Abstract
Cultivation of soybean sprouts in abiotic stress conditions, resulted from the presence of 5–25 mM FeSO4 in the culture media, causes a strong overexpression of ferritin. Accumulation of ferritin iron in sprouted seeds germinated in the 20 mM solution of FeSO4 was 67 times higher than in sprouts germinated in distilled water. The cultivation conditions also influence on another antioxidant content—mainly β-carotene content, which increased 28 times (in sprouts cultured in 10 mM FeSO4 solution) in comparison to the content in dry seeds. Obtained in stress conditions sprouted seeds contain less tocochromanoles than raw seeds. However, their total tocochromanol content was higher than in sprouted seeds cultured in distilled water in every examined concentration of Fe2+. A total antioxidant activity is increased only during culturing in 0–10 mM media, and it is positively correlated to the total phenolic compounds content (r = 0.8498). We concluded that germination in high abiotic stress also causes the increase in different antioxidants content, not only in ferritin, which is directly involved in the process of iron detoxification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Maintaining body iron homeostasis is essential for the proper functioning of human body. Lack of iron leads to anemia, and an excess to serious disorders resulting from primary (hemochromatosis), and secondary iron accumulation in the body (thalassemia, anemia of chronic disease, chronic viral hepatitis, alcoholic cirrhosis) [1–4]. Anemia is a common problem throughout the world and iron deficiency is the most prevalent nutritional deficiency in the world. It affects mainly the poorest segment of the population, particularly where malnutrition is predominant and the population is exposed to a high risk of water-related infection. According to World Health Organization (WHO), about 2 billion people suffer from anemia. The problem is particularly evident in developing countries where 90 % of people live with iron deficiency anemia [5]. Iron deficiency anemia is defined as the one that occurs when iron loss (often from intestinal bleeding or menses) occurs, and/or the dietary intake or absorption of iron is insufficient. In the second case, even if the supply of the microelement in the diet is quite high, the problem is often related to its bioavailability. In non-vegetarian diet, only 10–15 % of supplied iron is absorbed in the entherocytes of the duodenum. In the diet based on vegetables, the bioavailability is much lower [6]. The absorption of iron is strictly regulated, controlled, and enhanced in the time of increased demand for this microelement [7]. Therefore, the simplest method of preventing the development of anemia is iron supplementation. The supplementation may take various forms and is associated with various technical problems, such as proper selection of matrix, the balancing of inhibitors and enhancers, supplement stability, acceptability, costs and benefits of its intake [8, 9]. Therefore, an effective way to introduce a population level of iron in the food chain is still desired. The new, good source of iron should both limit its losses from food and increase its assimilation by the human body.
One of the directions indicated by the WHO for the prevention of anemia is the fortification of food in a readily absorbable form of iron [10]. Biofortification of edible parts of plants is postulated as the one of the alternative methods for the introduction of micronutrients (including iron) to the food chain [11]. Plants are an essential component of the food chain. The appropriate plants growth and thus the processes of synthesis of organic compounds are determined by the absorption of minerals from the soil. Due to the potential toxicity of many minerals present in soil, plants tightly regulate the processes of their imbibition and storage. Due to the adverse effects of both deficiency and excess of iron in the cell, the absorption of these ions involves various transporters and storage options [12]. Iron is stored in vacuoles, apoplasmic space, and first of all in plastids, as ferritin [13]. Ferritin is sphericeral protein cage around nanomineral of hydrated ferric oxide. The protein is able to accumulate up to 4,500 iron atoms in the apoferritin shell [14]. High accumulation of phytoferritin is observed especially in iron-rich organs (such as legume seeds) and synergistic bacteroids of leguminous root nodules [15].
Germination of soybean changes a level of almost all constituents present in raw seeds. As a result of proteolysis, the content of proteins decreases and simultaneously the content of amino acids and non-protein nitrogen increases [16, 17]. The lipids content is progressively lowered during germination [17]. Despite the fact that high activity of amylases is observed, an increase in starch content is noted [18]. Dietary fiber is reduced [19], but changes in mineral content depends on germination conditions [17]. The level of most vitamins is also increased [20]. Furthermore, sprouting decreases the content of antinutrients such as trypsin inhibitors, phytic acid, and galactosides [21, 22].
However, there is little data about the impact of biofortification during germination in abiotic stress conditions such important compounds as natural antioxidants. The aim of the experiment is to examine whether the strong concentration of iron during cultivation process influence not only on ferritin synthesis, but also on synthesis another antioxidants, such as tocopherols and tocotrienols, β-carotene, phenolic compounds.
Materials and methods
Chemicals
Methanol (HPLC-grade) and Folin–Ciocalteu reagent, 2,2′-diphenyl-1-picrylhydrazyl radical (DPPH), bovine serum albumin (BSA), caffeic acid, and quercetin were purchased from Sigma (St. Louis, MO). The tocopherols, tocotrienols, and β-carotene were purchased from Merck (Darmstadt, Germany). 1,4-Dioxane and n-hexane (both HPLC-grade) were obtained from Merck (Darmstadt, Germany). All other solvents and chemicals used in this study were of analytical grade. Deionized water was used in the resin-based column chromatography while ultrapure water purified via the Milli-Q system (Millipore, Bedford, USA) was used during analysis and germination soybean seeds. Stock and working standard of tocopherols, tocotrienols, and β-carotene were prepared by dissolving these analytes in n-hexane solution. The standard solutions stored at 4 °C were stable for at least 1 month.
Materials
Soybean seeds (Glycine max, cultivars Naviko) were obtained from the Department of Genetics and Plant Breeding, Poznan University of Life Sciences, Poland, harvested in the summer of 2009.
Soybean sprouts cultivation
Seeds were soaked in 70 % ethanol solution for 15 min at room temperature for disinfection. After washing out of ethanol from the seeds with tap and distilled water, dry soybean seeds were soaked for successive 12 h in FeSO4 solutions (0–25 mM FeSO4). Afterward, samples were cultured in the special germination dishes for 7 days at room temperature and 12 h of daylight illumination. They were watered every day with fresh FeSO4 solution with respective concentrations. During this time, the radicle of the seed came out, and the seed’s coat was torn. Finally, obtained sprouted seeds (radicle with cotyledons) were dried in a stream of warm (40 °C) circulating air to 8–10 % of moisture content. Samples of dried sprouted seeds were milled with an IKA M20 universal laboratory mill (IKA-Werke GmbH&Co, Staufen, Germany) and stored in the powder form in tightly sealed containers at room temperature. The preparation obtained from three replications of culture was mixed together.
Determination of ferritin iron content
1 g of milled powder was extracted with 20 mL of 6 M HCl for 30 min at 80 °C. Extracted inorganic iron, not chelated and not introduced into organic compounds, was determined after thiocyanate reaction spectrophotometrically (λ = 480 nm) [23] and is called in the text: free iron. Total iron content was determined after samples mineralization by atomic absorption spectrometry (λ = 248.3, slit of 0.15 nm) [24]. The difference between total iron and inorganic iron content is considered to be the organic bounded iron (the ferritin iron) content.
Determination of lipids content
Gravimetric determination of total lipids content was determined by multiple continuous sample extraction with petroleum ether (a mixture of pentanes and hexanes with a boiling point of 35–40 °C) (by 6 h). Extraction was performed using an automatic Soxhlet Büchi Extraction System B-811 (Büchi Labortechnik AG, Flawil, Switzerland). Total lipid content in a sample was measured as recommended in the [25].
Tocopherols, tocotrienols, and β-carotene analysis
In order to determine tocopherols, tocotrienols and β-carotene content, samples of dried soybean sprouted seeds (2 g) were saponified using 60 % KOH (2 mL), ethanol (20 mL), and pyrogallol (0.5 g). After 30 min of heating at the solvent boiling point (78 °C), 50 mL of 1 % NaCl solution were added and the samples were than cooled. The unsaponifiable substances were extracted using 50 mL n-hexane/ethyl acetate (90:10 v/v) [26, 27]. Tocopherols, tocotrienols, and β-carotene were qualitatively and quantitatively identified using liquid chromatography HPLC (Waters 600 Asc. Milford, MA, USA). The LiChrosorb Si60 column (250 × 4.6 mm; 5 μm) was used. The mobile phase consisted of n-hexane and 1,4-dioxane (97:3 v/v). Flow rate was 1.5 mL/min. The fluorometric detector (Waters 474 Asc. Milford, MA, USA) worked at excitation λ = 290 nm and emision λ = 330 nm for tocochromanols. β-carotene content was analyzed by UV–Vis spectrophotometry (450 nm) (PDA detector, Waters 2998 Asc. Milford, MA, USA). Concentrations of individual tocochromanol homologues and β-carotene were calculated from a previously prepared calibration curve. The limits of detection (LOD) and quantitation (LOQ) calculated at a signal-to-noise ratio of 3 and 10 (noise calculated peak to peak on a blank chromatogram at the tocopherols retention time) were 8 and 20 ng/mL, respectively.
Methanol extracts of phenolic compounds
All samples were defatted using an automatic Soxhlet Büchi Extraction System B-811 (Büchi Labortechnik AG, Flawil, Switzerland). The extraction with n-hexane was carried out for 2 h. To obtain soybean phenols, each sample was extracted three times with 80 % methanol. In brief, 5 g of sample were extracted with 50 mL methanol three separate stages overly for 30 min, at 50 °C temperature. After centrifugation (10 min at 5,000g) (model 6K15, Sigma, Osterode am Harz, Germany), the precipitate was re-extracted twice more following the same steps. The three supernatants were combined and evaporated under reduced pressure using an R-215 rotorvapor (Büchi Labortechnik AG, Flawil, Switzerland) to a volume 25 mL.
Total phenolics content
The total phenolics content in methanolic extract was determined by the Folin–Ciocalteu method [28]. An aliquot (0.2 mL) of the methanolic extract was placed in a volumetric flask (10 mL). Diluted Folin–Ciocalteu reagent (0.5 mL) was added. Saturated solution of sodium carbonate (1 mL) was added after 3 min. The flask was filled with water up to 10 mL. Absorbance at λmax 725 nm against a reagent blank was measured after 1 h using a UV–Vis spectrophotometer SP 8001 (Metertech Inc., Taipei, Taiwan). Caffeic acid (0–90 μg/mL) was used to produce standard calibration curve y = 117.48x (R 2 = 0.9986). The total phenolic content was expressed in mg of caffeic acid equivalents (CAE)/g dry plant material [29].
Total flavonoid content
Total flavonoid content was measured by the aluminum chloride colorimetric assay using a method based on the formation of complex flavonoid-aluminium. An aliquot (1 mL) of extracts was added to 10 mL volumetric flask containing 4 mL H2O. To the flask, 0.3 mL 5 % NaNO2 was added. After 5 min, 0.3 mL 10 % AlCl3 was added. At the 6th min, 2 mL 1 M NaOH solution was added and the total volume was made up to 10 mL with H2O. The solution was mixed well, and the absorbance was measured against prepared reagent blank at 510 nm, using a UV–Vis spectrophotometer SP 8001 (Metertech Inc., Taipei, Taiwan). Quercetin (0–30 μg/mL) was used to produce standard calibration curve y = 0.0396x (R 2 = 0.9897). The total flavonoid content was expressed in μg of quercetin equivalents (QE)/g dry plant material.
DPPH radical scavenging method for measuring antioxidant activity
The method consisted of spectrophotometric measurement of the intensity of the color change in solution depending on the amount of DPPH•(2,2-diphenyl-1-picrylhydrazyl radical). The reaction was initiated by mixing 1 mL of the methanolic extract with 3 mL methanol and then adding 1 mL of DPPH• (0.012 g/100 mL). Absorbance at λmax of 517 nm (UV–Vis spectrophotometer SP 8001 Metertech Inc., Taipei, Taiwan) was checked at 0, 0.5, and at every 0.5 min until the reaction reached a stable state. This plateau was reached within 60 min. The activity of the extract in scavenging DPPH• was calculated as follows:
Total antioxidant capacity is expressed also as Trolox equivalent on the basis of standard curve y = 0.3964x (R 2 = 0.9968). In order to compare, total antioxidant activity of obtained preparations additionally antiradical power (ARP) parameter was assayed [30]:
EC50–antioxidant activities were expressed as the EC50, that is, the concentration of antioxidant required to cause a 50 % reduction in the original concentration of DPPH. For ease of interpretation, antiradical powers were also calculated and defined as the inverse of the EC50 value.
Statistical analysis
Results are presented as means ± standard deviation from three replicates of each experiment. P value <0.05 was used to denote significant differences between mean values determined by the analysis of variance (ANOVA) using Statistica 7.0 (StatSoft, Inc., Tulsa, OK) software. Correlations were estimated using Pearson’s correlation coefficient (r).
Results and discussion
The soybean sprouted seeds fortified in iron may be proposed for preparation of iron supplements. In the presented experiment, the seeds were watered with FeSO4 solutions (5–25 mM) or only with water (reference sample) in order to obtain sprouted soybean seeds fortified in iron. The germinating seeds watered with FeSO4 solutions developed in abiotic stress conditions. They were both shorter and thicker and had a different color in comparison to the reference sample. In used higher concentrations of FeSO4 (20–25 mM FeSO4) in the culture medium, the germination process was strongly inhibited, however, not stopped. After 7 days of culturing, dried and ground samples of radicles with cotyledons were used for analysis.
Range of plant resistance to metal toxicity and deficiency is variable. It depends both the element as well as plant species. This range for sprouting soybean seeds was established experimentally and included concentration 0–25 mM of Fe2+ ions. Exceeding the tolerance level of a particular plant species to the examined elements (called a critical point) causes a decrease in biomass production. Extremally high doses of toxic elements may lead to death of the plant [31].
The presence of toxic metals (Fe, Cu, Zn, Cd) in cells leads to oxidative stress. In these conditions, more reactive oxygen species (ROS) and free radicals (FR) are generated than it is metabolized. Present in cells ROS and free radicals cause lipid peroxidation (which leads to damage of cell membranes and the membranes inside the cell), destroy the structure of nucleic acids (which may be a reason for mutagenesis), oxidize proteins (leading to changes in structure and their inactivation), and inactivate the photosynthetic electron transport chain [32, 33].
Under the conditions of abiotic stress, particularly induced by high levels of iron ions in the environment, overexpression of ferritin is observed. This protein is responsible for the uptake of toxic iron, preventing the formation of ROS. Only small amounts of iron can be made ready for use by −O2, H2O2 from phytoferritin shell and participate in radical chemistry [34]. Briat et al. [35] show that the lack of ferritin gene in the seeds leads to greater sensitivity to methylviologen (pro-oxidant compounds) during germination. Thus, it is evident that antioxidative function of ferritin in plants is very important for iron sequestration to avoid oxidative stress.
During germination in standard conditions, native ferritin is degraded and iron from mineral core is mobilized for the growth of the seedlings. Formation of free radical scavengers, which are induced by the free iron presence or ferrous iron chelators inhibit in vitro degradation of ferritin. The storage form of ferritin is expressed as a result of elevated free iron in cellular fluid. These data lead to the hypothesis that a similar mechanism occurs during seedling germination [36, 37].
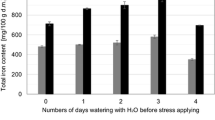
In standard germination conditions, production of ROS in cells is low [38] because the level of released iron from ferritin is also low and the ions are involved with synthesis of proteins, which takes part in photosynthesis, respiration or DNA synthesis processes [37]. Plant growth in high concentrated solution of FeSO4 leads to overexpression of ferritin. Increased concentration of ferrous iron in culture medium activates ferroxidase, which is responsible for uptake of iron by ferritin [39]. The changes in ferritin iron concentration were inferred from the observed changes in difference between the total iron content and free inorganic iron content built-in obtained sprouted seeds (Table 1). The level of ferritin iron was the highest in examined material obtained from cultures carried out in 20 mM of FeSO4. It was 67 times higher than for sprouts germinated in distilled water. It may be concluded that up to this concentration, the defense of plant against oxidative stress is connected with introducing iron into ferritin. Observed decrease of total iron content and simultaneous increase in inorganic Fe2+ content in sprouts cultured in 25 mM of FeSO4 confirm observed plant necrobiosis, which was the result of excessively high concentration of heavy metal in culturing medium.
During plants germination, reserves of lipids cumulated in seeds are utilized [34]. In the first stage of germination, storage proteins are degraded to amino acids. Next, synthesized enzymes (e.g., lipases) are used for the mobilization of the storage lipids. The glycerol formed by the hydrolysis of triacylglycerol can be fed into the gluconeogenesis pathway. Free fatty acids, which are simultaneously formed are first activated by CoA-thioesters. Next, in β-oxidation process is degraded to acetyl-CoA. As a result of β-oxidation and glyoxylate cycle, succinate particle is formed from two acetyl-CoA particles. The succinate is transferred to the mitochondria and converted there to oxaloacetate. Next, oxaloacetate in cytosol is converted into phosphoenolpyruvate, which is the precursor of hexoses synthesis (gluconeogenesis pathway) [40]. During this conversion, toxic H2O2 and glyoxylate are formed. These molecules are immediately degraded, because even a small amount of these substances causes complete inhibition of photosynthesis in chloroplasts. However, during examination of Arabidopsis development, it was observed that storage oil mobilization is not essential for seed germination but is essential for seedling establishment [41].
In the presented experiment, mobilization of lipids during plant development was observed. Changes in fats content during examined germination processes are presented in Table 2. At lower concentrations of FeSO4 in culture medium (0–10 mM), observed results are consistent with the general tendency. Fats content decreases slightly. However, increasing the concentration of Fe2+ causes a weak increase in lipids content in obtained sprouts in comparison to sprouts cultured in distilled water. No significant difference to raw seeds was observed.
Changes in the expression of ferritin and lipids content in abiotic stress conditions may influence the synthesis of fat-soluble antioxidant, such as tocopherols and tocotrienols and β-carotene. Soybean seeds are well known as a tocochromanol source, which are a group of major biological antioxidant. Tocopherols and tocotrienols are synthesized in higher plants plastids. During germination, the content of tocochromanol decreases, because they protect stored lipids from oxidation [42, 43]. For some species, decrease only of γ-tocopherol is observed and synthesis of others tocochromanols [44]. Some authors suggest that content of α-tocopherols changes depending on conditions of germination, as availability of light [21, 44–47]. The main tocopherol (Table 2) of seeds, it means γ-tocopherol, decreases in prepared sprouted soybean seeds (down to 78–88 %) and this is an ordinary trend observed during germination [48]. Even though the growth of sprouts is strongly limited in 25 mM FeSO4, the reduction of γ-tocopherol is still high. α-Tocopherol content in sprouted seeds enriched in Fe2+ achieved 82–114 % of α-tocopherol in seeds. The reduction of δ-tocopherol reaches only 8–14 % and content of β-tocopherol changes in range 87–123 % in comparison to seeds. β-Tocotrienol content is reduced to 77–93 % but γ-tocotrienol reaches 95–118 % of their content in seeds (Table 2).
However, even though the tocochromanol content in sprouts cultured in stress conditions is always lower than their level in seeds, it is also always higher than in sprouted seeds raising in distilled water. The highest content of total tocochromanols was achieved in sprouted seeds germinated in 10 mM FeSO4 solution (93 % of tocochromanol of seeds) (Table 2). It should be noted that observed changes may result both from culturing conditions and the way of obtained sprouts preparation, first of all drying in circulating air and storage at room temperature.
Another fat-soluble antioxidant is β-carotene. Carotenoids protect unsaturated lipids, triacylglycerols, proteins, and membranes from photooxidation [49]. During germination, depending on accessibility and type of illumination, β-carotene content is increased. In presented experiment, germination of seeds in ordinary conditions (water) caused more than 11-fold increase in β-carotene content (Table 2). Abiotic stress strongly affected synthesis of β-carotene. Raising in 10 mM of FeSO4 sprouts induced almost 28 times higher β-carotene accumulation than in raw seeds and 2.5 times higher than in sprouted seeds germinated in water. Further increasing of Fe2+ concentration in culture medium rapidly decreased the content of examined provitamin.
The elevated synthesis of β-carotene in the presence of iron ions was observed previously [50]. Kobayashi et al. [50] confirmed that generation of hydroxyl radical is important in the process of carotenogenesis activation.
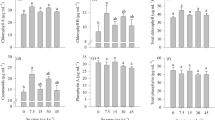
The impact of used stress conditions was not evident for the next group of examined antioxidant. An increase (~29 %) in total phenolic content was noted during germination in distilled water in comparison to the in raw seeds (Table 3). It was observed before that germination causes an increase in phenolic compound content up to 7–9 days of culturing [47, 51]. The total phenolic compounds content assayed for sprouts cultured in stress conditions was always smaller. The highest total phenolic compounds content was noted for sprouts raising in 5–10 mM FeSO4 solutions, and it was 119–123 % of total phenolic compound content assayed in raw seeds. However, the changes were statistically not significant.
Flavonoids are ~11 % of phenolic compounds, and this tendency is observed for every obtained preparation (Table 3).
Table 3 presents also the changes in antioxidant capacity expressed as Trolox (μM/L) and ARP. Generally, the germination of seeds is the process, which improves their antioxidant activity [21] as it was seen for soybean seeds cultured in 0–10 mM FeSO4 concentrations. However, in higher stress conditions (15–25 mM of FeSO4), it is continuously decreased and even lower than for raw seeds. The changes in antioxidant activity may also confirm that in concentrations of FeSO4 higher than 15 mM, oxidative stress is so high, that it starts to inhibit plant development. However, the growth of the sprouts was still observed. An increase in antioxidant activity may result from changes in total phenolic compounds content. The correlation between antioxidant activity and the content of total phenolic compounds reaches the value r = 0.8498.
Conclusion
In the present investigation, sprouts enriched in ferritin were cultured in order to obtain an edible part of plants fortified in ferritin. The highest accumulation of ferritin was observed during cultivation in 20 mM FeSO4 solutions.
The ferritin is a major antioxidant that protects cells against toxic iron in these conditions. However, stress conditions cause also an increase of β-carotene synthesis. Antioxidant activity was correlated to the total phenolic content and increased both in the sprouts cultured in water and in lower concentrations of FeSO4. Development of plant in high concentration of FeSO4 influences hydrolysis of lipids. Tocochromanols participation in protecting cells against oxidation is lower than observed in raw seeds. Germination of seeds in stress conditions strongly influences development of sprouts. Reactive oxygen species is formed not only as a consequence of hydrolytic processes, how it may be observed for germination in water.
References
Andrews NC (1995) Disorders of iron metabolism. N Engl J Med 341:1986–1995
Kushner JP, Porter JP, Olivieri NF (2001) Secondary iron overload. Hematology Am Soc Hematol Educ Program 1:47–61
Rouault TA (2003) Hepatic iron overload in alcoholic liver disease: why does it occur and what is its role in pathogenesis? Alcohol 30:103–106
Weiss G (2002) Pathogenesis and treatment of anaemia of chronic disease. Blood Rev 16:87–96
WHO (2000) Turning the tide of malnutrition: responding to the challenge of the 21st century. Geneva (WHO/NHD.007)
Hunt JR (2003) Supplements bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr 78(3):633S–639S
Fleming RE, Bacon BR (2005) Orchestration of iron homeostasis. NEJM 352(17):1741–1744
Hurrell RF (1997) Preventing iron deficiency through food fortification. Nutr Rev 55:210–222
Uauy R, Hertrampf E, Reddy M (2002) Iron fortification of foods: overcoming technical and practical barriers. J Nutr 132:849S–852S
Stoltzfus RJ, Dreyfuss ML (1998) Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia. ILSI Press, Washington
White PJ, Broadley MR (2009) Biofortification of crops with seven mineral elements often lacking in human diets—iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol 182:49–84
Curie C, Briat JF (2003) Iron transport and signaling in plants. Ann Rev Plant Biol 2003:183–206
Briat J-F, Lobreaux S (1998) Iron storage and ferritin in plants. Met Ions Biol Syst 35:563–583
Harrison PM, Arosio P (1996) The ferritins: molecular properties, iron storage function and cellular regulation. Biochem Biophys Acta 1275:161–203
Theil EC, Briat JF (2004) Plant ferritin and non-heme iron nutrition in humans. HarvestPlus technical monograph 1. Washington, DC
Savelkoul FHMG, Boer H, Tamminga S, Schepers AJ, Elbourg L (1992) In vitro enzymatic hydrolysis of protein and protein pattern change of soya and faba beans during germination. Plant Foods Hum Nut 42:275–284
Bau H-M, Villaume C, Nicolas J-P, Méjean L (1997) Effect of germination on chemical composition of biochemical constituents and antinutritional factors of soya bean (Glycine max) seeds. J Sci Food Agric 73:1–9
Suda M, Watanabe T, Kobayashi M, Matsuda K (1986) Changes in starch content and related enzymes activities during the growth of germinating soybeans. Agric Biol Chem 50:3195–3196
Chandrasiri V, Bau HM, Villaume C, Giannangeli F, Lorient F, Méjean L (1987) Effect de la germinationde la graine de soja sur la composition et la valeur nutritionnelle de sa farine. Sci Aliments 7:139–150
Khalil AH, Mansour EH (1995) The effect of cooking, autoclaving and germination on the nutritional value of faba beans. Food Chem 54:177–182
Fernandez-Orozco R, Piskula MK, Zieliński H, Kozłowska H, Frias J, Vidal-Valverde C (2006) Germination as a process to improve the antioxidant capacity of Lupinus angustofilus L. var. Zapaton Eur Food Res Technol 223:495–502
Torres A, Frías J, Granito M, Guerra M, Vidal-Valverde C (2007) Free α-galactosides lupine flour as pasta ingredient: chemical, biological and sensory evaluation. J Sci Food Agric 87:74–81
Sandell EB (1959) Colorimetric determination of traces of metals. Interscience Publishers Inc, New York
Tsalev DL, Zaprianov ZK (1984) Atomic absorption spectrometry in occupational and enviromental healths practice: determination of individual elements. CRC Press, Boca Raton
Current protocols in food analytical chemistry (2001) Extraction and measurement of total lipids. John Wiley & Sons, Inc. D1.1.1-D1.1.11
Ryynänen M, Lampi AM, Salo-Väänänen P, Ollilainen V, Piironen V (2004) A small-scale sample preparation method with HPLC analysis for determination of tocopherols and tocotrienols in cereals. J Food Comp Anal 17:749–765
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolibdicphosphotungstic acid reagent. Am J Enol Viticul 16:144–158
Siger A, Nogala-Kałucka M, Lampart-Szczapa E (2008) The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J Food Lipid 15:137–149
Zhishen J, Mengcheng T, Jianming W (1999) Research on antioxidant activity of flavonoids from natural materials. Food Chem 64:555–559
Suja KP, Jayalekshmy A, Arumughan C (2005) Antioxidant activity of sesame cake extract. Food Chem 91:213–219
Woźny A (1997) Responses of plant cells to trace (heavy) elements of ecosystems. Idee Ekol 10:35–57
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Physiol 17:21–34
Tripathi BN, Gaur JP (2004) Relationship between copper- and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 219:397–404
Gilbert DL, Colton CA (2002) Reactive oxygen species in biological systems: an interdisciplinary approach. Kluwer Academic Publishers, New York
Briet JF, Duc C, Ravet K, Gaymard F (2010) Ferritins and iron storage in plants. Bioch Bioph Acta 1800:806–814
Lobreaux S, Briat JF (1991) Ferritin accumulation and degradation in different organs of pea (Pisum sativum) during development. Biochem J 274:601–606
Briat JF, Lobreaux S, Grignon N, Vansuyt G (1999) Regulation of plant ferritin synthesis: how and why. Cell Mol Life Sci 56:155–166
Dubey RS (2010) In: Gupta SD (ed) Reactive oxygen species and antioxidants in higher plants. CRC Press, Boca Raton
Laulhère JP, Briat JF (1993) Iron release and uptake by plant ferritin as affected by pH, reduction and chelation. Biochem J 290:693–699
Heldt HW (2005) Plant biochemistry. Elsevier Academic Press, San Diego
Graham IA (2008) Seed storage oil mobilization. Annu Rev Plant Biol 59:115–142
Eitenmiller RR, Lee J (2004) Vitamin E: food chemistry, composition and analysis. Marcel Dekker Inc., New York
Sattler SE, Giliand LU, Magallanes-Lundback M, Pollard M, DellaPenna D (2004) Vitamin E is essential for seed longevity and for preventing lipid peroxidation during germination. Plant Cell 16:1419–1432
Zieliński H, Kozłowska H (2003) The content of tocopherols in Cruciferae sprouts. Pol J Food Nutr Sci 4:25–31
Frias J, Miranda ML, Doblado R, Vidal-Valverde C (2005) Effect of germination and fermentation on the antioxidant vitamin content and antioxidant capacity of Lupinus albus L. ver. Multolupa. Food Chem 92:211–220
Friedrich W (1988) Vitamins. Walter de Gruyter Inc., Berlin
Zieliński H (2003) Contribution of low molecular weight antioxidants to the antioxidant screen of germinated soybean seeds. Plant Food Hum Nutr 58:1–20
Smirnoff N (2005) In: Smirnoff N (ed) Antioxidents and reactive oxygen species in plant. Blackwell Publishing Ltd., Oxford
DellaPenna D, Pogson BJ (2006) Vitamin synthesis in plants: tocopherols and carotenoids. Annu Rev Plant Biol 57:711–738
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. App Environ Microbiol 59:867–873
White PJ, Xing Y (1997) In: Shahidi F (ed) Natural antioxidants: chemistry, health, effects and applications. AOCS Press
Acknowledgments
The authors acknowledge financial support by the Ministry of Science and Higher Education of the Republic of Poland (Project N312 029 31/2098).
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Zielińska-Dawidziak, M., Siger, A. Effect of elevated accumulation of iron in ferritin on the antioxidants content in soybean sprouts. Eur Food Res Technol 234, 1005–1012 (2012). https://doi.org/10.1007/s00217-012-1706-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1706-y