Abstract
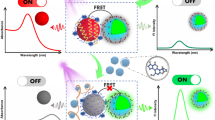
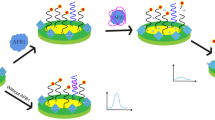
Aflatoxins represent an important class of mycotoxins that are known to be mutagenic, carcinogenic, and teratogenic. Here, we report the use of the systematic evolution of ligands by exponential enrichment technology to screen for a DNA aptamer that recognizes Aflatoxin B1 (AFB1) with high affinity and specificity. AFB1 was first attached to magnetic nanoparticles and then incubated with an ssDNA library. After ten rounds of screening and amplification, 30 aptamer sequences were obtained following enrichment. Combined with a homological and structural analysis and affinity and specificity experiments, aptamer sequence 1, possessing the best affinity and specificity toward AFB1, was finally obtained. The dissociation constants value for aptamer sequence 1 was 11.39 nM. And, the specificity experiment results showed the binding between AFB1 aptamer with five other toxins was very week (did not exceed 15 % compared with AFB1). To demonstrate the potential use of this aptamer for quantitative analysis, a fluorescent bioassay with aptamer 1 was developed. The assay showed a wide linear range, with the AFB1 concentration ranging from 50 to 1,500 ng/L and a detection limit of 35 ng/L. Additionally, the spiked recovery experiment of AFB1 in peanut oil sample exhibited a recovery ratio between 94.2 and 101.2 % which showed good accuracy of the proposed aptamer-based bioassay. This fluorescent method represents a powerful tool for use in the detection of AFB1 without complex sample treatments.




Similar content being viewed by others
References
Bennett JW, Klich M (2003) Mycotoxins. Clin Microbiol Rev 16:497–516
Bircan C (2009) Incidence of ochratoxin A in dried fruits and co-occurrence with aflatoxins in dried figs. Food Chem Toxicol 47:1996–2001
Mahnine N, Meca G, Elabidi A, Fekhaoui M, Saoiabi A, Font G, Manes J, Zinedine A (2011) Further data on the levels of emerging Fusarium mycotoxins enniatins (A, A1, B, B1), beauvericin and fusaproliferin in breakfast and infant cereals from Morocco. Food Chem 124:481–485
Meca G, Zinedine A, Blesa J, Font G, Manes J (2010) Further data on the presence of Fusarium emerging mycotoxins enniatins, fusaproliferin and beauvericin in cereals available on the Spanish markets. Food Chem Toxicol 48:1412–1416
Oueslati S, Meca G, Mliki A, Ghorbel A, Manes J (2011) Determination of Fusarium mycotoxins enniatins, beauvericin and fusaproliferin in cereals and derived products from Tunisia. Food Control 22:1373–1377
Yildiz O (2013) Physicochemical and sensory properties of mulberry products: gümüşhane pestil and köme. Turk J Agric For 37:762–771
Tabata S, Kamimura H, Ibe A, Hashimoto H, Iida M, Tamura Y, Nishima T (1993) Aflatoxin contamination in foods and foodstuffs in Tokyo-1986–1990. J AOAC Int 76:32–35
Koppen R, Koch M, Siegel D, Merkel S, Maul R, Nehls I (2010) Determination of mycotoxins in foods: current state of analytical methods and limitations. Appl Microbiol Biotechnol 86:1595–1612
Alexander L, Peter Z, Ada P, Jorg S, Andri PB, Sabine J, Elke A, Wolfgang L (2002) Comparison of methods for the determination of ochratoxin A in wine. Anal Chim Acta 453:33–41
Lee NA, Wang S, Allan RD, Kennedy IR (2004) A rapid Aflatoxin B1 ELISA: development and validation with reduced matrix effects for peanuts, corn, pistachio, and soybeans. J Agric Food Chem 52:2746–2755
Kamika I, Takoy LL (2011) Natural occurrence of Aflatoxin B1 in peanut collected from Kinshasa, democratic Republic of Congo. Food Control 22:1760–1764
Hussain I, Anwar J, Munawar MA, Asi MR (2008) Variation of levels of aflatoxin M1 in raw milk from different localities in the central areas of Punjab, Pakistan. Food Control 19:1126–1129
Jestoi M, Rokka M, Jarvenpaa E, Peltonen K (2009) Determination of Fusarium mycotoxins beauvericin and enniatins (A, A1, B, B1) in eggs of laying hens using liquid chromatography–tandem mass spectrometry (LC–MS/MS). Food Chem 115:1120–1127
Soblev VS, Dorner JW (2002) Cleanup procedure for determination of aflatoxins in major agricultural commodities by liquid chromatography. J AOAC Int 85:642–645
Saha D, Acharya D, Roy D, Shrestha D, Dhar T (2007) Simultaneous enzyme immunoassay for the screening of Aflatoxin B1 and ochratoxin A in chili samples. Anal Chim Acta 584:343–349
Chen YQ, Wang ZQ, Wang ZH, Tang SS, Zhu Y, Xiao XL (2008) Rapid enzyme-linked immunosorbent assay and colloidal gold immunoassay for kanamycin and tobramycin in swine tissues. J Agric Food Chem 56:2944–2952
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Andrew DE, Jack WS (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Famulok M, Hartig JS, Mayer G (2007) Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem Rev 107:3715–3743
Breaker RR (2004) Natural and engineered nucleic acids as tools to explore biology. Nature 432:838–845
Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W (2006) Aptamers evolved from live cells as effective molecular probes for cancer study. Proc Natl Acad Sci USA 103:11838–11843
Fang XH, Tan WH (2010) Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc Chem Res 43:48–57
Vinkenborg JL, Karnowski N, Famulok M (2011) Aptamers for allosteric regulation. Nat Chem Biol 7:519–527
Iliuk AB, Hu L, Tao WA (2011) Aptamer in bioanalytical applications. Anal Chem 83:4440–4452
Famulok M, Mayer G (2011) Aptamer modules as sensors and detectors. Acc Chem Res 44:1349–1358
Ito Y, Kawazoe N, Imanishi Y (2000) In vitro selected oligonucleotides as receptors in binding assays. Methods 22:107–114
Ellington AD, Szostak JW (1992) Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 355:850–852
Cox JC, Ellington AD (2001) Automated selection of anti-protein aptamers. Bioorg Med Chem 9:2525–2531
Huang CJ, Lin HI, Shiesh SC, Lee GB (2010) Integrated microfluidic system for rapid screening of CRP aptamers utilizing systematic evolution of ligands by exponential enrichment (SELEX). Biosens Bioelectron 25:1761–1766
Berezovski M, Musheev M, Drabovich A, Krylov SN (2006) Non-SELEX selection of aptamers. J Am Chem Soc 128:1410–1411
Gendloff EH, Casale WL, Ram HP, Tai JH, Pestka JJ, Hart LP (1986) Hapten–protein conjugates prepared by the mixed anhydride method: cross-reactive antibodies in heterologous antisera. J Immunol Methods 92:15–20
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 21375049), National S&T Support Program of China (2012BAK08B01), the S&T Supporting Project of Jiangsu (BE2011621, BE2012614), the Research Fund for the Doctoral Program of Higher Education (20110093110002), JSCIQ_2012IK166, JUSRP51309A and NCET-11-0663, the Fundamental Research Funds for the Central Universities (Grant JUSRP11224).
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ma, X., Wang, W., Chen, X. et al. Selection, identification, and application of Aflatoxin B1 aptamer. Eur Food Res Technol 238, 919–925 (2014). https://doi.org/10.1007/s00217-014-2176-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-014-2176-1