Abstracts
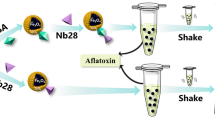
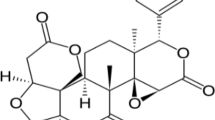
A rapid and sensitive aptasensor was established for the dual-readout determination of aflatoxin B1 (AFB1) utilizing an electrostatically mediated fluorescence resonance energy transfer (FRET) signal amplification strategy. In the presence of AFB1, the aptamer preferentially bound to AFB1, resulting in the aggregation of bare gold nanoparticles (AuNPs) induced by NaCl, accompanied by a change of AuNP solution from wine-red to purple. This color change was used for colorimetric channel analysis. Then, the positively charged quantum dots were introduced into reaction system and interacted with negatively charged AuNPs, which successfully converted the color signal into a more sensitive fluorescence signal through FRET. The fluorescence quenching efficiency decreased with increasing concentrations of AFB1, and the fluorescence of aptasensor gradually recovered. The variation of fluorescence intensity was employed for fluorometric channel analysis. Under the optimal conditions, the color and fluorescence signals exhibited excellent response to AFB1 concentration within the ranges 10–320 ng·mL−1 and 3–320 ng·mL−1, respectively, and the limit of detection was as low as 7.32 ng·mL−1 and 1.48 ng·mL−1, respectively. The proposed aptasensor exhibited favorable selectivity, good recovery (85.3–113.4% in spiked corn and wheat samples), stable reproducibility (RSD<13.3%), and satisfactory correlation with commercial kits (R2=0.998). The aptasensor developed integrates advantages of modification-free, dual-readout, self-calibration, easy operation, and cost-effectiveness, while providing a simple and universal strategy for rapid and sensitive detection of mycotoxins in foodstuffs.
Graphical Abstract






Similar content being viewed by others
References
Goud KY, Kailasa SK, Kumar V, Tsang YF, Lee SE, Gobi KV, Kim K-H (2018) Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: a review. Biosens Bioelectron 121:205–222. https://doi.org/10.1016/j.bios.2018.08.029
Xu N, Xiao Y, Xie Q, Li Y, Ye J, Ren D (2021) Occurrence of aflatoxin B1 in total mixed rations and aflatoxin M1 in raw and commercial dairy milk in northern China during winter season. Food Control 124:107916. https://doi.org/10.1016/j.foodcont.2021.107916
IARC (1993) IARC monographs on the evaluation of carcinogenic risks to humans: some naturally occurring substances: Food Items and Constituents. In: Heterocyclic Aromatic Amines and Mycotoxins, vol 56. international Agency for Research on Cancer, UK
U.S. Food and Drug Administration (FDA) (2021) Manual of compliance policy guides-694 chapter 5-food, colors, and cosmetics [EB/OL] [2021-06-29].
Maragos CM, Busman M (2010) Rapid and advanced tools for mycotoxin analysis: a review. Food Addit Contam Part A 27(5):688–700. https://doi.org/10.1080/19440040903515934
Matabaro E, Ishimwe N, Uwimbabazi E, Lee BH (2017) Current immunoassay methods for the rapid detection of aflatoxin in milk and dairy products. Compr Rev Food Sci Food Saf 16(5):808–820. https://doi.org/10.1111/1541-4337.12287
Chen A, Yang S (2015) Replacing antibodies with aptamers in lateral flow immunoassay. Biosens Bioelectron 71:230–242. https://doi.org/10.1016/j.bios.2015.04.041
Lerdsri J, Chananchana W, Upan J, Sridara T, Jakmunee J (2020) Label-free colorimetric aptasensor for rapid detection of aflatoxin B1 by utilizing cationic perylene probe and localized surface plasmon resonance of gold nanoparticles. Sens. Actuators B 320:128356. https://doi.org/10.1016/j.snb.2020.128356
Vijitvarasan P, Cheunkar S, Oaew S (2022) A point-of-use lateral flow aptasensor for naked-eye detection of aflatoxin B1. Food Control 134:108767. https://doi.org/10.1016/j.foodcont.2021.108767
Xiong J, He S, Zhang S, Qin L, Yang L, Wang Z, Zhang L, Shan W, Jiang H (2023) A label-free aptasensor for dual-mode detection of aflatoxin B1 based on inner filter effect using silver nanoparticles and arginine-modified gold nanoclusters. Food Control 144:109397. https://doi.org/10.1016/j.foodcont.2022.109397
Zhu T, Li N, Huang J, Xu X, Su X, Ma Y, Yang R, Ruan J, Su H (2022) An electrochemical aptasensor based on target triggered multiple-channel DNAzymes cycling amplification strategy with PtFe@Co-MOF as signal amplifier. Microchim Acta 189(10):388. https://doi.org/10.1007/s00604-022-05478-0
Zhao Y, Yang Y, Luo Y, Yang X, Li M, Song Q (2015) Double detection of mycotoxins based on SERS labels embedded Ag@Au core–shell nanoparticles. ACS Appl. Mater. Interfaces 7(39):21780–21786. https://doi.org/10.1021/acsami.5b07804
Hou Y, Jia B, Sheng P, Liao X, Shi L, Fang L, Zhou L, Kong W (2022) Aptasensors for mycotoxins in foods: recent advances and future trends. Compr Rev Food Sci Food Saf 21(2):2032–2073. https://doi.org/10.1111/1541-4337.12858
Quesada-González D, Stefani C, González I, de la Escosura-Muñiz A, Domingo N, Mutjé P, Merkoçi A (2019) Signal enhancement on gold nanoparticle-based lateral flow tests using cellulose nanofibers. Biosens Bioelectron 141:111407. https://doi.org/10.1016/j.bios.2019.111407
Zhou Y, Huang X, Hu X, Tong W, Leng Y, Xiong Y (2021) Recent advances in colorimetry/fluorimetry-based dual-modal sensing technologies. Biosens Bioelectron 190:113386. https://doi.org/10.1016/j.bios.2021.113386
Wu S, Duan N, Ma X, Xia Y, Wang H, Wang Z, Zhang Q (2012) Multiplexed fluorescence resonance energy transfer aptasensor between upconversion nanoparticles and graphene oxide for the simultaneous determination of mycotoxins. Anal Chem 84(14):6263–6270. https://doi.org/10.1021/ac301534w
Pehlivan ZS, Torabfam M, Kurt H, Ow-Yang C, Hildebrandt N, Yuce M (2019) Aptamer and nanomaterial based FRET biosensors: a review on recent advances (2014-2019). Microchim Acta 186(8):563. https://doi.org/10.1007/s00604-019-3659-3
Lu X, Wang C, Qian J, Ren C, An K, Wang K (2019) Target-driven switch-on fluorescence aptasensor for trace aflatoxin B1 determination based on highly fluorescent ternary CdZnTe quantum dots. Anal Chim Acta 1047:163–171. https://doi.org/10.1016/j.aca.2018.10.002
Xiang L, Tang J (2017) QD-aptamer as a donor for a FRET-based chemosensor and evaluation of affinity between acetamiprid and its aptamer. Rsc Advances 7(14):8332–8337. https://doi.org/10.1039/c6ra26118c
Hildebrandt N, Spillmann CM, Algar WR, Pons T, Stewart MH, Oh E, Susumu K, Diaz SA, Delehanty JB, Medintz IL (2017) Energy transfer with semiconductor quantum dot bioconjugates: a versatile platform for biosensing, energy harvesting, and other developing applications. Chem Rev 117(2):536–711. https://doi.org/10.1021/acs.chemrev.6b00030
Li JF, Tian XD, Li SB, Anema JR, Yang ZL, Ding Y, Wu YF, Zeng YM, Chen QZ, Ren B, Wang ZL, Tian ZQ (2013) Surface analysis using shell-isolated nanoparticle-enhanced Raman spectroscopy. Nat Protoc 8(1):52–65. https://doi.org/10.1038/nprot.2012.141
Xiong J, He S, Wang Z, Xu Y, Zhang L, Zhang H, Jiang H (2022) Dual-readout fluorescence quenching immunochromatographic test strips for highly sensitive simultaneous detection of chloramphenicol and amantadine based on gold nanoparticle-triggered photoluminescent nanoswitch control. J Hazard Mater 429:128316. https://doi.org/10.1016/j.jhazmat.2022.128316
Liu J (2012) Adsorption of DNA onto gold nanoparticles and graphene oxide: surface science and applications. Phys Chem Chem Phys 14(30):10485–10496. https://doi.org/10.1039/c2cp41186e
Chen S, Yu Y-L, Wang J-H (2018) Inner filter effect-based fluorescent sensing systems: a review. Anal Chim Acta 999:13–26. https://doi.org/10.1016/j.aca.2017.10.026
Singuru MMR, Sun SC, Chuang MC (2020) Advances in oligonucleotide-based detection coupled with fluorescence resonance energy transfer. TrAC Trends Anal Chem 123:115756. https://doi.org/10.1016/j.trac.2019.115756
Goryacheva OA, Beloglazova NV, Goryacheva IY, De Saeger S (2021) Homogenous FRET-based fluorescent immunoassay for deoxynivalenol detection by controlling the distance of donor-acceptor couple. Talanta 225:121973. https://doi.org/10.1016/j.talanta.2020.121973
Chen L, Wen F, Li M, Guo X, Li S, Zheng N, Wang J (2017) A simple aptamer-based fluorescent assay for the detection of Aflatoxin B1 in infant rice cereal. Food Chem 215:377–382. https://doi.org/10.1016/j.foodchem.2016.07.148
Shim W-B, Kim MJ, Mun H, Kim M-G (2014) An aptamer-based dipstick assay for the rapid and simple detection of aflatoxin B1. Biosens Bioelectron 62:288–294. https://doi.org/10.1016/j.bios.2014.06.059
Xia X, Wang H, Yang H, Deng S, Deng R, Dong Y, He Q (2018) Dual-terminal stemmed aptamer beacon for label-free detection of aflatoxin B1 in broad bean paste and peanut oil via aggregation-induced emission. J Agric Food Chem 66(46):12431–12438. https://doi.org/10.1021/acs.jafc.8b05217
Shim W-B, Mun H, Joung H-A, Ofori JA, Chung D-H, Kim M-G (2014) Chemiluminescence competitive aptamer assay for the detection of aflatoxin B1 in corn samples. Food Control 36(1):30–35. https://doi.org/10.1016/j.foodcont.2013.07.042
Funding
We gratefully acknowledge the support of Beijing Natural Science Foundation of China (Grant No. 6222021).
Author information
Authors and Affiliations
Contributions
Jincheng Xiong: conceptualization, data curation, formal analysis, writing—original draft. Shuang He: investigation, methodology, data analysis. Linqian Qin: investigation, methodology. Shuai Zhang: methodology, data curation. Wenchong Shan: investigation, data curation, formal analysis. Haiyang Jiang: resources; supervision; writing—review and editing; funding acquisition; project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 1.50 MB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, J., He, S., Qin, L. et al. Aptasensor-based assay for dual-readout determination of aflatoxin B1 in corn and wheat via an electrostatic force–mediated FRET strategy. Microchim Acta 190, 80 (2023). https://doi.org/10.1007/s00604-023-05641-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-023-05641-1