EMF-2) that caused an average decrease of − 23% in the concentration of flavan-3-ols and procyanidins.
The aggregation of data in groups 3 and 4 involved stress elicitors that increased the concentration of the analysed compounds.
Cluster 3 included the effects of low-frequency electromagnetic field (L-EMF-60), ultrasounds (US-20, US-40) and high-frequency electromagnetic field (H-EMF-0.5), which caused an increase of the concentration level of flavan-3-ols and procyanidins by + 20% as compared to the control sample. Single element group 4 included the stress factor (US-30) that increased the concentration of the analysed compounds by + 48% on average. The observed changes of flavonol concentration applied only to pear skin, whereas the determined levels in the flesh were below detection limit. The concentration of flavonol in pear skins under the influence of abiotic stress factors is graphically presented in Fig. 5 and Tables 2, 3, 4, 5.
The variability of flavonol concentration in pear skins under the influence of stress elicitors ranged from 751.00 mg/100 g dry matter for the control sample to 300.00 mg/100 g dry matter for skins of fruit exposed to high-frequency electromagnetic field (H-EMF-0.5). In every case, the procedures resulted in a statistically significant decrease of the concentration of flavonol compounds in pear skins.
Discussion
The concentration changes of polyphenolic compounds were mostly caused by heat stress whose effects can be observed during exposure to electromagnetic field.
Vibrations of the electromagnetic field increase the temperature inside cells and activate antioxidant protection mechanisms. According to Shabrangi et al. [19], by changing the electromagnetic field power and exposure time, various biological results can be achieved. Low-frequency fields have a more destructive effect on mitochondria by disrupting calcium channels and thus, reducing the synthesis of antioxidant compounds [20]. The effects of electromagnetic field also depend on the content of dry mass. According to Nabizadeh et al. [21], exposure of dry and soaked pumpkin seeds to electromagnetic field of 2 mT for 15 and 30 min resulted in changes of the determined parameters. Exposure to the field for 15 and 30 min contributed to a decrease in the content of protein and the activity of peroxidase and dismutase in both dry and soaked pumpkin seeds. In the case of catalase, its activity increased only in dry seeds exposed to electromagnetic field for 15 min.
Abiotic stress factors stimulate cells to produce ROS (reactive oxygen species) as well as ABA (abscisic acid), SA (salicylic acid), JA (jasminic acid), ET (ethylene), which are then responsible for the synthesis of MAF kinases, which directly induce the synthesis of genes, MYC, MYB, NAC, ZF, HFS, responsible for initiating the synthesis pathways of secondary metabolites. A too intense stress signal leads to the generation of high concentration of kinases, thus damaging the cell’s genetic material, which determines the synthesis of secondary metabolites [22, 23]. Stress elicitors initiate in plant cells the mechanisms responsible for modifying metabolic pathways towards increased synthesis of secondary metabolites whose main task is to scavenge free radicals [24, 25]. Plant metabolic response to stress conditions is a dynamic process corresponding to the intensity of the stress factor. Adaptation to stress conditions requires extra energy necessary to synthesise protein for antioxidant protection [26, 27]. Many authors [24, 28, 29] showed, that exposure to UVC radiation results in an increased synthesis of polyphenolic compounds, which is a defence response related to increased temperature in plant cells. An increase of temperature inside plant cells also occurs under the influence of waveform factors, i.e. appropriate stimulation with ultrasounds or electromagnetic radiation, leading to the initiation of antioxidant protection mechanisms [30,31,32,33]. Gill and Tuteja [24] showed that a number of flavonoid biosynthesis genes are induced in stress conditions, in particular, when exposed to UV radiation. The most thoroughly analysed abiotic stress factor has been the influence of UV radiation and ultrasounds on the amount of polyphenolic compounds in plants. A research conducted by Chang-Hong et al. [13] showed that after exposure to various doses of UV radiation, tomatoes stored for 35 days demonstrated an increase in the content of gallic acid by 21%, catechins by 26%, chlorogenic acid by 14%, caffeic acid by 37%, quercetin by 38% and p-coumaric acid by 36%. Exposure to ultrasounds causes cavitation in cell organelles, leading to increased permeability of cell membranes by activating calcium channels, which contributes to an increase of enzymatic activity, which probably stimulates an increase of the synthesis of secondary metabolites [34]. Wu and Lin [33] showed that a 2-min exposure to 38.5 Hz US (ultrasound) in a water bath resulted in an increase of the level of polyphenolic compounds in the in vitro cell culture of ginseng suspension and at the same time caused increased activity of enzymes (PPO) and (PO) and decreased water content in cells. Similar results were arrived at by Santos et al. [18] who used 25 kHz ultrasounds for 30 min on freshly sliced mango and achieved a general increase in the polyphenol content by approximately 20%. In turn, Yu et al. [29] used 25 kHz 26 W ultrasounds on romaine lettuce leaves for 1, 2 and 3 min and observed the highest increase of phenolic compounds after 60 h of storage, by 22.5%, 16.3% and 17.9%, respectively. The variability of flavan-3-ols concentration in skins depended on the stress factor. The influence of abiotic stress factors, including ultrasounds, activates the synthesis of secondary metabolites (polyphenolic compounds). The impact, time and frequency are the factors modelling the intensity of enzyme synthesis of the flavonoid pathway. In the discussed case, the observed phenomenon presented in Table 3, related to an increase and then a slight decrease of the polyphenol content, can be a result of changes in the enzyme system of the flavonoid pathway. The direct cause can be the depletion of substrates for enzyme production, but due to insufficient research, further studies are required to establish the reason of such changes.
Conclusion
Pear fruit is valued for its high amount of polyphenolic compounds. The use of abiotic stress factors such as UVC radiation, low-frequency electromagnetic field (L-EMF), high-frequency electromagnetic field (H-EMF) and ultrasounds (US) for various periods resulted in differences in the content of phenolic acids, flavan-3-ols, and procyanidins, and flavonols. Changes in the concentration of phenolic acids in the skin and flesh of pears depended on the abiotic stress factor. The effects of abiotic stress factors differentiated the content of polyphenolic compounds in the skin and flesh of the fruit in question. The presence of flavonols was only observed in pear skin and the level of changes was similar to phenolic acids. The stress used in fractions of pear in all cases lowered the sum of polyphenolic compounds in the peel, while the effect of US noticed the increase in the total polyphenols content in the flesh of pears. The research results show that it is possible to produce juice with a higher content of polyphenolic compounds, because juice is obtained mainly from the flesh; however, there is a need for further research to confirm the observed tendencies in the changes of polyphenolic compounds in fractions of pears.
References
Kaur R, Arya V (2012) Ethnomedicinal and phytochemical perspectives of Pyrus communis Linn. J Pharmacogn Phytochem 1(2):14–19
O’Callaghan N (2015) Final report review on the health benefits and nutritional properties of pear. South Australian Health and Medical Research Institute, North Terrace
Cebulak T, Oszmiański J, Kapusta I, Lachowicz S (2017) Effect of UV-C radiation, ultra-sonication electromagnetic field and microwaves on changes in polyphenolic compounds in chokeberry (Aronia melanocarpa). Molecules 22(7):1161
Mahammad MU, Kamba AS, Abubakar L, Bagna EA (2010) Nutritional composition of pear fruits (Pyrus communis). Afr J Food Scien Tech 1(3):76–81
Öztürk A, Demirsoy L, Demirsoy H, Asan A, Gül O (2015) Phenolic compounds and chemical characteristics of pears (Pyrus Communis L.). Int J Food Prop 18(3):536–546
Parle M, Arzoo (2001) Why is pear so dear. Int J Res Ayurveda Pharm 6 7(Suppl 1):108–113
Simirgiotis M, Quispe C, Bórquez J, Areche C, Sepúlveda B (2016) Fast detection of phenolic compounds in extracts of Easter Pears (Pyrus communis) from the Atacama Desert by ultrahigh-performance liquid chromatography and mass spectrometry (UHPLC–Q/Orbitrap/MS/MS). Molecules 21(1):92
Wang T, Li X, Zhou B, Li H, Zeng J, Gao W (2015) Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J Funct Food 13:276–288
Li X, Wang T, Zhou B, Gao W, Cao J, Huang L (2014) Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem 152:531–538
Đurić G, Žabić M, Rodić M, Stanivuković S, Bosančić B, Pašalić B (2015) Biochemical and pomological assessment of European pear accessions from Bosnia and Herzegovina. Hort Sci 42(4):176–184
Milošević T, Milošević N, Glišić I, Mladenović J (2012) Fruit quality attributes of blackberry grown under limited environmental conditions. Plant Soil Envi 58(7):322–327
Ayala-Zavala JF, Wang SY, Wang CY, González-Aguilar GA (2004) Effect of storage temperatures on antioxidant capacity and aroma compounds in strawberry fruit. LWT Food Sci Technol 37(7):687–695
Liu CH, Cai LY, Lu XY, Han XX, Ying TJ (2012) Effect of postharvest UV-C irradiation on phenolic compound content and antioxidant activity of tomato fruit during storage. J Integr Agric 11(1):159–165
Jagadeesh SL, Charles MT, Gariepy Y, Goyette B, Raghavan GSV, Vigneault C (2011) Influence of postharvest UV-C hormesis on the bioactive components of tomato during post-treatment handling. Food Bioprocess Technol 4(8):1463–1472
Yu J, Engeseth NJ, Feng H (2016) High intensity ultrasound as an abiotic elicitor—effects on antioxidant capacity and overall quality of romaine lettuce. Food Bioprocess Technol 9(2):262–273
Kolniak-Ostek J, Oszmiański J (2015) Characterization of phenolic compounds in different anatomical pear (Pyrus communis L.) parts by ultra-performance liquid chromatography photodiode detector-quadrupole/time of flight-mass spectrometry (UPLC-PDA-Q/TOF-MS). Int J Mass Spectrom 392:154–163
Schieber A, Keller P, Carle R (2001) Determination of phenolic acids and flavonoids of apple and pear by high-performance liquid chromatography. J Chromatogr A 910(2):265–273
Santos JG, Fernandes FAN, de Siqueira Oliveira L, de Miranda MRA (2015) Influence of ultrasound on fresh-cut mango quality through evaluation of enzymatic and oxidative metabolism. Food Bio Tech 8(7):1532–1542
Shabrangi A, Majd A (2009) Effect of magnetic fields on growth and antioxidant systems in agricultural plants. In: PIERS Proceedings, Beijing, China, March, 23–27
Belyavskaya NA (2004) Biological effects due to weak magnetic field on plants. Adv Space Res 34(7):1566–1574
Nabizadeh S, Majd A, Arbabiyan S, Mirzai M, Sharifnia F (2014) Assessment of the effect of electromagnetic fields on biochemical and antioxidant parameter changes of Cucurbita maxima Duchesne. Adv Environ Biol 8:185–194
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Cur Opinion Plant Biol 9(4):436–442
Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017) Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8:161
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48(12):909–930
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Kosová K, Vítámvás P, Prášil IT, Renaut J (2011) Plant proteome changes under abiotic stress—contribution of proteomics studies to understanding plant stress response. J Proteom 74(8):1301–1322
Mirouze M, Paszkowski J (2011) Epigenetic contribution to stress adaptation in plants. Cur Opinion Plant Biol 14(3):267–274
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. Crop stress and its management: perspectives and strategies. Springer, Dordrecht, pp 261–315
Yu J, Engeseth NJ, Feng H (2016) High intensity ultrasound as an abiotic elicitor—Effects on antioxidant capacity and overall quality of romaine lettuce. Food Bio Tech 9(2):262–273
Monselise EBI, Parola AH, Kost D (2003) Low-frequency electromagnetic fields induce a stress effect upon higher plants, as evident by the universal stress signal, alanine. Biochem Bioph Res Commun 302(2):427–434
Da Silva JAT, Dobránszki J (2016) Magnetic fields: how is plant growth and development impacted? Protoplasma 253(2):231–248
Wang C, Yang A, Yin H, Zhang J (2008) Influence of water stress on endogenous hormone contents and cell damage of maize seedlings. J Integr Plant Biol 50(4):427–434
Wu J, Lin L (2002) Ultrasound-Induced Stress Responses of Panax ginsengCells: enzymatic Browning and Phenolics Production. Biotech Progress 18(4):862–866
Kwiatkowska B, Bennett J, Akunna J, Walker GM, Bremner DH (2011) Stimulation of bioprocesses by ultrasound. Biotech Adv 29(6):768–780
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethics requirement
This article does not contain any studies with human or animal subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cebulak, T., Oszmiański, J., Kapusta, I. et al. Effect of abiotic stress factors on polyphenolic content in the skin and flesh of pear by UPLC-PDA-Q/TOF-MS. Eur Food Res Technol 245, 2715–2725 (2019). https://doi.org/10.1007/s00217-019-03392-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-019-03392-z