Abstract
The release of volatile compounds, such as aroma, from a food material during hydration is of wide relevance to the food industry. To this end, dry powders of varying chemical composition were hydrated in a controlled system to investigate the impact of varying composition (protein, lipid and carbohydrate) on the delivery rate of volatile compounds to the headspace. Additional lipid and carbohydrate reduced the concentration of volatile compounds in the headspace and accelerated their rate of delivery to the headspace. Protein had no measurable impact. Of the volatile compounds measured, 2,3 butanedione and acetaldehyde were shown to be released slowly into the headspace, and pyrrol, methyl acetate and pyridine were released rapidly; this differential release rate was strongly correlated with hydrophobicity and would indicate that during hydration there is a temporal dimension to the relative abundance of volatile compounds in the headspace.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human perception of food flavour during the preparation–consumption cycle is driven by a number of interacting sensory modalities; these may include taste, vision, mouthfeel, olfaction, auditory and trigeminal stimuli [1, 2]. It is the combination of these stimuli, prior experiences [3, 4] and the temporal framework within which they innervate the brain that creates overall perception [5].
Within the olfactory modality, the principle stimulus, aroma, is perceived in one of two ways. The inhalation of volatile compounds through the nose (orthonasal delivery) and the delivery of volatile compounds to the nasal cavity during exhalation (retronasal delivery), both routes deliver volatile compounds to odour-binding proteins [6] and aroma receptors in the nasal cavity allowing perception to occur through combinatorial coding [7], but both achieve this in different ways [8]. The resulting impact is a significant difference in perception when comparing the inhaled aroma and the aroma associated with consumption of a food material [8, 9].
The work detailed herein focusses on the headspace delivery of volatile compounds during the hydration of a food powder; this is designed to be representative of aroma inhaled during the preparation of products such as instant coffee, soups, gravies or beverages. The aroma perceived during this stage of the preparation process is a key consumer liking step and is often overlooked when evaluating or designing products [10].
When a powder is dissolved, volatile compounds are delivered to the headspace via a complex kinetic partitioning process. For a volatile compound to be available in the headspace it has to be available in the continuous phase, and for it to be available in the continuous phase it has to be first dissolved from the dispersed phase into the continuous phase. The rate of delivery of volatile compounds from the dispersed phase to the continuous phase is dependent on a number of factors, which may include interactions with hydrophobic regions such as lipids [11, 12], entropic restrictions to the dispersion of carbohydrates, the breaking of transient bonds with proteins and wetting kinetics of the powder. To simplify a complex process, it is proposed that there are three main components within food systems that play a role in dispersion, these being lipids, carbohydrates and proteins.
The presence of lipid in a dispersing system will play a number of roles. Lipid will change the polarity of the product and act as a sink for hydrophobic volatile compounds [13, 14], and additionally, lipid may be present as natural emulsions [15–18] or form emulsion droplets [19–21], oil layers or micelles [22, 23] within the product during dispersion, which will impact the spatial distribution of lipophilic compounds and modify mixing behaviour [24, 25]. Simple carbohydrates drive the partitioning of volatile compounds through salting-in and salting-out phenomena [26–28]; in an aqueous system, this is associated with the relative availability of water molecules in the continuous phase [22]. Complex carbohydrates can form structures that sterically entrap compounds [29] and, if present in the glassy state, can form an immobile matrix that restricts the mobility of volatile compounds and limits migration to the gaseous phase [30, 31]. Protein can also reduce the concentration of some volatile compounds in the headspace [26], although proteins generally have a lesser impact on the partitioning of volatile compounds to the headspace than lipid or carbohydrate. Proteins may modify the availability of volatile compounds through the formation of reversible hydrophobic and hydrogen bonds [32] or irreversible covalent linkages [33]. Physical long distance networks of lipid, carbohydrate or protein that impart macroscopic structural changes such as complex emulsions [34], gel networks [35], carbohydrate structures [36] and fibrous protein networks (e.g., muscle) [37] are not discussed within this work, but will clearly impact the delivery of volatile compounds.
In summary, the impact of ingredient composition on the delivery of volatile compounds from powdered food systems is complex; therefore, to prepare for reformulation and product development challenges, aroma chemists and product developers require an insight into the relative partitioning of volatile compounds between foods and their headspace; this can only be achieved by combining fundamental knowledge, modelling data and applied case studies with the experience of flavourists. The aim of this research program was therefore to develop an understanding of the relative delivery rate of volatile compounds to the headspace from hydrating powders. The specific objective of this study was to identify the impact of ingredient modification (carbohydrates, proteins and lipids) on the intensity (IMAX) and time to reach maximum intensity (TMAX) of volatile compounds delivered to the headspace of a model system containing a standardized dry powder (soluble coffee) dissolving in an aqueous solvent (water).
Materials and methods
Spray-dried coffee powder (n = 3) and sunflower oil were obtained from a local supermarket, Loughborough, UK. Casein and fructose were purchased from Acros Organics (Geel, Belgium).
Dissolution cell
A bespoke dissolution cell (Fig. 1) was designed for evaluating the delivery of volatile compounds to the headspace during powder dispersion. The dissolution cell, was cuboid in shape, had an internal elevation of 70 mm, an internal volume of 450 mL and contained a sample introduction port and a headspace analysis port for analysis via an APcI-MS headspace sampling interface [34, 38]. The sample was premixed by shaking to homogeneity and then introduced quickly through a large gauge syringe into the continuous phase (40 °C) without contacting the headspace. Remote stirring of the dissolution liquid was achieved through the use of a magnetic stirrer and magnetic flea.
Headspace analysis
Gaseous headspace above the sample within the dissolution cell was analysed by APcI-MS (Micromass, Manchester, UK) for 10 min at a sampling flow rate of 40 mL/min with an MS nose interface (Micromass, Manchester, UK) operating in full scan mode (m/z range 20–220) with a 4 kV corona discharge. The maximum intensity (IMAX) and time to maximum intensity (TMAX) were measured [34] as illustrated in Fig. 2.
Model powder matrix
A model powder matrix was developed including protein, carbohydrate, lipid and spray-dried coffee powder, and the spray-dried coffee powder being present as the source of volatile compounds. The relative concentration of protein, carbohydrate and lipid was varied according to Table 1 to accommodate for enhancement in protein, carbohydrate or lipid within the sample which would be characteristic of changes in the nutritional composition of dry soup powders, the inclusion of milk powder in a coffee mix or changes in the formulation of instant gravy. In all samples analysed, the weight of the instant coffee remained the same (12.525 g).
Molecular modelling
Physicochemical properties of the volatile compounds of interest were estimated using KOWWIN v1.67 within EPISuite ver. 3.20 (U.S. Environmental Protection Agency).
Moisture analysis
Samples were taken on day of opening of commercial products, the product was mixed to homogeneity and triplicate samples (1.5 g) were taken for analysis. Moisture content was measured in predried aluminium trays by evaporation to dryness at 105 °C for 48 h (Convection oven MOV-112F, Sanyo, Japan). Samples were weighed until constant weight. Water activity was determined at 24.6 °C using an AquaLab 3TE water activity meter (Decagon Devices Inc, Pullman, USA).
Statistical analysis
All samples were presented in randomized order and analysed in triplicate. Data were analysed by XLSTAT 2009 (Addinsoft, USA), using analysis of variance with Tukey’s post hoc test (P < 0.05) to identify significant differences between samples sets [39].
Results and discussion
The delivery of volatile compounds to the headspace in this closed system (Fig. 1) is driven by two independent factors, firstly the dispersion and dissolution of the powder into the dissolving solvent and secondly the equilibrium partitioning of the volatile compound across the air-product barrier. The rate (TMAX) and intensity (IMAX) of delivery of volatile compounds to the headspace above a dissolving powder was therefore measured to correlate with changes in the dissolution kinetics and the static equilibrium state, respectively (Fig. 2).
After the addition of the sample powder (instant coffee with a moisture content ± SD of 5.58 % ± 0.31 % and water activity ± SD of 0.25 ± 0.00 at 24.6 °C) to the solvent within the dissolution cell (40 °C), the concentration of volatile compounds in the headspace rapidly increased, and then effectively plateaued after 1.0–1.5 min. The curve formed was of a smooth shape, was reproducible and was dependent on the chemical composition of the dissolving powder. A typical headspace hydration curve is illustrated in Fig. 2, shows the development of the total headspace ion count over time and clearly identifies the two extracted parameters, TMAX and IMAX (as previously described).
Headspace volume and the stirring speed of the magnetic flea were optimized for analysis using pure coffee powder; the method optimization results are detailed in Figs. 3 and 4 and show variation in response and repeatability with varying headspace volume and stirring speed. A stir speed of 400 rpm and a headspace volume of 225 mL were chosen as stable reproducible process parameters. Higher stir speeds gave more reproducible data, but the introduction of chaotic vortexes within the dissolution cell limited the interpretation of results formed. Headspace volume had little effect on the resultant IMAX and TMAX. Although higher volumes gave a slight enhancement in IMAX reproducibility, a mid-range headspace volume was chosen as this minimized variation and maximized the signal to noise ratio of the APCI-MS. Further investigations were conducted to optimize air flow rate into the APCI-MS (Table 2) and a flow rate of 40 mL min−1 was chosen for its stability and reproducibility. Calibration curves were prepared using coffee preparations (0.25–30.0 g 450 mL−1) at a range of concentrations to evaluate the linearity of the delivery curve; the coefficient of determination was chosen to estimate how well a linear model will fit future data and was acceptable at R 2 > 0.95.
Within the volatile mix, different compounds were delivered to the headspace at different rates (Fig. 5); this is shown by evaluating the changes in individual mass to charge ratios (m/z) over time. Previous studies have shown that delivery of volatile compounds from a matrix to the headspace on hydration is strongly dependent on the physicochemical properties (e.g., hydrophobicity, volatility, molecular size) of the molecule of interest [40, 41].
Studies on coffee have shown a tentative identification of compounds by m/z with acetaldehyde, 2,3 butanedione, pyridine, methylacetate and pyrrole predicted to be assigned to m/z of 45, 87, 80, 75 and 68, respectively [42, 43]. When evaluating across the calibration curves (n = 3), 68, 75 and 80 were shown to be released to the headspace quickly and 87 and 45 were shown to be released more slowly. Assuming that the mass to charge ratio markers indicated by Lindinger et al. [42, 43] are correct, this would indicate that pyrrol, methylacetate and pyridine could be classified as “rapid releasers” and 2,3 butanedione and acetaldehyde as “slow releasers.” The TMAX ± SD for the model coffee sample is shown in Fig. 5, and the corresponding mass spectrum (histogram of the mass to charge ratios) of the model coffee sample is shown in Fig. 6.
Of the five compounds discussed, there is a marked difference in hydrophobicity (log P) when comparing the rapid releasers with the slow releasers (Table 3). Log P is the logarithmic function of the concentration ratio when a compound is allowed to equilibrate between two phases of varying hydrophobicity, typically the two solvents being octanol and water, with the assumption that the two phases behave as ideal solutions and that temperature is maintained constant. Log P of the rapid releasers (pyrrol, methylacetate and pyridine), as estimated by molecular modelling, is greater (Table 1) than those of the slow releasers (2,3 butanedione and acetaldehyde). This indicates that the more lipophilic compounds (larger log P) are generally quicker to be delivered and to equilibrate with the headspace than the hydrophilic compounds (smaller log P). This supports previous research in static systems, which demonstrate that in a disturbed system the rate kinetics to equilibrium for lipophilic compounds are faster than those of hydrophilic compounds [44]. The data presented in this paper are the first to report on the impact of chemical composition and a volatile compounds physicochemical properties on volatile delivery during hydration of food powders.
To investigate the impact of changes of bulk chemical composition (lipid, protein, carbohydrate) on the powder dissolution kinetics and the headspace delivery of volatile compounds, three compounds (sunflower oil, casein and fructose) were added to instant coffee powder at varying concentration levels. The maximum headspace intensity (IMAX) and time to reach maximum headspace intensity (TMAX) were directly compared.
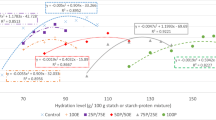
The inclusion of lipid had a marked impact on IMAX (Fig. 7); IMAX reduced significantly (P < 0.05) with 11.8 % lipid inclusion, and further reduced (P < 0.05) with 50 % additional lipid inclusion. The significant reduction in the availability of volatile compounds in the headspace is driven by the change in polarity of the matrix, which may have included the formation of emulsion droplets or surface oil (although free oil could not be visibly observed). The change in bulk polarity will reduce the air-product partition coefficient of hydrophobic compounds and therefore will reduce the concentration of total volatile compounds in the headspace. There will be a compound-specific effect that is driven by polarity, although this has not been further detailed as this study is not solely focused on the static partitioning of volatile compounds, a topic that is well covered by previous authors [14, 19, 45–48].
Impact of a lipid (0.0, 6.6, 11.8, 50 %), b carbohydrate (0.0, 8.5, 15.6, 50 %) and c protein (0.0, 6.6, 12.4, 50 %) concentration on maximum headspace intensity (IMAX ± SD, mV). Different letters indicate statistically significant differences within a figure as measured by ANOVA-TUKEYS post hoc test (P > 0.05, n = 3) (protein = casein, carbohydrate = fructose, lipid = sunflower oil). In all cases, the weight of coffee powder was constant
Carbohydrate had a suppressive effect (P < 0.05) on the maximum headspace volatile concentration (Fig. 7) at 15.6 % carbohydrate inclusion, but at higher concentrations no further statistical impact of additional inclusion was demonstrated. The carbohydrate was chosen for its value within a model system, but it does imply that addition of simple sugars to a coffee mix will modify the headspace volatile concentration. A previous study with a reduced concentration range showed no impact of fructose on the partitioning of a range of volatile compounds [49], but the work used a different simple sugar and did not include the concentration range of interest of this study.
Protein had no statistically significant impact on the headspace volatile concentration in the system under study (Fig. 7). Although there is some indication of suppression of the maximum headspace intensity, which would support previous studies that have shown casein to interact selectively and transiently with some aroma compounds [50, 51].
Lipid reduced (P < 0.05) the time to achieve maximum equilibrium state (Fig. 8), although this was only statistically significant at a lipid concentration of 50 %. An accelerated time to equilibrium would indicate that dispersion was faster and more effective with increased lipid inclusion. This may indicate that additional lipid may drive increased effectiveness of mixing or promote a more rapid headspace equilibrium, which may be achieved through the transient delivery of insoluble oil droplets directly to the air-product interface prior to emulsification.
Impact of a lipid (0.0, 6.6, 11.8, 50 %), b carbohydrate (0.0, 8.5, 15.6, 50 %) and c protein (0.0, 6.6, 12.4, 50 %) concentration on time to maximum headspace intensity (TMAX ± SD, min). Different letters indicate statistically significant differences within a figure as measured by ANOVA-TUKEYS post hoc test (P > 0.05, n = 3) (protein = casein, carbohydrate = fructose, lipid = sunflower oil). In all cases, the weight of coffee powder was constant
The addition of carbohydrate reduced (P < 0.05) the time to achieve maximum headspace intensity. This was significant at a concentration of 15.6 % and was surprising as additional fructose would increase both the viscosity of the solution [52] and the surface tension [53], thereby reducing mobility and the kinetics associated with mixing phenomena. The results demonstrated contradict this and show that mixing kinetics are enhanced by the addition of fructose and that the delivery of volatile gasses to the headspace occurs more rapidly in a fructose-enriched system. This may be due to enhanced solubility and dissolution kinetics of the powder when enriched in the soluble sugar fructose.
Protein had no impact on the time to maximum headspace intensity (Fig. 8) and there were no underlying trends that could be observed.
In conclusion, the addition of lipid or carbohydrate has a significant impact on the delivery of aroma during hydration. It should be noted that although previous studies have shown similar impacts of lipid on the static partitioning of aroma during the preparation of coffee brews [54], there are no studies investigating the delivery of aroma during the hydration of instant or soluble coffee.
Kim et al. [55] showed a suppressive effect of both fat and protein on coffee aroma headspace partitioning, and Bucking and Steinhart [56] similarly showed that carbohydrates and protein depress the static headspace aroma concentration above coffee brew. Chiralertpong et al. [57] showed a selective impact of non-dairy creamer on the partitioning of non-polar compounds, although no impact was shown on the polar aroma compounds, and there was no impact of sucrose addition (10 % w/w) on aroma partitioning in a coffee brew preparation.
The impact of lipid and carbohydrate on the availability of aroma during the hydration of dried food powders highlights the importance of a true understanding of aroma chemistry during reformulation and product development exercises. Simple product changes such as fat reduction, nutritional enhancement (e.g., addition of protein) and replacement of carbohydrates for materials of enhanced functionality will not only impact the static partitioning of the aroma compounds into the headspace, but will also have a significant impact on other quality attributes, such as the delivery of aroma during hydration and product preparation.
Conclusion
In summary, both lipid and carbohydrate reduce the headspace volatile concentration significantly and reduce the time that is required to achieve equilibrium. Protein has no impact on the headspace volatile concentration or the time to achieve equilibrium. In addition, the delivery rate of the volatiles to the headspace is dependent on the physicochemical properties (log P) of individual volatiles.
References
Meiselman HL, MacFie HJH (1996) Food choice, acceptance and consumption. Blackie Academic and Professional, London
Visschers RW, Jacobs MA, Frasnelli J, Hummel T, Burgering M, Boelrijk AEM (2006) Cross-modality of texture and aroma perception is independent of orthonasal or retronasal stimulation. J Agric Food Chem 54:5509–5515
Beaver JD, Lawrence AD, Van Ditzhuijzen J, Davis MH, Woods A, Calder AJ (2006) Individual differences in reward drive predict neural responses to images of food. J Neurosci 26:5160–5166
Small DM, Veldhuizen MG, Felsted J, Mak YE, McGlone F (2008) Separable substrates for anticipatory and consummatory food chemosensation. Neuron 57:786–797
Xian T, Fisk ID (2012) Salt delivery from potato crisps. Food Funct 3:376–380
Pelosi P (1994) Odorant-binding proteins. Crit Rev Biochem Mol 29:199–228
Malnic B, Hirono J, Takaaki S, Buck L (1999) Combinatorial receptor codes for odors. Cell 96:713–723
Diaz ME (2004) Comparison between orthonasal and retronasal flavour perception at different concentrations. Flavour Frag J 19:499–504
Small DM, Gerber JC, Mak YE, Hummel T (2005) Differential neural responses evoked by orthonasal versus retronasal odorant perception in humans. Neuron 47:593–605
Fisk ID, Kettle A, Hofmeister S, Virdie A, Silanes Kenny J (2012) Discrimination of roast and ground coffee aroma. Flavour
Forss DA (1969) Role of lipids in flavors. J Agric Food Chem 17:681–685
Bayarri S, Taylor AJ, Hort J (2006) The role of fat in flavor perception: effect of partition and viscosity in model emulsions. J Agric Food Chem 54:8862–8868
Gonzalez-Tomas L, Bayarri S, Taylor AJ, Costell E (2007) Flavour release and perception from model dairy custards. Food Res Int 40:520–528
Linforth R, Cabannes M, Hewson L, Yang N, Taylor A (2010) Effect of fat content on flavor delivery during consumption: an in vivo model. J Agric Food Chem 58:6905–6911
Fisk ID, White DA, Carvalho A, Gray DA (2006) Tocopherol—an intrinsic component of sunflower seed oil bodies. J Am Oil Chem Soc 83:341–344
Fisk ID, Gray DA (2011) Soybean (glycine max) oil bodies and their associated phytochemicals. J Food Sci 76:C1349–C1354
Iwanaga D, Gray DA, Fisk ID, Decker EA, Weiss J, McClements DJ (2007) Extraction and characterization of oil bodies from soy beans: a natural source of pre-emulsified soybean oil. J Agric Food Chem 55:8711–8716
White DA, Fisk ID, Makkhun S, Gray DA (2009) In vitro assessment of the bioaccessibility of tocopherol and fatty acids from sunflower seed oil bodies. J Agric Food Chem 57:5720–5726
Carey ME, Asquith T, Linforth RST, Taylor AJ (2002) Modeling the partition of volatile aroma compounds from a cloud emulsion. J Agric Food Chem 50:1985–1990
Hodgson MD, Langridge JP, Linforth RST, Taylor AJ (2005) Aroma release and delivery following the consumption of beverages. J Agric Food Chem 53:1700–1706
Arancibia C, Jublot L, Costell E, Bayarri S (2011) Flavor release and sensory characteristics of o/w emulsions. Influence of composition, microstructure and rheological behavior. Food Res Int 44:1632–1641
Rabe S, Krings U, Berger RG (2004) Dynamic flavour release from miglyol/water emulsions: modelling and validation. Food Chem 84:117–125
Taylor AJ, Mottram DS (1996) Flavour science: recent developments. Woodhead Publishing, Cambridge
de Roos KB (2005) Food lipids. American Chemical Society, Washington, pp 145–158
Brauss MS, Linforth RST, Cayeux I, Harvey B, Taylor AJ (1999) Altering the fat content affects flavor release in a model yogurt system. J Agric Food Chem 47:2055–2059
Voilley A, Étiévant P (2006) Flavour in food. Woodhead Publishing, Cambridge
Hansson A, Andersson J, Leufven A (2001) The effect of sugars and pectin on flavour release from a soft drink-related model system. Food Chem 72:363–368
Friel EN, Linforth RST, Taylor AJ (2000) An empirical model to predict the headspace concentration of volatile compounds above solutions containing sucrose. Food Chem 71:309–317
Kant A, Linforth RST, Hort J, Taylor AJ (2004) Effect of beta-cyclodextrin on aroma release and flavor perception. J Agric Food Chem 52:2028–2035
Anese M, Manzocco L, Maltini E (2005) Effect of coffee physical structure on volatile release. Eur Food Res Technol 221:434–438
Levi G, Karel M (1995) Volumetric shrinkage (collapse) in freeze-dried carbohydrates above their glass transition temperature. Food Res Int 28:145–151
Guichard E (2002) Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev Int 18:49–70
Denker M, Parat-Wilhelms M, Drichelt G, Paucke J, Luger A, Borcherding K, Hoffmann W, Steinhart H (2006) Investigation of the retronasal flavour release during the consumption of coffee with additions of milk constituents by ‘oral breath sampling’. Food Chem 98:201–208
Fisk ID, Linforth R, Taylor A, Gray D (2011) Aroma encapsulation and aroma delivery by oil body suspensions derived from sunflower seeds (helianthus annus) Eur Food Res. Technology 232:905–910
Blee N, Linforth R, Yang N, Brown K, Taylor A (2011) Variation in aroma release between panellists consuming different types of confectionary. Flavour Frag J 26:186–191
Hau MYM, Gray DA, Taylor AJ (1998) Binding of volatiles to extruded starch at low water contents. Flavour Frag J 13:77–84
Pérez-Juan M, Flores M, Toldrá F (2007) Binding of aroma compounds by isolated myofibrillar proteins: effect of protein concentration and conformation. Food Chem 105:932–939
Taylor AJ, Linforth RST, Harvey BA, Blake B (2000) Atmospheric pressure chemical ionisation mass spectrometry for in vivo analysis of volatile flavour release. Food Chem 71:327–338
Fisk ID, White DA, Lad M, Gray DA (2008) Oxidative stability of sunflower oil bodies. Eur J Lipid Sci Technol 110:962–968
Brauss MS, Balders B, Linforth RST, Avison S, Taylor AJ (1999) Fat content, baking time, hydration and temperature affect flavour release from biscuits in model-mouth and real systems. Flavour Frag J 14:351–357
Clawson AR, Linforth RST, Ingham KE, Taylor AJ (1996) Effect of hydration on release of volatiles from cereal foods. Food Sci Technol Leb 29:158–162
Lindinger C, Pollien P, Ali S, Yeretzian C, Blank I, Mark T (2005) Unambiguous identification of volatile organic compounds by proton-transfer reaction mass spectrometry coupled with gc/ms. Anal Chem 77:4117–4124
Lindinger C, Labbe D, Pollien P, Rytz A, Juillerat MA, Yeretzian C, Blank I (2008) When machine tastes coffee: instrumental approach to predict the sensory profile of espresso coffee. Anal Chem 80:1574–1581
Fisk ID, Linforth RST, Taylor AJ, Gray DA (2011) Aroma encapsulation and aroma delivery by oil body suspensions derived from sunflower seeds (helianthus annus). Eur Food Res Technol 232:905–910
Deleris I, Zouid I, Souchon I, Trelea IC (2009) Calculation of apparent diffusion coefficients of aroma compounds in dairy emulsions based on fat content and physicochemical properties in each phase. J Food Eng 94:205–214
Ghosh S, Peterson DG, Coupland JN (2008) Temporal aroma release profile of solid and liquid droplet emulsions. Food Biophys 3:335–343
Relkin P, Fabre M, Guichard E (2004) Effect of fat nature and aroma compound hydrophobicity on flavor release from complex food emulsions. J Agric Food Chem 52:6257–6263
van Ruth SM, King C, Giannouli P (2002) Influence of lipid fraction, emulsifier fraction, and mean particle diameter of oil-in-water emulsions on the release of 20 aroma compounds. J Agric Food Chem 50:2365–2371
Decourcelle N, Lubbers S, Vallet N, Rondeau P, Guichard E (2004) Effect of thickeners and sweeteners on the release of blended aroma compounds in fat-free stirred yoghurt during shear conditions. Int Dairy J 14:783–789
Fares K, Landy P, Guilard R, Voilley A (1998) Physicochemical interactions between aroma compounds and milk proteins: effect of water and protein modification. J Dairy Res 81:82–91
Guichard E (2006) Flavour retention and release from protein solutions. Biotechnol Adv 24:226–229
Nikam PS, Ansari HR, Hasan M (2000) Density and viscosity studies of glucose and fructose solutions in aqueous and 0.5 mol dm3 aqueous nh 4 cl. J Mol Liq 87:97–105
Adhikari B, Howes T, Shrestha A, Bhandari BR (2007) Effect of surface tension and viscosity on the surface stickiness of carbohydrate and protein solutions. J Food Eng 79:1136–1143
Bücking M, Steinhart H (2002) Headspace gc and sensory analysis characterization of the influence of different milk additives on the flavor release of coffee beverages. J Agric Food Chem 50:1529–1534
Kim KJ, Rho JM, Kim SY (1995) The effect of creamer/milk addition to coffee aroma retention in coffee solution. Colloque Scientifique International sur le Cafe, Kyoto
Bucking M, Steinhart H (1999) Frontiers of flavour science. WB-Druck, Freising
Chiralertpong A, Acree T, Barnard J, Siebert K (2008) Taste–odor integration in espresso coffee. Chemosens Percept 1:147–152
Acknowledgments
Margarida Carvalho Da Silva and Jenny Drury are acknowledged for their assistance in the moisture content analysis and during proof reading.
Conflict of Interest
The authors declare that they have no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Fisk, I.D., Boyer, M. & Linforth, R.S.T. Impact of protein, lipid and carbohydrate on the headspace delivery of volatile compounds from hydrating powders. Eur Food Res Technol 235, 517–525 (2012). https://doi.org/10.1007/s00217-012-1776-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1776-x