Abstract
Production of high-quality tinned meat requires optimisation of sterilisation and pasteurisation processes. Changes in conditions during heating (a w , pH) affect thermoresistance of microorganisms and the possibility of their regeneration following the heating process. Inactivation models of microorganisms used in practice do not take into account these factors. The aim of this study was to determine the influence of the decreasing water activity of the medium during heating and recovery on the thermoresistance of Enterococcus faecium and determine z aw (the distance of a w from a wopt = 1 which leads to a tenfold reduction in D value) and z′ aw (the distance of a w from a′ w of the recovery medium which leads to a tenfold reduction in D value) parameters. The performed experiments revealed that Enterococcus faecium PCM 1859 thermoresistance increased during heating in the environment characterised by reduced a w . Statistically significant differences occurred when aw was reduced to the value of <0.97. The impact of a w on the Enterococcus faecium PCM 1859 thermoresistance was characterised by the following coefficients: z aw = 0.14−0.28, z′ aw = 0.18−0.44.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Although Nicolas Appert published the principles regarding production of tinned articles, including meat, over 200 years ago, they continue to enjoy high popularity. The high temperature the products are exposed to during heating causes a series of unfavourable phenomena: reduced quantities of thermolabile vitamins (primarily B1), changes and, frequently, deterioration of the product sensory quality, greater concentration of Maillard’s reaction products [1] as well as greater quantity of the thermal drip [2]. Hence, attention to the quality of tinned articles consists, primarily, in the attenuation of heating parameters. Tinned meat, due to high meat pH, should be subjected to sterilisation. However, the application of “hurdle” technology during the manufacture of these products may attenuate considerably the heating process. Shelf stable products (SSP) canned meat obtained in this way is characterised by microbiological stability in the course of long storage employing only the process of pasteurisation (Tc > 90 °C) at a simultaneous application of other conservation factors such as reduction in the aw and/or pH value as well as addition of sodium nitrite [3–7].
Meat and meat products are characterised by high water activity, making it possible to control this parameter. However, it should be remembered that lower water activity can affect thermoresistance of microorganisms.
The requirements of food safety as well as of the best possible quality of the finished product make it necessary for the models of microorganism inactivation to be optimised and increasingly precise. Although the control of heating of canned meat should continue to be based on the determination in the course of the process of the sterilisation value F, the current model which allows calculation of the coefficient of inactivation velocity L is far from satisfactory:
where T—temperature of critical zone of canned meat during heating T r —reference temperature z—increase in temperature which leads to a tenfold reduction in the decimal reduction time (D)
The published research results indicate that this value must take into account both pH and a w of the heated raw material [8].
where T—temperature in centre of canned meat, in critical zone T*—reference temperature D*—D value at T*, pH* and aw = 1 z T —conventional z value pH—pH of heated product pH*—pH of maximal heat resistance of bacteria (generally 7) z pH —the distance of pH from pH* which leads to a tenfold reduction in decimal reduction time (D) a w —water activity of heated product z aw —the distance of a w from awopt = 1 which leads to a tenfold reduction in D value pH′—pH of the recovery medium pH′ opt —optimal pH value of the recovery medium z′ pH —the distance of pH from pH′ of the recovery medium which leads to a tenfold reduction in D valuea′ w —aw of the recovery medium a′ wopt —optimal aw of recovery medium z′ aw —the distance of aw from a′ w of the recovery medium which leads to a tenfold reduction in D value
According to Mafart [9]:
That is why a new model of calculation of the L value was elaborated:
Explanations as in Eq. (2).
The presented model was developed for sporulating bacteria which become inactivated during sterilisation. Mafart et al. [9] demonstrated that for food articles of pH 4–7, this model can be utilised also for processes which run at lower temperatures (pasteurisation). In addition, it also makes allowances for the regeneration of thermally damaged bacterial cells of such pH and water activity that occurred during heating because it corresponds to conditions which occurred in canned materials during their storage. Until recently, the probability of regeneration of microorganisms was checked on media with optimal parameters (pH and a w ), and therefore, the obtained D values were overestimated [10–13].
The application of the above-presented model requires appropriate coefficients (z pH , z aw , z′ pH , z′ aw ) for the indicator microorganism. So far, such coefficients were determined for bacteria from Bacillus and Clostridium genus, that is, for sporulating bacteria which should be taken into consideration during the sterilisation process [8, 9, 11, 13, 14].
However, no information is available regarding the pasteurisation process of canned meat which should take into consideration survivability of thermoresistant non-sporulating bacteria. Enterococci are considered to be the most thermoresistant among vegetative bacteria, and in the case of pasteurised meat products, they should be treated as test bacteria, especially Enterococcus faecium and Enterococcus faecalis which dominate among them [15].
The aim of this paper was to ascertain thermoresistance and regeneration possibilities of thermally damaged Enterococcus faecium PCM 1859 cells depending on the water activity of the medium during heating and incubation following the thermal process and to determine z aw and z′ aw values.
Materials and methods
The bacterial strain used in the described experiments was that of Enterococcus faecium PCM 1859 derived from the Strain Collection of the Polish Academy of Sciences in Wrocław. Experimental bacteria were cultured on Slanetz and Bartley medium with differing water activity. Substrate water activity was reduced with the assistance of NaCl, and the following variants were obtained:
(A) basic medium (optimal), containing peptone—20 g, dipotassium phosphate—4 g, yeast ekstrakt—5 g, glucose—2 g, sodium azide—0.4 g, TTC—0.1 g, agar—15 g, distilled water—1 dm3, pH of ready medium 7.2, a w = 1.0; (B) basic medium +1 % NaCl, pH = 7.2, a w = 0.98; (C) basic medium +1.5 % NaCl, pH = 7.2, a w = 0.97; and (D) basic medium +3 % NaCl, pH = 7.2, a w = 0.95.
Following inoculation, samples were incubated for 48 h at the temperature of 37 °C. Bacteria collected from media A–D were placed in small flasks with physiological fluid containing the same quantity of NaCl as during culturing. Their initial concentration amounted to 106−108 cfu/cm3. Next, 10 cm3 suspension was collected from each flask, transferred to three test tubes and heated in a water bath at the temperature of 55 °C, respectively, for 10, 20 and 30 min. The same procedures were followed when bacteria were heated at the temperature of 60 °C for 1, 3 and 5 min and at 65 °C for 1, 2 and 3 min. Each experiment was repeated three times. Next, 10 cm3 suspension was collected from each flask, transferred to nine test tubes and heated in a water bath at the temperature of 55 °C for 10, 20 and 30 min; 60 °C for 1, 3 and 5 min; and 65 °C—for 1, 2 and 3 min. After the appropriate time of heating, the bacteria were inoculated in order to ascertain the number of survived microorganisms. Bacteria cultured on medium A after heating were flushed with medium A; bacteria collected from the medium of reduced water activity were incubated on medium A (optimal) and, simultaneously, on the medium characterised by the same parameters as during heating. A survival curve was plotted for a given heating temperature from which time of decimal reduction D was determined for both the bacteria incubated on the basic medium and on the modified medium (of reduced a w value), and then from the curve of log D−a w dependence, z aw and z′ aw coefficients were determined.
Measurements of water activity
Measurements of water activity were conducted employing the analyser of water activity Aquaspector—1 (NAGY Meßsysteme GmbH, Gäufel, Germany). Samples were closed in plastic container and placed in the measuring chamber, at 20 °C. The result was read from the electronic display with the accuracy of up 0.005.
DSC analysis
Bacteria for investigations were obtained by delicate scratching of colonies that developed on the medium of definite a w . Samples were weighed with ±0.01 mg accuracy and placed in aluminium sample pans (Perkin-Elmer, Norwalk, USA; No. 0219-0062). The mass of samples ranged from 10 to 15 mg. Sealed capsules were heated in a differential scanning calorimeter DSC 7 by Perkin-Elmer (Norwalk, USA) from the temperature of 5–110 °C with 5 °C/min velocity, that is, the temperature similar to that reached during high-temperature pasteurisation of canned materials. After heating, samples were immediately cooled to the initial temperature and heated again in order to evaluate the reversibility of the process. Five replications were performed with each sample. As a result of DSC analyses, thermal curves were obtained. The enthalpy of denaturation ΔH (J/g) and temperatures of initial (T 0 ) and maximum transition (T max ) (°C) were calculated with the data analysis software supplied by Perkin-Elmer. Enthalpy Δ(H) was determined by measuring the area under the DSC curve and expressed in J/g of sample.
It was impossible to avoid some contamination of samples with the medium during experiments, and therefore, for control, measurements were also made using only the pure medium. Identical thermograms were obtained demonstrating that slight contamination with the medium exerted no influence on the result.
Statistical analysis
Results obtained in this study were subjected to statistical analysis. One-factorial analysis of variance and post hoc Tukey’s tests were applied for multiple comparisons of mean values. The level of significance was p ≤ 0.05. All computations were performed using STATISTICA PL v. 10 software by StatSoft.
Results and discussion
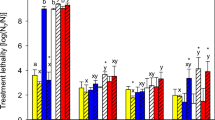
Table 1 presents the impact of the medium aw during heating on D values (variants A, B, C and D regeneration on optimal medium). Statistically significant differences in E. faecium thermoresistance measured by the D value with respect to the control sample (variant A) were observed in the case of bacteria heated in the environment with a w = 0.95 at the temperature of 55 °C (variant D) as well as a w = 0.95 and a w = 0.97 at the temperature of 60 °C and 65 °C (variant C and D). Also, some literature data regarding the effect of water activity on thermoresistance corroborate that the lower the water activity, the higher the thermoresistance of microorganisms [16–19]. The impact of environmental water activity during heating on spores and vegetative cells depends on the substance used for its reduction. Microorganisms are characterised by different metabolic pathways and behave differently in an environment of identical water activity depending on the solution employed for its regulation [20]. In the study discussed here, a w reduction was achieved by means of the addition of sodium chloride whose impact on thermoresistance remains debatable. According to some researchers, increased concentrations of NaCl do not affect thermoresistance of microorganisms [13, 21], while others reported a protective influence of sodium chloride on microbes [19, 22, 23]. Such effect was observed, for example, in the case of Streptococcus faecium isolated from luncheon meat and heated in the presence of sodium chloride. There are also reports describing reduced thermoresistance of bacteria from Bacillus and Clostridium genus together with the increase in sodium chloride concentrations [11, 13, 24].
Increased thermoresistance of Enterococcus faecium PCM 1859 depending on the water activity of the medium was confirmed by the results obtained with the assistance of the scanning calorimeter.
Table 2 presents characteristics of endothermic transformations. In the course of heating of bacteria cultured on media with different water activities, the following peaks were recorded: m, n, p, q and on media with a w = 0.97 and a w = 0.95, additionally, peak r. The appearance of peak r could be the result of differences in bacteria thermoresistance because certain elements of the thermogram can occur in resistant organisms and be absent in organisms sensitive to heat. The observed presence on the thermogram of combined m and n peaks is associated with ribosome denaturation, while that of peak p—of intracellular DNA [25]. Ribosome denaturation during heating was reported also in other papers [26–28].
Table 2 illustrates denaturation enthalpy for individual changes taking place in a bacterial cell under the influence of heating. The results presented in this Table show that the enthalpy of these transformations depends on the water activity of the medium on which the bacteria were cultured and assumed the highest values for a w = 0.98. Peaks m and n were not clearly separated. According to Mackey et al. [27], their appearance is the result of a complex phenomenon, namely ribosome denaturation. Results presented in Table 2 indicate that enthalpy of these transformations depends on the activity of the medium water on which the bacteria were cultured, and it assumes the greatest values for a w = 0.98 and a w = 0.97. Consecutive changes, recorded on the thermogram as peak p with T max ranging from 86.8 to 88.6 °C, characterise destruction of second-order DNA which is associated with the absorption of light of 259 nm wavelength. The temperature in which half of the maximum extinction increases is achieved is referred to as the so-called melting point (T m ). At T m temperature, denaturation of isolated and appropriately purified DNA takes place. In the course of heating of bacterial cells, this phenomenon is connected with the denaturation of cell wall constituents [25, 27]. In the case of heating of the E. faecium strain, the highest value of enthalpy was calculated for bacteria cultured on the medium with a w = 0.98. Continued heating of bacteria resulted in the appearance of peak q associated with melting of consecutive DNA segments and denaturation of successive constituents of the cell wall. The highest ∆H values were determined during the DSC analysis, bacteria cultured on the medium with a w = 1.00 and a w = 0.98 (Table 3).
Increased temperatures cause disruption of hydrogen bridges and expansion of polynucleotide chains. This kind of DNA denaturation can be reversed by slow cooling down which allows fresh evaporation and reassociation of complementary segments. Therefore, heating of the examined bacteria, cooling them down to the temperature of 5 °C and repeated heating led to the development on the thermogram of a small p′ peak with the enthalpy of 0.08–0.09 J/g (bacteria cultured on media with a w = 0.97; 0.98 and 1,00) and 0.05 J/g (bacteria cultured on the medium with a w = 0.95). According to Miles et al. [25], the appearance of the p′ peak is associated with the melting of the intracellular DNA.
The performed investigations revealed that maximal temperatures of individual peaks attained in the course of the first heating differed between one another, although no directly proportional relationships between thermoresistance of bacteria cultured on the media of varying water activity and T max of individual peaks were demonstrated. On the other hand, repeat heating after cooling down to the temperature of 5 °C resulted in the development of peak p′ whose maximum temperature (T max ) increased together with the increasing thermoresistance of the examined bacteria, and the performed statistical analysis exhibited significant differences between all the obtained values (Table 2).
At the present time, one of the basic concepts explaining thermoresistance mechanisms of microorganisms is rapid resynthesis of denaturised constituents under the influence of heat, that is, synthesis of the so-called shock proteins [29, 30]. Cooling down of samples after heating and repeat heating ensures irreversible DNA denaturation. Therefore, the initial temperature of irreversible denaturation (T 0 ) was calculated as the difference between the extrapolated initial temperatures obtained during the primary and repeat heating. The results presented in Table 2 indicate that the lower the water activity of the substrate on which the bacteria were cultured, the higher was the initial denaturation temperature (T 0 ). The performed statistical analysis showed that T 0 values for a w = 1.00 and a w = 0.98 differed from T 0 for a w = 0.95. Literature data indicate unequivocally that T 0 temperature is associated with thermoresistance of microorganisms; more thermoresistant microorganisms are characterised by higher T 0 temperatures. It was also demonstrated that T 0 increases with the amount of NaCl addition to the medium which means that microorganisms increase their thermal resistance under the influence of salt [25, 27].
Calculated D values for the examined bacteria made it possible to present a dependence of log D on the a w of the environment during heating and to determine the z aw coefficient (Table 3). The obtained results revealed that bacteria from the E. faecium genus were less sensitive to changes in environmental water activity during heating at the temperature of 55 °C (z aw = 0.28) than at the temperature of 60 °C and 65 °C (z aw = 0.14).
Due to unfavourable changes taking place during the process of heating, it is never assumed that thermal treatment of canned materials will kill off all microorganisms. The stability of the finished product results from the fact that some part of cells is really killed, whereas another part is only damaged and during storage of canned articles is unable to regenerate and develop and that is why no colonies are developed in cultures [10]. On the other hand, it is stressed that many microorganisms causing food deterioration are capable of adapting to unfavourable environmental conditions and, by doing so, become more resistant to stress factors. Investigations into food shelf life and safety are conducted most frequently employing microorganisms which have not been exposed to stress. It is, therefore, probable that prognostic models do not take into account resistance of microorganisms exposed to factors limiting their growth [23].
Regeneration of bacterial cells damaged during the action of high temperature depends on the incubation conditions. Until now, this quantity was determined on media characterised by optimal parameters (pH, a w ), and therefore, the concept of the thermoresistance assessment of microorganisms on the basis of D and z values reflecting only heating conditions appears to be inadequate [10]. It was demonstrated that thermally damaged cells were more sensitive to sodium chloride [16, 24] and, therefore, they could regenerate on optimal media used in laboratories but not in canned material of reduced a w during storage. The number of cells capable to regenerate following thermal treatment should be determined on the medium with such pH and a w which occurred during heating because this reflects the conditions occurring in canned materials during their storage [31].
That is why, after heating bacterial cells in the environment of different water activities, the number of cells capable for regeneration was assessed employing the medium of such a w as during heating. The obtained results are collated in Table 1 (variant B′, C′ and D′). These results show that on the modified medium, microorganisms had smaller possibilities of regeneration and the determined D values—characterised thermoresistance of the Enterococcus faecium PCM 1859 strain incubated after heating on a modified medium—were always smaller in comparison with D values obtained when the bacteria were regenerated on the optimal medium. After heating the bacteria in the environment with a w = 0.95 at the temperature of 60 °C, statistically significantly higher thermoresistance of Enterococcus faecium was demonstrated when the strain was regenerated on the optimal and not on the modified medium. The increase in the temperature to 65 °C caused that the strain incubated after heating on the optimal medium was characterised by higher thermoresistance in comparison with the modified medium and statistically significant differences were found for bacteria inoculated on the media with a w = 0.97 and a w = 0.95. Shortening of the time of bacterial decimal reduction under the influence of sodium chloride present in the regeneration medium was also determined in the case of Bacillus cereus bacteria [13]. Also Mafart and Leguerinel [10] demonstrated that when incubation conditions were not optimal, the so-called apparent value D′ was determined which was lower than the D value determined in optimal conditions.
On the basis of the log D versus a w dependence of the medium during bacterial incubation after heating, z′ aw coefficient was determined characterising E. faecium thermoresistance determined on the modified medium (Table 3). The sensitivity of the examined bacteria to sodium chloride concentration in the regeneration medium increased with the increase in the heating temperature. The determined z′ aw coefficient equalled 0.44; 0.23 and 0.18 for temperatures of 55, 60 and 65 °C, respectively.
Conclusions
The introduction of management systems of food–health security as well as growing consumer requirements regarding quality causes that models describing conditions of can sterilisation must be more and more precise. They should envisage survivability of microorganisms not only on the basis of temperature changes in the source of thermal processing but should also take into account changes in their thermal resistance depending on water activity and pH of the environment. Cells damaged in the course of the thermal process are capable of regeneration, but this ability is restricted if a w and pH of the can are not optimal. The performed experiments demonstrated that the reduction in the water activity of the medium in the course of heating increased thermal resistance of the PCM 1859 strain of Enterococcus faecium, and the z aw coefficient determining the impact of a w for the time of the decimal reduction D depends on the heating temperature and amounts from 0.14 to 0.28. Similarly, the z′ aw coefficient determining the influence of a w of the regenerating medium on the value of D depends on the heating temperature and ranges from 0.18 to 0.44. This means that the examined bacterial strain was characterised by a greater tolerance to changes in water activity of the environment during heating if the rate of survivability of these microorganisms following pasteurisation was assessed on a modified and not optimal medium (coefficient z′ aw greater than z aw ). The results obtained in the present study indicate possibilities of control of meat can pasteurisation taking into consideration the new model of determination of lethal rates L.
References
Langourieux S, Escher FE (1998) Sulfurous off-flavor formation and lipid oxidation in heat-sterilized meat in trays. J Food Sci 63:716–720
Honikel KO (2004) Vom Fleisch zum Produkt. Reifen-Erhitzen-Zerkleinern-Salzen. Fleischwirtsch 5:228–234
Leistner L, Wirth F, Takács J (1970) Einteilung der Fleischkonserven nach der Hitzebehandlung. Fleischwirtsch 50:216–217
Reichert JE, Stiebing A (1977) Herstellung von längerfristig haltbaren Leberwurstkonserven durch Pasteurisieren infolge aw—wertsenkung. Fleischwirtsch 57:910–921
Wirth F (1979) The present stage of development in the manufacture of canned meats. Fleischwirtsch 59(4):536–541
Gould GW (1996) Methods for preservation and extension of shelf life. Int J Food Microbiol 33:51–64
Vuković IK (1999) Major hygienic and technological procedures in prevention of botulism from meat products. Technol Mesa 40:51–59
Leguérinel I, Spegagne I, Couvert O, Gaillard P, Mafart P (2005) Validation of an overall model describing the effect of three environmental factors on the apparent D-value of Bacillus cereus spores. Int J Food Microbiol 100:223–229
Mafart P, Couvert O, Leguérinel I (2001) Effect of pH on the heat resistance of spores. Comparison of two models. Int J Food Microbiol 63:51–56
Mafart P, Leguérinel I (1997) Modelling the heat stress and the recovery of bacterial spores. Int J Food Microbiol 37:131–135
Leguerinel I, Couvert O, Mafart P (2000) Relationship between the apparent heat resistance of Bacillus cereus spores and pH and NaCl concentration of the recovery medium. Int J Food Microbiol 55:223–227
Mafart P (2000) Taking injures of surviving bacteria into account for optimising heat treatments. Int J Food Microbiol 55:175–179
Coroller L, Leguérinel I, Mafart P (2001) Effect of water activities of heating and recovery media on apparent heat resistance of Bacillus cereus spores. Appl Environ Microbiol 67:317–322
Gaillard S, Leguerinel I, Mafart P (1998) Model for combined effects of temperature, pH and water activity on thermal inactivation of Bacillus cereus spores. J Food Sci 63:887–889
Franz CMAP, Stiles ME, Schleiferk H, Holzapfel WH (2003) Enterococci in foods-a conundrum for food safety. Int J Food Microbiol 88:105–122
Hutton MT, Koskinen MA, Hanlin JH (1991) Interacting effects of pH and NaCl on heat resistance of bacterial spores. J Food Sci 56:821–822
Blackburn CW, Curtis LM, Humpheson L, Billon C, Mcclure PJ (1997) Development of thermal inactivation models for Salmonella enteritidis and Escherichia coli O157:H7 with temperature, pH and NaCl as controlling factors. Int J Food Microbiol 38:31–44
Raccach M, Henningsen EC (1997) The effect of chloride salts on Yersinia enterocolitica in meat. Food Microbiol 14:431–438
Mazas M, Martinez S, López M, Alvarez AB, Martin R (1999) Thermal inactivation of Bacillus cereus spores affected by the solutes used to control water activity of the heating medium. Int J Food Microbiol 53:61–67
Vittadini E, Chinachoti P, Lavoie P, Pham X (2005) Correlation of microbial response in model food systems with physico-chemical and “mobility” descriptors of the media. Inn Food Sci Emerging Technol 6:21–28
Chumney RK, Adams DM (1980) Relationship between heat the increased sensitivity of heat injured Clostridium perfringens spores to surface active antibiotics and to sodium chloride and sodium nitrite. J Appl Bacteriol 49:55–63
Vrchlabsky J, Leistner L (1971) Hitzeresistenz von Laktobazillen bei unterschiedlichen aw-Werten. Fleischwirtsch 51(9):1368–1370
Rowan NJ (1999) Evidence that inimical food-preservation barriers alter microbial resistance, cell morphology and virulence. Trends Food Sci Technol 10:261–270
González I, López M, Mazas M, González J, Bernardo A (1997) Thermal resistance of Bacillus cereus spores as affected by additives in the recovery medium. J Food Safety 17:1–12
Miles CA, Mackey BM, Parsons SE (1986) Differential scanning calorimetry of bacteria. J Gen Microbiol 132:939–952
Anderson WA, Hedges ND, Jones MV, Cole MB (1991) Thermal inactivation of L. Monocytogenes studied by differential scanning calorimetry. J Gen Microbiol 137:1419–1424
Macket BM, Miles CA, Parsons SE, Seymour DA (1991) Thermal denaturation of whole cells and cell components of Escherichia coli examined by differential scanning calorimetry. J Gen Microbiol 137:2361–2374
Earnschaw RG, Appleyard J, Hurst RM (1995) Understanding physical inactivation processes: combined preservation opportunities using heat, ultrasound and pressure. Int J Food Microbiol 28:197–219
Laport MS, Silva MR, Silva CC, Bastsos MCF, Giambiagi-De Marval M (2003) Heat-resistance and heat-shock response in the nosocomial pathogen Enterococcus faecium. Curr Microbiol 46:313–317
Gawande PV, Griffiths MW (2005) Effects of environmental stresses on the activities of the uspA, grpE and rpoS promoters of Escherichia coli O157:H7. Int J Food Microbiol 99:91–98
Bell RG, De Lacy KM (1984) Heat injury and recovery of Streptococcus faecium associated with the souring of chub-packed luncheon meat. J Appl Bacteriol 57:229–236
Conflict of interest
None.
Compliance with Ethics Requirements
This article does not contain any studies with human or animal subjects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Danyluk, B., Stangierski, J., Gajewska-Szczerbal, H. et al. Impact of environmental water activity on E. faecium thermoresistance and possibility of regeneration of heat-damaged cells in pasteurised canned meat. Eur Food Res Technol 236, 1041–1047 (2013). https://doi.org/10.1007/s00217-013-1963-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-013-1963-4