Abstract
LuxS-mediated quorum-sensing mechanism, which was based on the production of universal signal molecule called autoinducer-2 (AI-2), regulates important physiological traits and a variety of adaptive processes in different bacteria. But little was known about AI-2/LuxS of lactobacillus bacteria from fermented meat. Bacterial strains of the species Lactobacillus sakei; Lactobacillus sp. and Lactobacillus plantarum, all isolated from Chinese fermented meat, were found to possess AI-2 activity and LuxS every 3 h during 36 h. In contrast, all the strains were found to have AI-2 activity and the full-length cDNA sequences of LuxS gene were obtained. The qRT-PCR analysis indicated that all LuxS gene showed strongly up-regulated after high nitrate concentration shock. The changes of LuxS during 36 h were shown in wavy curve rather than parabolic curve different from the lasted reports. The fact that AI-2 activity and LuxS gene are produced by bacterial strains found in the Chinese fermented meat. And also increased by meat-relevant stress conditions, indicated that AI-2/LuxS signaling might be important in regulation of microbial succession during ripened of Chinese fermented meat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nitrite was approved for use in most countries in fermented meat for its helpful to product color and antiseptic effect. Because of its current use of additives also could not be substituted by other alternative, nitrite was still widely used in meat processing. So the adaption of strains after high nitrate concentration shock was important selected criteria to the starter in fermented meat.
Quorum sensing (QS) was the process of cell-to-cell signaling that enables bacteria to collectively control gene expression, thereby coordinating activities that were productive only at a high population density. It was further shown that bacterial processes [1–7, 13] such as biofilm formation [10], bioluminescence, motility, virulence factor secretion, antibiotic production, and sporulation could be controlled by QS systems.
Autoinducer-2 (AI-2)-mediated QS had been extensively studied in relation to the regulation of microbial behavior [8, 9], which played a unique role as it was the only presently known species-nonspecific signaling molecule produced by both Gram-negative and Gram-positive bacteria. AI-2 was produced by LuxS, an enzyme found in many bacterial species and thus proposed to enable interspecies communication [17]. The LuxS gene was present in several Gram-positive and Gram-negative bacterial species, but few species have been definitively shown to use an AI-2/LuxS quorum-sensing system, especially as fermented starter in food [11, 12, 14, 16].
Among food-relevant bacteria, AI-2 had been found to control biofilm formation in Listeria monocytogenes and virulence in both Staphylococcus aureus and Escherichia coli. However, AI-2-controlled behaviors were not only restricted to pathogenic bacteria [18, 19] but are also found among food-relevant starter cultures and probiotic bacteria such as acid stress regulation in Lactococcus lactis and Lactobacillus spp., respectively [13, 30].
Lactic acid bacteria (LAB) constitute a huge and heterogeneous group of food-grade microorganisms, including many genera, such as Lactobacillus, Enterococcus, Lactococcus, Leuconostoc, and Streptococcus that played an essential role in the fermentation of animal and vegetable raw materials, including milk, meat and fish, vegetables, cocoa, and coffee.
Lactobacillus was the most wildly used food starter. Previous studies had shown that LuxS homologs had been found in the genome of different lactobacilli [20–22] and production of AI-2 by some Lactobacillus species had been investigated earlier [8] had investigated the functional role of the LuxS gene in the probiotic bacterium Lactobacillus rhamnosus GG. They found that mutation of this gene had apheliotropic physiological effects on the growth of L. rhamnosus GG and also the mutation made a defect in mono-species biofilm formation. The complex nutritional requirements of the mutant showed disruption of many interdependent metabolic fluxes, which indicates a central metabolic role for the LuxS gene in L. rhamnosus GG. Involvement of LuxS gene in the regulation of environmental stresses such as acidic stress has been also proven in lactobacillus spp. [30].
To the best of our knowledge, no previous studies had focused on AI-2/LuxS-controlled behaviors in fermented food bacteria. The aim of the present study was to determine whether bacterial strains belonging to species involved in fermented food had the ability to produce AI-2/LuxS and how they played the rule in fermented procession.
In the case of AI-2/LuxS, the influence of important fermented-relevant stress conditions as high nitrate concentration shock on the AI-2/LuxS activity was investigated.
Materials and methods
Strains and propagation
The LAB strains used in this study were isolated from samples taken from Chinese fermented meat (6 samples). The LAB strains used in this study were identificated by Sangon Biotech Ltd. (Shanghai, China) to be (1) Lactobacillus plantarum strain F, (2) Lactobacillus sakei strain L4 and (3) Lactobacillus plantarum strain R based on biochemical tests, and a 1.5-kbp sequence of the 16S rRNA gene region which was fully determined and analyzed by the Clustal W program.
Samples were spread-plated on the following agar media within 12 h after collection: de Man–Rogosa–Sharpe (MRS) agar, modified MRS (mMRS) agar containing 50 g/L of sucrose instead of glucose. Agar media were incubated at 37 °C for 12 h. Subsequently, mucoid and ropy colonies were picked up, purified, and tested for catalase activity. Catalase-negative bacteria displaying a mucoid or ropy phenotype were stored at −80 °C in their corresponding isolation medium supplemented with 25 % (v/v) of glycerol as a cryoprotectant.
Growth of bacterial strains
After the second propagation step, the cultures were used for inoculation of 100 mL of MRS (OD600 nm 0.03 corresponding to 1 × 10−6 cfu). Growth was performed at 37 °C with shaking at 120 rpm and followed by measurement of the optical density at 600 nm using a PUXI UV spectrophotometer (PUXI, Bei Jin, China). Lag phases (l) were determined by extrapolation of the intercept between the line of OD0 h and the exponential phase line.
Preparation of cell-free supernatants
Cell-free supernatants from the smear bacteria were prepared by removing cells from growth medium by centrifugation (4,000g for 10 min) followed by passing the supernatants through 0.20 mm filters (DGS02025SO, Millipore, Billerica, MA, USA). Cell-free supernatants were stored at 4 °C before use.
Autoinducer-2 bioluminescence assay [24]
AI-2 activity was determined by the AI-2 bioluminescence assay, which relies on the ability of a Vibrio harveyi reporter strain (BB170) to produce bioluminescence in response to AI-2. Briefly, the V. harveyi BB170 reporter strain (AI-1 sensor negative and AI-2 sensor positive) was grown with aeration (120 rpm) at 30 °C for 16 h in AB medium [14]. The culture was diluted 1:5000 into fresh AB medium and cell-free supernatants from the strains listed in Table 1 were added to a final concentration of 10 % (v/v). Cell-free supernatant obtained from V. harveyi BB152 (expresses AI-2 but not AI-1) grown in AB media was used as positive control, whereas sterile growth medium (TSB) was used as negative control. The 96-well plate (237108 k, Nunc, Roskilde, Denmark)was incubated at 30 °C, and light measurement was performed every 15 min using the FLUOstar Optima Microplate Reader (BMG LABTECH, Durham, NC, USA). Maximal stimulation of V. harveyi BB170 bioluminescence occurred between 4 and 5 h after addition of cell-free supernatant. Data are reported as relative bioluminescence (%), where the positive and negative controls are set to 100 and 0 %, respectively.
Effect of high nitrate concentrations on expression of LuxS gene [23]
At late exponential phase (OD600 nm 2.0e4.0), cultures of bacteria were divided into 3 aliquots, centrifuged (4,000g for 10 min) and supernatants were removed. For determination of the influence of high nitrate concentrations on expression of LuxS, cells were resuspended in MRS with 0 %, 0.005 %, 0.01 % (w/v) nitrate (NaNO3, 306 C). After 3, 9, 15, 21, 27, 33 and 36 h of incubation, cell were prepared and tested for expression of LuxS gene. The GAPDH of lactobacillus was used as an internal control for transcript normalization.
RNA isolation and qRT-PCR [33]
Total RNA was extracted from Lactobacillus using the RNAprep Pure Kit (TaKaRa) following the manufacturer’s instruction. CDNA was transcribed from 0.5 µg of each total RNA using a PrimeScript RT Reagent Kit (TaKaRa). qRT-PCR was performed using 2 µg of CDNA product and each primer of 0.4 µM in a 25 µL reaction volume with SYBR Premix Ex TaqTM(Perfect Real Time; TaKaRa) on a Mini-Opticon real-time PCR system(Bio-Rad, Hercules, USA). The specific primers used for qRT-PCR analysis were designed on the basis of the 3′ or 5′-untranslated regions (UTR) of individual genes using the Primer Premier 5 software (http://www.premieriosoft.com/primerdesign/), and the primer sets finally were run using a program: 95 °C for 30 s, followed by 40 cycles (95 °C for 5 s and 60 °C for 30 s).The amplification efficiencies for all genes tested in this study ranged 95–110 %.Data were analyzed according to the threshold cycle (C t).The relative changes in gene expression were quantified using the 2−△△Ct method. Differences indicated in Fig. 5 were based on Duncan’s multiple range tests using DPS software(www.chinadps.net).
Determination of nitrate reduction from the three strains [15]
The capability of the three strains to reduce nitrate was evaluated by determining their residue amount in each model-medium (0.005 and 0.01 %). The quantitative evaluation was carried out with the cuvette tests according to the enclosed instructions. A test tube containing 1 mL sample solution from each model-medium was first led in water bath at 80 °C for 15 min to stop enzymatic processes and then centrifuged (16RS, Beckman, USA) at 10,000 g for at least 1 min at room temperature(25 °C).The supernatant was filtered with filter paper No.595 1/2 in a funnel. The supernatant was kept at −72 °C in Safe-Lock tubes (Eppendorf-Netheler-Hanz GmbH, Germany) until used for the enzymatic analysis. The test for nitrate was based on the photo metric measurement of NADPH. In the presence of the enzyme nitrate reductase, nitrate is reduced to nitrite by NADPH. The amount of NADPH oxidized during the reaction is linearly proportional to the amount of nitrate. The amount of reduced nitrate was determined by measuring the decrease in NADPH by means of its light absorbance at 340 nm. The samples of test were evaluated every 3, 9, 15, 21, 27, 33 and 36 h.
Results and discussion
The QS system involving AI-2/LuxS appeared to be the most widespread WS system among bacterial species. Many bacteria had been reported previously to use QS systems to adapt to environmental changes [26, 28, 30]. In this study, increased LuxS expression was observed when Lactobacillus plantarum strain F, Lactobacillus sakei strain L4, Lactobacillus plantarum strain R were transferred to an environment with high nitrate concentrations (0.005 % and 0.01 %, w/v).
Alignment of LuxS and phylogenetic relationship analysis
In our studies, three LuxS sequences were isolated from lactobacillus bacteria in Chinese fermented meat, and BLAST similarity searching against the GenBank database showed they had some similarities to each other. To obtain the complete LuxS sequences corresponding to the three bacteria in fermented meat, 3′ and 5′ RACE were performed. After sequencing, three full-length LuxS of 452, 446, and 443 bp were found (Figs. 1, 2, 3).
Alignment of protein sequences revealed that functional protein domains were retained in all LuxS sequences (Fig. 3). Not only were three highly conserved sites critical for zinc binding (His 58, and His 61) observed clearly in this suspected LuxS specie from strains (Fig. 3), but also a recently reported amino acid (Gly82)was present that is required for AI-2 production (Plummer et al. 2011).
Predicted by the Protean program in DNAstar software, the deduced translation product of LuxS from strain F consisted of 158 amino acids with a relative molecular mass of 17,320.8 Da and a theoretical isoelectric point of 6.19. In case of LuxS from strain R, it encoded a 156 amino acid and 17,102.4 Da protein, whose theoretical isoelectric point was 5.68. The deduced translation products of LuxS from L4 strain consisted of 157 amino acids with a relative molecular mass of 17,350.7 Da and a theoretical isoelectric of 5.93.
No typical signal peptides were found in all LuxS after analyzing their N-terminals using the SignalP software. Moreover, the transmembrane topology predictions of three LuxS were carried out with TMHMM 2.0 software. The results showed that there was a weak internal transmembrane segment in them (Figs. 1, 2, 3).
AI-2 activity at standard growth conditions in 36 h
With respect to AI-2 activity at standard growth conditions, Gori et al. [19] and Moslehi-Jenabian [30] found that five strains including Arthrobacter nicotianae 20123, Corynebacterium ammoniagenes 20305, ammoniagenes 20306, Corynebacterium casei 44701 and Microbacterium barkeri 20145 have AI-2 activity. The AI-2 activity in them increased during 8 to 14 h and then decreased after that every 2 h. However, the AI-2 activity was not found after 16 h.
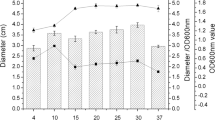
As seen in Fig. 1, AI-2 activity was determined in all the three supernatants of the bacterial strains fermented meat. AI-2 of (a) and (b) strain augments dramatically in the mid-log phase and decreases during the stationary phase. While, the AI-2 activity of (c) increased before the mid-log phases, then also decreases during the stationary phase.
The AI-2 activity patterns may be depended upon their own growth curve [25, 27, 29] (Fig. 4). It was noticed that strain (a) and strain (b) had the similar AI-2 activity curves different from curves of strain (c) the same as their similar growth curve (Fig. 4).
Strain (c) was found to have the higher AI-2 activity rather than in strain (a) and strain (b). The maximum AI-2 activity was found in strain (c) could be detected at 3-6 h of fermentation, whereas for the remaining two strains, the maximum AI-2 activity was detected at 18–21 h of fermentation. It was also worth noting that the AI-2 of the three tested strains was seemed like wavy curve rather than parabolic curve. AI-2 activity was may be correlated to the maximum specific growth rate, when AI-2 activity was observed earlier in the bacterial strains growing with the fastest maximum specific growth rate (Fig. 4).
Multiple alignments of LuxS homolog from (a) Lactobacillus plantarum strain F, (b) Lactobacillus sakei strain L4, (c) Lactobacillus plantarum strain R with the related LuxS proteins. The multiple alignment was conducted using ClustalW2 (http://www.ebi.ac.uk/Tools/clustalw2/index.html), and the final output was expressed through processing of program ESPript 2.2 (http://www.espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi). The protein sequences of LuxS protein used here are sampled from a collection of selected bacteria, which included Lactobacillus plantarum strain 1, Lactobacillus plantarum strain SQ332, Lactobacillus reuteri strain 100-23, Lactobacillus reuteri strain RC-14, Lactobacillus reuteri 2. Three conserved zinc-binding sites (H57 and H61) are indicated with black triangles. A critical amino acid G81 was also very conserved (highlighted with an arrow), which was recently found to be an active site for AI-2 production in Campylobacter jejuni
Transcriptional analysis of the LuxS gene after high nitrate concentration shock
To further investigate the effect of high nitrate concentration shock on the AI-2 activity at transcription level, the transcription level of the LuxS gene was monitored in (1) Lactobacillus plantarum strain F, (2) Lactobacillus sakei strain L4 and (3) Lactobacillus plantarum strain R after exposure to high nitrate concentration shock with qRT-PCR. The housekeeping gene GAPDH was used as internal control. It was verified that nitrate stress has obvious influence on the transcription level of this gene (Fig. 5, Table 2).
Autoinducer-2 (AI-2) activity in supernatants of (a) Lactobacillus plantarum strain F, b Lactobacillus sakei strain L4, c Lactobacillus plantarum strain R determined by the Vibrio harveyi BB170 bioluminescence assay. Supernatants were collected after 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33 and 36 h of growth. The level of AI-2 activity for V. harveyi BB170 was set to 100 %, whereas sterile growth medium was set to 0 %. Results are the average of three independent determinations (the error bars indicate standard deviations). Growth curves are included to illustrate that the observed AI-2 activities were produced in a growth-dependent manner
After nitrate attack, the transcript levels of all strains obviously increased than without attack. In all the three strains, the transcription level of the LuxS gene was found to increase to high nitrate concentration evidently. And the increased level of the LuxS gene was correlated with the concentration of nitrate. As seen in Fig. 5, for the bacterial strains, the higher increases in LuxS gene for were observed in higher nitrate concentration. However, the increase of LuxS gene in the three strains was not at equal level under the same nitrate shock, which maybe subject to different tolerance to nitrates of the strains. For crest, if the strain had better adaptability, the peaks appeared less. To the strain (a) and (b), there were only one main peak (at 15 h) and anther small peak (at 33 h),while three peaks (at 3, 21, 36 h), were found in 36 h in strain (c). And when the nitrate concentration reached 0.01 %, three peaks also were found in strain (b).From all above, bacterial strains belonging to lactobacillus found on fermented meat were found to produce AI-2 activity and express LuxS gene every 3 h from 0 to 36 h exposed to high nitrate concentration stress in this study.
Relative levels of LuxS expression of strain (a), (b) and (c) after various inductions. GAPDH was used as internal control. Data represent the mean ± SD (n = 6) of six samples. Different letters above bars represent significant differences at P < 0.05. The experiments were repeated 3 times with similar results
The exact consequences of AI-2 production and LuxS genes by fermented remain unclear. However, as a nonspecific QS molecule involved in stress regulation, AI-2/LuxS might play an important role in the succession of the complex microbial flora found on fermented meat.
Increased knowledge of how starter cultures as well as lactobacillus in fermented products use QS to control growth may be of particular importance to obtain high quality and safety. Starter cultures might be stimulated by the addition of the so-called pro-QS compounds resulting in increased microbial communication, which could be used to ensure the establishment and growth of starter cultures and thus stimulate important technological properties. AI-2/LuxS seems most relevant for bacterial species such as lactobacillus present in the early stages of ripening, where the Chinese fermented meat is characterized by high concentration of nitrate. In most publications to date, LuxS was assumed to be dedicated to AI-2 production and cell-to-cell communication, and further physiological functions had rarely been taken into consideration. Only a few reports have pointed out that LuxS played a role in a recycling pathway linked to methionine metabolism, which may, in fact, be its most important function in many organisms. Indeed, when Miller and Duerre (1968) first analyzed this enzyme in E. coli more than three decades ago, it was in the context of SAH degradation and methionine biosynthesis [31–33]. It was now clear that LuxS fulfills a role in a metabolic cycle termed the “activated methyl cycle” [34, 35] (Fig. 5, Table 2).
Nitrate reduction of the three strains after high nitrate concentration shock
In order to investigate the nitrate reductase activity of the three strains further, the remaining of nitrate content was also frequently measured during the period of 36 h. The results were calculated as a percentage of the start concentration as presented in Fig. 6.
In the present work, the tested three strains all showed nitrate reductase capabilities with the remnants of nitrate ranged from 78.21 to 87.53 % of the start concentration (0.005 %) after 36 h fermentation. While the remnants of nitrate ranged from 91.13 to 94.10 % of the start concentration (0.01 %) after 36 h fermentation. Both in the two nitrate broth, nitrate reductase capabilities with the remnants of nitrate of (a) (b) and (c) strains all decreased during 36 h. However, to all the three strains, the remnants of nitrate were found to increase in high nitrate concentration evidently, which was may be due to the decrease of OD value of the strain. However, the regulation of nitrate reductase from the three strains was not inconsistent with the LuxS genes in the same fermentation time (36 h).
These nitrate reduction was mainly achieved during the initial phase of fermentation where a shardly any further nitrate was reduced after 15 h. It was found there were negative correlation between AI-2 and the nitrate reduction of (a) and (b) strain during 15 h, the positive correlation of strain (c) during 12 h. To the LuxS genes, there were very little relation between the reduction of nitrate with LuxS genes. There was one point was sure that both of the reduction of nitrate and the LuxS genes were all decreased in 0.01 % (w/v) than in 0.005 % (w/v).The reason for this may have been the pH decrease, the tolerance of strains and the concentration nitration in the medium all influencing the activity of the nitrate reductase. It was still need further study about the LuxS genes how to regulate activity of nitrate reductase.
Conclusions
In conclusion, AI-2/LuxS activity was shown in supernatants from strains of three lactobacillus strains (all found in the Chinese fermented meat). Expression of LuxS was increased by meat-relevant stress conditions as high nitrate concentrations indicating that AI-2/LuxS signaling has a regulating role in stress adaptation in these bacteria. The results indicate that AI-2/LuxS signaling might take place different change law of bacteria found in Chinese fermented meat. These results indicate that LuxS-mediated QS via AI-2 activity could be also involved in nitrate stress response in Lactobacillus.
References
Azcarate-Peril MA, McAuliffe O, Altermann E, Lick S, Russell WM, Klaenhammer TR (2005) Microarray analysis of a two component regulatory system involved in acid resistance and proteolytic activity in Lactobacillus acidophilus. Appl Environ Microbiol 71:5794–5804
Buck BL, Azcarate-Peril MA, Klaenhammer TR (2009) Role of autoinducer-2 on the adhesion ability of Lactobacillus acidophilus. J Appl Microbiol 107:269–279
Challan Belval S, Gal L, Margiewes S, Garmyn D, Piveteau P, Guzzo J (2006) Assessment of the roles of LuxS, S-ribosyl homocysteine, and autoinducer 2 in cell attachment during biofilm formation by Listeria monocytogenes EGD-e. Appl Environ Microbiol 72:2644–2650
Chiang-Ni Chuan, Zheng Po-Xing (2012) Environmental pH changes, but not the LuxS signaling pathway, regulate SpeB expression in M1 group A streptococci. J Med Microbiol 61:16–22
Cotter PD, Hill C (2003) Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol Mol Biol Rev 67:429–453
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus :a review. Proteomics 4:106–122
De Angelis M, Bini L, Pallini V, Cocconcelli PS, Gobbetti M (2001) The acid-stress response in Lactobacillus sanfranciscensis CB1. Microbiology 147:1863–1873
DeKeersmaecker SCJ, Vanderleyden J (2003) Constraints on detection of autoinducer-2 (AI-2) signalling molecules using Vibrio harveyi as a reporter. Microbiol Comment 149:1953–1956
Ding W, Wang H, Griffiths MW (2005) Probiotics down-regulate flaAσ28 promoter in Campylobacter jejuni. J Food Prot 68:2295–2300
Domka J, Lee J, Bansal T, Wood TK (2007) Tempora l gene expression in Escherichia coli K-12 bio films. Environ Microbiol 9:332–346
Elvers KT, Park SF (2002) Quorum sensing in Campylobacter jejuni: detection of a luxS encoded signalling molecule. Microbi ology 148:1475–1481
Federle MJ, Bassler BL (2003) Interspecies communication in bacteria. J Clin Invest 112:1291–1299
Frees D, Vogensen FK, Ingmer H (2003) Identification of proteins induced at low pH in Lactococcus lactis. Int J Food Microbiol 87:293–300
Greenberg EP, Hastings JW, Ulitzur S (1979) Induction of luciferase synthesis in Beneckea harveyi by other marine bacteria. Arch Microbiol 120:87–91
Paik Hyun-Dong, Lee Joo-Yeon (2014) Investigation of reduction and tolerance capability of lactic acid bacteria isolated from kimchi against nitrate and nitrite in fermented sausage condition. J Meat Sci 97:609–614
Isolauri E, Salminen S, Ouwehand AC (2004) Microbial–gut interactions in health and disease probiotics. Best Pract Res Clin Gastroenterol 18:299–313
Kaper JB, Sperandio V (2005) Bacterial cell-to-cell signaling in the gastrointestinal tract. Infect Immun 73:3197–3209
Kim Y, Oh S, Ahn EY, Imm JY, Oh S, Park S, Kim SH (2007) Proteome analysis of virulence factor regulated by autoinducer-2-like activity in Escherichia coli O157:H7. J Food Prot 70:300–307
Gori Klaus, Moslehi-Jenabian Saloomeh, Purrotti Micol, Jespersen Lene (2011) Autoinducer-2 activity produced by bacteria found in smear of surface ripened cheese. J Dairy Sci 21:48–53
Klein G, Pack A, Bonaparte C, Reuter G (1998) Taxonomy and physiology of probiotic lactic acid bacteria. Int J Food Microbiol 41:103–125
Lebeer S, Claes IJ, Verhoeven TL, Shen C, Lambrichts I, Ceuppens JL, Vanderleyden J, De Keersmaecker SC (2008) Impact of luxS and suppressor mutations on the gastrointestinal transit of Lactobacillus rhamnosus GG. Appl Environ Microbiol 74:4711–4718
Lebeer S, De Keersmaecker SCJ (2007) Functional analysis of luxS in the probiotic strain Lactobacillus rhamnosus GG reveals a central metabolic role important for growth and biofilm formation. J Bacteriol 189:860–871
Marco ML, Kleerebezem M (2008) Assessment of real-time RT-PCR for quantification of Lactobacillus plantarum gene expression during stationary phase and nutrient starvation. J Appl Microbiol 104:587–594
McNab R, Ford SK, El-Sabaeny A, Barbieri B, Cook GS, Lamont RJ (2003) LuxS-based signaling in Streptococcus gordonii: autoinducer-2 controls carbohydrate metabolism and biofilm formation with porphyromonas gingivalis. J Bacteriol 185:274–284
Medellin-Peña MJ, Griffiths MW (2009) Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Appl Environ Microbiol 75:1165–1172
Cao Min, Feng Youjun (2011) Functional definition of LuxS, an autoinducer-2 (AI-2) synthase and its role in full virulence of Streptococcus suis Serotye 2. J Microbiol 49:1000–1011
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45
Phongsisay V, Perera VN, Fry BN (2007) Expression of the htrB gene is essential for responsiveness of Salmonella typhimurium and Campylobacter jejuni to harsh environments. Microbiology 153:254–262
Rogers PD, Liu TT (2007) Gene expression profiling of the response of Streptococcus pneumoniae to penicillin. J Antimicrob Chemother 59:616–626
Moslehi-Jenabian Saloomeh, Purrotti Micol, Jespersen Lene, Gori Klaus (2009) AI-2 signaling is induced by acidic shock in probiotic strain of Lactobacillus spp. J Food Microbiol 135:295–302
Sakarya S, Gokturk C, Ozturk T, Ertugrul MB (2010) Sialic acid is required for nonspecific adherence of Salmonella enterica ssp. enterica serovar typhi on caco-2 cells. FEMS Immunol Med Microbiol 58:330–335
Schauder S, Shokat K, Surette MG, Bassler BL (2001) The LuxS family of bacterial autoinducers: biosynthesis of a novel quorum-sensing signal molecule. Mol Microbiol 41:463–476
Schmittgen TD, Zakrajsek BA (2000) Effect of experimental treatment on housekeeping gene expression: validation by real time, quantitative RT-PCR. J Biochem Biophys Methods 4:69–81
Shao H, Lamont RJ, Demuth DR (2007) Autoinducer 2 is required for biofilm growth of aggregatibacter (actinobacillus) actinomycetemcomitans. Infect Immun 75:4211–4218
Siller M, Janapatla RP, Pirzada ZA, Hassler C, Zinkl D (2008) Functional analysis of the group a Streptococcal LuxS/AI-2 system in metabolism, adaptation to stress a interaction with host cells. BMC Microbiol 8:188
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lin, M., Zhou, GH., Wang, ZG. et al. Functional analysis of AI-2/LuxS from bacteria in Chinese fermented meat after high nitrate concentration shock. Eur Food Res Technol 240, 119–127 (2015). https://doi.org/10.1007/s00217-014-2313-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2313-x