Abstract
The aim of this study was to investigate the activity of lipoxygenase extracted from Lupinus angustifolius seeds and its interactions with native lupin polyphenols. The examinated enzyme is active at pH 7.5 causing peroxidation of linoleic acid. The main hydroperoxide produced in the presence of investigated lupin lipoxygenase was 13-hydroperoxy-octadecadienoic acid (13-HPODE t-t). In the presence of the phenolic compounds extracted from L. angustifolius seeds, the changes of lipoxygenase activity were observed. A distinct antioxidant effect of lupin polyphenols observed at their low concentration (0.58 μg/ml) was almost completely overcome at higher concentration of phenolic compounds (2.91 μg/ml). It comes probably from oxidation of polyphenols in the presence of lupin lipoxygenase. In this mechanism, polyphenol radical formation is possible. The formed radicals decrease the antioxidant efficiency of the phenolic compounds. Eventual phenoxyl radical-mediated peroxidation may be inhibited by the presence of antioxidants able to regenerate flavonoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lipoxygenase (LOX EC 1.13.11.12) is a nonheme iron-containing enzyme belonging to dioxygenase that catalyzes the hydroperoxidation of linoleic acid as well as other polyunsaturated fatty acids containing cis, cis-1,4-pentadiene double bond [1]. Lipoxygenase activity requires presence of free polyunsaturated fatty acids. Linoleic acid is the most commonly available plant-originated food substrate for lipoxygenase. This enzyme occurs as multiple isoform that differs in case of its optimum pH as well as reaction substrates and products. In soybean seeds, four lipoxygenase isoenzymes were isolated [2]. The first of them interacts with free fatty acids at optimal pH 9.0, and as a result of reaction at room temperature, 9- and 13-hydroperoxides are produced in a ratio of 1–9. The second enzyme acts on triacylglycerides as well as free fatty acids and has optimum pH at 6.8. At room temperature, the second isoform of lipoxygenase produced 9- and 13-hydroperoxides in a ratio 1–1. The third isoform is very similar to the second, but its activity is inhibited by calcium ions. Fourth lipoxygenase isoenzyme is very similar to the third isoenzyme, but it is activated by calcium ions [2]. Most lipoxygenase isoenzyme occurring in plants are classified as first type that acts in an alkaline pH on free fatty acids or as second type that has optimum activity at neutral pH and contributes to co-oxidation of carotenoids. This enzyme might play a key role in utilizing of lupin as a valuable source of protein for human consumption and animal feedstuff [3]. The hydroperoxide products resulting from action of lipoxygenase on polyunsaturated fatty acids can lead to reactions of formed peroxyl radical complexes with vitamins, pigments, proteins and polyphenols which may cause reduction in the quality of lupin as a material for food industry.
Lupin, like other legumes, is a rich source of phenolic compounds. Presence of these compounds form, a very desirable from a nutritional point of view, antioxidant properties of lupin seeds [4, 5]. Phenols occurring in lupin seeds can be divided into several groups, but main phytochemicals belong to apigenin C-glycosides derivatives and were identified to be apigenin-6,8-di-C-β-glucopyranoside as well as apigenin 7-O-β-apiofuranosyl-6,8-di-C-β-glucopyranoside [6]. These compounds were noted in all of three economically important lupin seeds species, namely L. albus, L. luteus and L. angustifolius. During germination of lupin seeds, qualitative and quantitative changes in the content of phenolic compounds may take place [7]. Therefore, these two apigenin glucosides have not been detected in leaves and roots of 3-week-old plants [8].
Undesired effect of lipoxygenase activity may be minimized by utilizing LOX inhibitory properties of phenolic compounds [9]. Several mechanisms explaining this phenomenon have been reported. Soybean isoflavones inhibit lipoxygenase by iron reduction in active site of the enzyme [10]. Semi-quinones as well as quinones occuring during the oxidation of polyphenols may bind to sulfhydryl or amino groups of the enzyme that leads also to inhibition [11]. Well-known properties of phenolic compounds which prevent oxidation of linoleic acid by free radical scavenging can cause LOX inhibition. Pea seeds lipoxygenase could be effectively inhibited by caffeic acid as well as flavonoids like catechin and quercetin [12].
There are many studies providing information about lupin seed lipoxygenase that mainly focus on purification and characteristic of enzyme [3, 13, 14]. Also many authors demonstrate results on health promoting properties of phenolic compounds that occur in lupin seeds [4–6]. However, a detailed study of lipoxygenase inhibitory activities by native flavonoids from lupin seed has not been studied, yet. Therefore, in the presented work, we investigated these interactions.
Materials and methods
Reagents
Vitexin (5,7,4’-trihydroxyflavone-8-C-glucoside), gallic acid, Folin–Ciocalteu reagents, 2,2’-diphenyl-1-picrylhydrazyl radical (DPPH·), tris(hydroxymethyl) aminomethane (TRIS), dithiothreitol (DDT), ethylenediaminetetraacetic acid (EDTA), sodium metabisulphite, Triton X-100, linoleic acid were purchased from Sigma-Aldrich (St. Louis, MO). Acetonitrile, methanol and orthophosphoric acid (all HPLC-grade) were obtained from Merck (Darmstadt, Germany). All other solvents and chemicals used in this study were of analytical grade. Redistilled water was used in the resin-based column chromatography, while ultrapure water purified via the Milli-Q system (Millipore, Bedford, USA) was used during the HPLC analysis.
Plant material
Narrow-leaf lupin seeds (L. angustifolius, cv. Zeus) were obtained from the Plant Breeding Station Smolice, Przebędowo branch. All samples were milled with an IKA M20 universal laboratory mill (IKA-Werke GmbH&Co, Staufen, Germany), sieved to obtain a fraction below 1.6 mm and defatted using an automatic Soxhlet Büchi Extraction System B-811 (Büchi Labortechnik AG, Flawil, Switzerland). The extraction with n-hexane was carried out for 2 h. Samples were stored in closed polyethylene bags at −18 °C until the analysis.
Lipoxygenase extraction
Lipoxygenase from L. angustifolius seeds was partially purified using the procedure described by Yoshie-Stark & Wäsche [3] with some modifications. To ensure optimum enzyme activity, extraction buffer was 50 mM Tris–HCl pH 7.5 containing additionally 0.3 mM DDT, 0.2 mM EDTA, 10 mM sodium metabisulphite, 0.1 % Triton X-100 and 5 g of PVPP. The defatted ground lupin seeds were suspended with this buffer in proportion 1:20 (w/v) and mechanical stirring for 1 h at 4 °C. The resulting suspension was centrifuged for 30 min at 20,000g and 4 °C, the pellet was discard, and the supernatant was subjected to second additional centrifugation step (30 min at 20,000g and 4 °C). Partial purification of the enzyme was performed by ammonium sulfate precipitation of obtained supernatant. The supernatant was mixed with 75 % saturated ammonium sulfate for 1 h at 4 °C. The precipitate was obtained by centrifugation at 20,000g for 30 min. The pellet was re-suspended in small volume of 50 mM Tris–HCl buffer at pH 7.5. The obtained enzyme extract was desalted by applying HiTrap Desalting column (Pharmacia, Upssala, Sweden) equilibrated with 50 mM Tris–HCl buffer at pH 7.5. The absorbance of the fractions was measured at 280 nm, and desalted proteins were collected in 2 ml fractions. Desalting step was carried using automatic FPLC system (Pharmacia, Upssala, Sweden).
Protein concentration measurements
Protein concentration was determined by colorimetric Bradford method [15]. Analyses were carried out at wavelength λ = 595 nm (UV–Vis spectrophotometer SP 8001, Metertech Inc. Taipei, Taiwan). To determine protein concentration, standard curve based on BSA has been made (y = 0.6192x; R 2 = 0.9966).
Methanol extracts of polyphenols
To obtain polyphenols from narrow-leaf lupin seeds (L. angustifolius, cv. Zeus), sample was extracted three times with 80 % methanol. In brief, 5 g of sample was extracted with 50 ml methanol in three separate stages for 30 min, at 50 °C temperature. After centrifugation (10 min at 5,000g) (model 6K15, Sigma, Osterode am Harz, Germany), the precipitate was re-extracted twice more following the same steps. The three supernatants were combined and evaporated under reduced pressure using an R-215 rotorvapor (Büchi Labortechnik AG, Flawil, Switzerland) to a volume 25 ml.
Total phenolics content
The total phenolics content in methanolic extract was determined by the Folin–Ciocalteu method [16, 17]. An aliquot (0.2 ml) of the methanolic extract was placed in a volumetric flask (10 ml). Diluted Folin–Ciocalteu reagent (0.5 ml) was added. Saturated solution of sodium carbonate (1 ml) was added after 3 min. The flask was filled with water up to 10 ml. Absorbance at λmax 725 nm against a reagent blank was measured after 1 h using a UV–Vis spectrophotometer SP 8001 (Metertech Inc., Taipei, Taiwan). Gallic acid (0–20 μg/ml) was used to produce standard calibration curve y = 0.0109x (R 2 = 0.9959). The total phenolic content was expressed in mg of gallic acid equivalents/100 g dry plant material.
Determination of the flavonoids composition
Identification and quantification of L. angustifolius seeds’ flavonoids was carried out by combining for identification characteristic MS fragmentation patterns as well as for quantitative UV-DAD analyses utilizing vitexin as an equivalent as it was described previously [6].
Radical scavenging activity
The method consisted of spectrophotometric measurement of the intensity of the color change in solution depending on the amount of DPPH·. The reaction was initiated by mixing polyphenol methanolic extract with 3 ml methanol and then adding 1 ml of DPPH· (0.012 g/100 ml). Absorbance at λmax = 517 nm (UV–Vis spectrophotometer SP 8001, Metertech Inc. Taipei, Taiwan) was checked after 30 min of incubation in the darkness. The activity of the extract in scavenging DPPH· was expressed as ARP (antiradical power), according to formula:
where EC 50 is the concentration of antioxidant required to cause a 50 % reduction in the original concentration of DPPH.
Linoleic acid peroxidation in the presence of lupin lipoxygenase and polyphenols extracts
For linoleic acid peroxidation experiments, a solution containing 23 μg/ml of lipoxygenase from L. angustifolius seeds, 96 μM of linoleic acid and 0.58–2.91 μg/ml of lupin polyphenols in 50 mM Tris–HCl buffer (pH 7.5) was prepared. Each sample was completed with buffer to final volume of 2.13 ml. The samples were incubated in the darkness at the room temperature (22 °C). The peroxidation was stopped by adding 15 μl of 0.2 M hydrochloric acid solution.
HPLC analysis of linoleic acid hydroperoxides (FAOOHs)
The procedures of Banni et al. [18] were adopted for HPLC analysis of the linoleic acid hydroperoxides. Identification and quantification of hydroperoxides was achieved using analytical reversed-phase high-performance liquid chromatography (HPLC—Waters, Milford, MA, USA) using a LiChrosorb RP-18 (250 × 4.6 mm; 5 μm) (Merck, Germany). An isocratic program was used with the mobile phase, combining acetonitrile, water and acetic acid (60:40:0.12 v/v). The flow rate was 1.5 ml/min. Signal was monitored at 200–300 nm with the diode array detector (PDA detector 2998 Waters, Milford, MA, USA). The content of individual fatty acid hydroperoxides (FAOOHs) in all samples was calculated on the basis of calibration curves made for pure 13-hydroperoxy-octadecadienoic acid (13-HPODE) and 13-hydroperoxy-octadecatrienoic acid (13-HPOTE) standards that were synthesized as described by Nogala-Kalucka et al. [19].
Spectroscopic measurements
The absorption spectra were measured in the range 260–500 nm with a UV–Vis SP 8001 spectrophotometer (Metertech Inc. Taipei, Taiwan) in a 1 × 1 cm quartz cuvette. The concentration of lupin lipoxygenase dissolved in 50 mM Tris–HCl buffer (pH 7.5) was 23 μg/ml, linoleic acid 96 μM and lupin phenolic compounds 0.58–2.91 μg/ml. All above mentioned components were placed into quartz cuvette where the reaction was carried out.
Statistical analysis
Results are presented as means ± standard deviation from three replicates of each experiment. A P value <0.05 was used to denote significant differences between mean values determined by the analysis of variance (ANOVA) with the assistance of Statistica 10.0 (StatSoft, Inc., Tulsa, OK) software.
Results and discussion
Phenolics content and DPPH radical scavenging ability
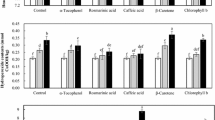
The content of phenolic compounds in narrow-leaf lupin seeds (L. angustifolius, cv. Zeus) was described in details in our previous work [6]. The dominant flavonoid compounds in L. angustifolius seeds are apigenin-6,8-di-C-β-glucopyranoside and apigenin 7-O-β-apiofuranosyl-6,8-di-C-β-glucopyranoside. The content of apigenin glycosides was expressed as vitexin (5,7,4’-trihydroxyflavone-8-C-glucoside) equivalents because vitexin is structurally closely related to these two compounds. The content of those compounds in the lupin seeds utilized in this study was 40.08 and 66.90 mg/100 g d.m., respectively (Table 1). The total phenolic compounds content determined using Folin–Ciocalteu method was 162.58 mg/100 g d.m. as a gallic acid equivalent. Lampart-Szczapa et al. [4] showed that the total content of phenolic compounds (as caffeic acid equivalent) was smaller in the testa than in the cotyledons. It was demonstrated that the highest content was noted in two bitter cultivars, L. albus cv. Bac and L. angustifolius cv. Mirela. In cotyledons, it was 131.9 and 187.8 mg/100 g, respectively, and in testa, 41.7 and 28.8 mg/100 g, respectively. In cotyledons of the remaining cultivars, the total phenol content ranged from 31.8 (L. luteus cv. Piast) to 41.5 mg/100 g (L. luteus cv. Popiel), and in testa, from 16.0 (L. luteus cv. Popiel) to 24.5 mg/100 g (L. luteus cv. Piast). Pastor-Cavada et al. [20] show the total content of phenolic compounds (expressed as catechin equivalent) in different species of lupin from 8.7 mg/g in L. micranthus flour up to 11.0 mg/g in L. angustifolius flour. Similar polyphenol contents were observed in different genotypes of cultivated L. angustifolius [21]. Sosulski and Dabrowski [22] reported that defatted flours of 10 legumes contained only soluble esters of trans-ferulic, trans-p-coumaric and syringic acids. Among these legumes, mung bean, field pea, faba bean, lentil and pigeon pea contained from 18 to 31 mg of total phenolic acid esters per kilogram, while in Navy bean, lupin, lima bean, chickpea and cowpea, the total amount of these compounds ranged from 55 to 163 mg/kg. Dueñas et al. [7] studied the effect of germination process on the level of phenolic compounds in lupin. In the analyzed sample extracts, several phenolic compounds, nonflavonoids hydroxybenzoics, hydroxycinnamics and flavonoids, such as flavones, isoflavones and dihydroflavonols, were identified. Among hydroxybenzoic and hydroxycinnamic compounds, these authors have identified such compounds as protocatechuic acid glycoside (an infrequent form found in legumes), trans-p-coumaroyl-glutaric acid (is reported for the first time in lupin), ferulic acid glycoside and ferulic acid derivative (corresponding to a feruloyl malic acid). Concentration of compounds studied by them in lupin seeds were 4.6 μg/g hydroxybenzoics, 0.30 μg/g hydroxycinnamics, 19.1 μg/g flavones, 0.1 μg/g dihydroflavonols and 0.9 μg/g isoflavones, respectively [7]. This confirms that the main phenolic compounds that occur in lupin seeds are classified as flavonoids.
The antioxidant properties of phenolic compounds are of great importance and are related to their qualitative and quantitative composition. DPPH radical scavenging activity of lupin seeds extract was also establish at 7.20 μM Trolox Eq/g d.m (Table 1). This result is close to values obtained in our previous study for L. angustifolius (6.89–7.47 μM Trolox Eq/g d.m.) and other lupin species (L. albus—from 3.51 to 6.78 μM Trolox Eq/g d.m.; L. luteus—from 8.12 to 9.03 μM Trolox Eq/g d.m) [6]. Data provided by various authors about antioxidant capacity of lupin seeds slightly differ [23–25]. However, these differences are mainly due to varietal characteristics, climate change, agrotechnical and storage conditions as well as factors related to the method of extraction of compounds showing antioxidant activity form the seeds.
Linoleic acid peroxidation in the presence of lupin lipoxygenase and phenolic extracts
In the presence of lupin lipoxygenase, linoleic acid undergoes peroxidation, and linoleic acid hydroperoxides are formed. The investigated lipoxygenase was active at pH 7.5. This is consistent with the results obtained by Yoshie-Stark and Wasche [3], who reported that lipoxygenase isolated from L. angustifolius reaches maximum of its activity at this pH. After 3 min of incubation at 22 °C, the concentration of total hydroperoxides in the sample containing 23 μg/ml of lipoxygenase and 96 μM of linoleic acid reached 216.73 μM/g fatty acid (Table 2). The main hydroperoxide produced in the presence of investigated lupin lipoxygenase was 13-hydroperoxy-octadecadienoic acid (13-HPODE t-t) (Fig. 1). This isomer is typical for many plant lipoxygenases [9, 26]. The presence of lupin polyphenols in the samples containing lipoxygenase and linoleic acid caused inhibition of lipoxyganase activity. Similarly, the main hydroperoxide produced by lupin lipoxygenase in the presence of polyphenols was 13-hydroperoxy-octadecadienoic acid (13-HPODE t-t) (Fig. 2). After 3 min of incubation at 22 °C in the presence of 0.58 μg/ml polyphenols, the content of hydroperoxides decreased to 86.58 μM/g fatty acid in comparison with 216.73 μM/g fatty acid in the absence of phenolic compounds (Table 2). Surprisingly, the increase in polyphenols content caused a reduction in inhibition effect. The concentration of hydroperoxides after 3 min of incubation in the samples containing 1.45 and 2.91 μg/ml of polyphenols amounted to 149.42 and 199.46 μM/g fatty acid, respectively (Table 2). The observed effect may come from pro-oxidant properties of polyphenols. In order to check polyphenol changes during incubation with lipoxygenase, UV–Vis spectra were obtained (Fig. 3a, b). In the samples containing polyphenols (0.58–2.91 μg/ml) with lipoxygenase and linoleic acid, the increase in band with maximum at about 276 nm and decrease in band with maximum at about 375 nm during incubation were observed. The band with maximum at about 270–280 nm is attributed to oxidized polyphenols. Formation of the band with the maximum at 283 nm was noticed during oxidation of rapeseed lipoxygenase with rapeseed native polyphenolic compounds [9]. This is also in accordance with the conclusion of Chedea et al. [11], who observed the formation of oxidized polyphenolic compounds after incubation of catechins and polyphenols from grape in the presence of lipoxygenase in primary leukocyte culture. The authors assigned arising band at 282–288 nm to free and oxidized polyphenols. Jimenez and Garcia-Carmona [27] observed that quercetin in the presence of sodium periodate or polyphenol oxidase undergoes oxidation, and the products of quercetin oxidation were characterized by the increase in absorption at 291 nm and decrease at 372 nm in comparison with quercetin. According to Pinto and Macias [28], quercetin incubated in the presence of lipoxygenase and linoleic acid may be oxidized to pro-oxidant species. During oxidation, a decrease in the band centered at 375 nm and a slight increase in absorbance in the region 330–340 nm were observed. The changes of spectra observed during our experiments (increase of band with maximum at about 276 nm and decrease of band with maximum at about 375 nm) may therefore come from oxidation of lupin polyphenols in the presence of native lipoxygenase and linoleic acid. This oxidation may be a reason of reduction in antioxidant effects observed when higher concentration of polyphenols (1.45 and 2.91 μg/ml) was present in the samples. It leads to decrease in polyphenols concentration able to scavenge free radicals. On the other hand, the polyphenol radical formation is possible. In this mechanism proposed by Patel et al. [29], the flavonoids react with the peroxyl radicals forming the corresponding phenoxyl radicals and hydroperoxides. The arising radicals decrease the antioxidant efficiency of the compounds. In our case, the peroxyl radicals were formed during linoleic acid oxidation. Such pro-oxidant properties have been shown for apigenin—flavonoid presents in lupin as well as in bamboo leaves extract containing vitexin and isovitexin [6, 30–33]. Apigenin B-ring phenoxyl radical may be responsible for the observed pro-oxidant effect [31]. This moiety is present in vitexin and isovitexin molecules as well as in the apigenin C-glucosides found in lupin seeds.
Conclusions
The lipoxygenase extracted from L. angustifolius seeds is active at pH 7.5 causing peroxidation of linoleic acid. The main hydroperoxide produced in the presence of investigated lupin lipoxygenase was 13-hydroperoxy-octadecadienoic acid (13-HPODE t-t). In the presence of the phenolic compounds extracted from L. angustifolius seeds, the changes of lipoxygenase activity were observed. A distinct antioxidant effect of lupin polyphenols observed at their low concentration (0.58 μg/ml) was almost completely overcome at higher concentration of phenolic compounds (2.91 μg/ml). It comes probably from oxidation of polyphenols in the presence of lupin lipoxygenase. The formation of polyphenol radicals is possible. The formed radicals decrease the antioxidant efficiency of the phenolic compounds. Eventual phenoxyl radical-mediated peroxidation may be inhibited by the presence of antioxidants able to regenerate flavonoids.
References
Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenase from soybeans. Met Enzymol 91:441–451
Gordon MH (2001) In: Pokorny J, Yanishlieva M, Gordon MH (eds) Antioxidants in food. Practical applications. CRC Press LLC, Boca Raton
Yoshie-Stark Y, Wäsche A (2004) Characteristics of crude lipoxygenase from commercially de-oiled lupin flakes for different types of lupins (Lupinus albus, Lupinus angostifolius). Food Chem 88:287–292
Lampart-Szczapa E, Siger A, Trojanowska K, Nogala-Kałucka M, Małecka M, Pachołek B (2003) Chemical composition and antibacterial activities of lupin seeds extracts. Nahrung/Food 47:286–290
Lampart-Szczapa E, Korczak J, Nogala-Kalucka M, Zawirska-Wojtasiak R (2003) Antioxidant properties of lupin seed products. Food Chem 83:279–285
Siger A, Czubinski J, Kachlicki P, Dwiecki K, Lampart-Szczapa E, Nogala-Kalucka M (2012) Antioxidant activity and phenolic content in three lupin species. J Food Compos Anal 25:190–197
Dueñas M, Hernandez T, Estrella I, Fernandez D (2009) Germination as a process to increase the polyphenol content and antioxidant activity of lupin seeds (Lupinus angustifolius L.). Food Chem 117:599–607
Muth D, Marsden-Edwards E, Kachlicki P, Stobiecki M (2008) Differentiation of isomeric malonylated flavonoid glyconjugates in plant extracts with UPLC-ESI/MS/MS. Phytochem Anal 19:444–452
Dwiecki K, Siger A, Czubinski C, Nogala-Kałucka M, Lampart-Szczapa E (2012) The interactions between rapeseed lipoxygenase and native polyphenolic compounds in a model system. J Am Oil Chem Soc 89:379–387
Mahesha HG, Singh SA, Rao AG (2007) Inhibition of lipoxygenase by soy isoflavones: evidence of isoflavones as redox inhibitors. Arch Biochem Biophys 461:176–185
Chedea VS, Braicu C, Socaciu C (2010) Antioxidant/prooxidant activity of a polyphenolic grape seed extract. Food Chem 121:132–139
Szymanowska U, Jakubczyk A, Baraniak B, Kur A (2009) Characterization of lipoxygenase from pea seeds (Pisum sativum var. Telephone L.). Food Chem 116:906–910
Olias JM, Valle M (1988) Lipoxygenase from lupin seed: purification and characterization. J Sci Food Agric 45:165–174
Jacobo-Velázquez DA, Hernández-Brenes C, Cisneros-Zevallos L, Benavides L (2010) Partial purification and enzymatic characterization of avocado (Persea americana Mill, cv. Hass) lipoxygenase. Food Res Int 43:1079–1085
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye-binding. Anal Biochem 72:248–254
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolibdicphosphotungstic acid reagent. Am J Enol Vitic 16:144–158
Siger A, Nogala-Kałucka M, Lampart-Szczapa E (2008) The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipid 15:137–149
Banni S, Day BD, Evans RW, Corongiu FP, Lombardi B (1995) Detection of conjugated diene isomers of linoleic acid in liver lipids of rats fed a choline-devoid diet indicates that the diet does not cause lipoperoxdation. J Nutr Biochem 6:281–289
Nogala-Kałucka M, Kupczyk B, Polewski K, Siger A, Dwiecki K (2007) Influence of native antioxidants on the formation of fatty acid hydroperoxides in model systems. Euro J Lipid Sci Tech 109:1028–1037
Pastor-Cavada E, Juan R, Pastor JE, Alaiz M, Vioque J (2010) Antioxidant activity in the seeds of four wild lupinus species from southern Spain. J Food Biochem 34:149–160
Oomah BD, Tiger N, Olson M, Balasubramanian P (2006) Phenolics and antioxidative activities in narrow-leafed lupins (Lupinus angustifolius L.). Plant Food Hum Nutr 61:91–97
Sosulski F, Dabrowski K (1984) Composition of free and hydrolyzable phenolic acids in the flours and hulls of ten legume species. J Agri Food Chem 32:131–133
Martínez-Villaluenga C, Zielinski H, Frias J, Piskuła MK, Kozłowska H, Vidal-Valverde C (2009) Antioxidant capacity and polyphenolic content of high-protein lupin products. Food Chem 112:84–88
Wang S, Clements J (2008) In: Palta JA, Berger JB (eds) Lupins for health and wealth, Proceedings of the 12th international lupin conference, 14–18 September 2008, Fremantle, Western Australia. International Lupin Association, Canterbury, New Zealand
Ranilla LG, Genovese MI, Lajolo FM (2009) Isoflavones and antioxidant capacity of Peruvian and Brazilian lupin cultivars. J Food Compos Anal 22:397–404
Allen CL, Lancaster JE, Robinson DS (1999) Lipoxygenase activity in seeds from New Zealand native plants. New Zeal J Bot 37:737–745
Jiménez M, García-Carmona FJ (1999) Oxidation of the flavonol quercetin by polyphenol oxidase. J Agric Food Chem 47:56–60
Pinto MC, Macias P (2005) Oxidation of dietary polyphenolics by hydroperoxidase activity of lipoxygenase. J Agric Food Chem 53:9225–9230
Patel RP, Boersma BJ, Crawford JH, Hogg N, Kirk M, Kalyanaraman B, Parki DA, Barnes S, Darley-Usmar V (2001) Antioxidant mechanisms of isoflavones in lipid system: paradoxical effects of peroxyl radical scavenging. Free Rad Biol Med 31:1570–1581
Galati G, Sabzevari O, Wilson JX, O’Brien PJ (2002) Pro-oxidant activity and cellular effects of the phenoxyl radicals of dietary flavonoids and other polyphenolics. Toxic 177:91–104
Miyoshi N, Naniwa K, Yamada T, Osawa T, Nakamura Y (2007) Dietary flavonoid apigenin is a potential inducer of intracellular oxidative stress: the role in the interruptive apoptotic signal. Arch Biochem Bioph 466:274–282
Tsuji PA, Walle T (2008) Cytotoxic effects of the dietary flavones chrysin and apigenin in a normal trout liver cell line. Chem-Biol Inter 171:37–44
Zhang Y, Zhang Y (2008) Effect of natural antioxidants on kinetic behavior of acrylamide formation and elimination in low-moisture asparagine–glucose model system. J Food Engin 85:105–115
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Czubinski, J., Dwiecki, K., Siger, A. et al. Interactions between Lupinus angustifolius seeds lipoxygenase and native phenolic compounds in the model system. Eur Food Res Technol 235, 67–73 (2012). https://doi.org/10.1007/s00217-012-1737-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1737-4