Abstract
The effect of the fungal infection Fusarium graminearum and Fusarium culmorum on naked barley cultivars (n = 7) with respect to the barley’s total protein content and the content of the protein fractions albumins + globulins, prolamins (hordeins) and glutelins (hordenins) was investigated. A summer barley cultivar (n = 1) was used for comparison. The total protein content of the whole grain flours was very variable, ranging from 125 to 225 g kg−1. The influence of Fusarium infection showed that the content of hordeins and hordenins was slightly reduced, while the albumins and globulins were not affected. In addition, the effect of the two different growing locations on the protein content of the naked barley was also evaluated. It could be shown that the C-hordeins, γ-hordeins and D-hordenins were significantly positively affected by increasing nitrogen supply, whereas the B-hordenin content was significantly negatively influenced. Nitrogen availability seems to be a factor that promotes gene expression for hordeins but reduces the synthesis of the main B-hordenins.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Fusariumgraminearum and Fusariumculmorum infection of cereal grains, such as wheat and barley, leads to pathogenic effects on the plant and spike. These can result in dramatic yield and quality losses [1, 2]. Fusarium head blight (FHB) is the visible effect of this fungal infection and a problem known throughout the world [1, 3, 4]. The two aforementioned Fusarium species are producers of trichothecene mycotoxins such as deoxynivalenol (DON), 3- and 15-acetyldeoxynivalenol (3-Ac-DON and 15-Ac-DON), and others [1]. The trichothecene mycotoxins are potential inhibitors of protein biosynthesis. In mammals, they lead to unspecific effects in the intestines causing diarrhoea, vomiting, a reduced food intake and a raised bleeding tendency of the intestines. Their specific effects include a reduced leucocyte count connected with a loss of immune function and an increase in free radicals in the liver [5–8].
So far, little is known about naked barley [hull-less barley] (Hordeum vulgare nudum) cultivars, which are normally grown in organic farming systems, and the influence of Fusarium infection on the protein fractions in this cereal. The total protein content has been documented for naked barley as being between 12 and 16% of dry mass [9]. The influence of Fusarium infection on grain protein composition has been mainly investigated on wheat cultivars. The results of these studies have suggested that the infection degree had either no impact on the total protein content or it just caused a moderate increase in the total protein concentration [10, 11]. However, an influence on protein fractions, such as a rise in the prolamin (gliadins) and a reduction in the glutelin (glutenins) content, has been recorded. A change in the synthesis behaviour at different maturation stages has been postulated as an explanation for this observation [11]. However, a study focusing on the synthesis of cereal seed storage proteins did not show any alterations in the synthesis stages during grain maturation; only a belated polymerization of glutenins was noted [12].
As stated above, barley storage proteins have been much less investigated in the context of Fusarium infection, and the focus of this work was directed at determining the proteins that are expressed as a response to Fusarium infection [13]. Such proteins include pathogenesis-related proteins and defence-response proteins corresponding to the albumin and globulin fractions [13–16].
Increasing the nitrogen supply to wheat cultivars leads to an increase in their total protein content. The content of both prolamins and glutelin fractions is also increased. In contrast, no influence has been documented for the albumin and globulin fractions [17, 18]. Barley shows partly the same response to nitrogen with respect to its protein components as wheat [19, 20]. However, in contrast, the glutelins are not affected by nitrogen availability, while the albumin and globulin fraction increases [19]. No studies have been done on naked barley so far.
The present study focuses on the influence of artificial Fusarium infection on the Osborne protein fractions in naked barley [21]. Here, the aim was to study the impact of Fusarium on the fractions of prolamins (hordeins) and glutelins (hordenins) and their different protein types in naturally and artificially infected samples. Our investigations were concentrated on harvested grains to determine the conditions in fully developed grains. Additionally, the effect of two different growing locations with respect to their nitrogen supply on the total protein content and quantitative protein composition was also monitored. Finally, the question as to which modifications in protein composition and protein synthesis occur in connection to nitrogen availability and exposition to Fusarium infection was addressed.
Materials and methods
Experimental design and sample preparation
Seven naked barley cultivars (Lawina, Linz, Frealishe, Yonas, ZFS, Taiga and 00/900/5N) and, for comparison, one summer barley cultivar (Barke) were grown in two field trials at Reinshof [22] and Sattenhausen [SH] near Göttingen in the centre of Germany. The trials were randomized with eight replications. In each block, the second row was artificially inoculated during flowering three times with a mixed F. culmorum and F. graminearum spore suspension (50 mL m−2; 1 × 105 spores/mL). Three DON-producing strains of F. culmorum (FC34, FC35 and FC36) and F. graminearum (FG142; FG143; and FG144) were used for conidiospore production. After harvest, the grains without inoculation (natural infection) and with inoculation (artificial infection) of the plots (each four replications) from each field trial were pooled. Whole grain flour was obtained by milling (Retsch ZM 100, Haan, Germany) at a particle size of 0.5 mm.
The location conditions at RH are 152 m above sea level, wind sheltered and dale area near a river border. The N min content was 145 kg ha−1 recorded in 90 cm depth dry soil. At SH, the conditions are 260 m above sea level, hilly and windy area. The N min content was 95 kg ha−1 in 90 cm depth dry soil, and an additional fertilization with 40 kg N ha−1 was conducted 2 months after sowing.
Quantitative LC–MS/MS of Fusarium mycotoxin DON and 3-Ac-DON
Whole grain flour (5 g) was extracted with 40 ml of acetonitrile–water mixture (80:20) over night on a reciprocal shaker. The extracts were centrifuged for 12 min at 5,000×g, and 4 mL of the supernatant were used for solid-phase extraction (Bond-Elut Mycotoxin, Varian GmbH, Darmstadt, Germany) according to the manufacturer’s instructions. Two millilitres of the cleaned extract were evaporated to dryness under vacuum, redissolved in 200 μL of methanol–water (50:50) containing 0.2 mmol ammonium acetate, and ten microlitres of the solution were injected onto a C18 column (100 × 2 mm, 3 μm particle size) filled with polar modified material (Polaris Ether, Varian GmbH, Darmstadt, Germany). The analytes were eluted with a methanol–water gradient (15–70% methanol during 20 min) containing 0.2 mmol ammonium acetate at a flow rate of 0.2 ml min−1. DON and 3-Ac-DON were detected by tandem mass spectrometry as described by Adejumo et al. [23].
Quantitative nitrogen analysis
The nitrogen content was quantitatively measured with a C/N-analyser (Vario MAX CN, Elementar Analysesysteme GmbH, Hanau, Germany). Each 100-mg dry sample was analysed for its N content and converted into protein with the factor 6.25 for barley.
Quantitative protein analysis with RP-HPLC
Protein extraction from 100-mg flour samples was realized stepwise. In the first step, 1-mL extraction with solution A (phosphate buffer containing 97 parts of 0.067 mol L−1 Na2HPO4; 0.4 mol L−1 NaCl and 3 parts 0.067 mol L−1 KH2HPO4; 0.4 mol L−1 NaCl; pH 7.6) by vortexing for 2 min and shaking at room temperature (RT) for 10 min for albumins/globulins fractions, the extraction being repeated twice. The samples were centrifuged for 20 min at 6,000×g, and the supernatants were combined and filled up to 2 mL. The pellet was than extracted three times with 0.5 mL extraction solution B [60% ethanol (v/v)], vortexed for 2 min and exposed to 10-min shaking at RT. The samples were centrifuged for 20 min at 6,000×g, and the prolamin-containing supernatants were combined and filled up to 2 mL. In the third step, the remaining pellet was extracted two times with 1 mL extraction solution C (50% 1-Propanol (v/v) and 0.05 mol L−1 Tris/HCl (pH 7.5) containing 2 mol L−1 urea as well as 1% dithioerythritol) under N 2, with 2-min vortexing and 30-min shaking at 60 °C. The samples were centrifuged for 20 min at 6,000×g, and these glutelin-containing supernatants were combined and filled up to 2 mL. All extracts were filtered with 0.45-μm Filter: FP 30/0.45 CA Whatman (schleicher + schnell) before HPLC injection.
For the RP-HPLC, a Nucleosil 300-5 C8 250 × 4.6 silica column (Macherey–Nagel, Dueren, Germany) was used. As mobile phases, A = 0.1% in H2O (v/v) and B = 0.1% TFA in acetonitrile (v/v) were applied. The flow rate was 1 mL min−1 with the column temperature maintained at 50 °C. For the albumins/globulins detection (Fig. 1), 150 μL of sample solution were injected and separated by using the following gradient: 0 min, 20% B; 20 min, 60% B; 21 min, 90% B, 26 min, 90% B; 37 min 20% B; whereas for prolamins and glutelins (Fig. 1), 50 and 100 μL were injected, respectively, and the separation was performed by applying the following gradient: 0 min, 24% B; 50 min, 56% B; 51 min, 90% B, 56 min, 90% B; 67 min 24% B [24]. The external PWG (Prolamin Working Group) gliadin standard was used for the quantification of the protein fractions [25].
Statistical analyses were performed using Microsoft Excel 2003 for mean value, standard deviation and significance (P). For a more clear illustration of the results, just the two of the naked barley cultivars with the strongest differences are presented separately in a table.
Results and discussion
The influence of Fusarium infection on the toxin concentration and the quantitative protein composition of naked barley
Tables 1, 2, 3, 4 and 5 summarize the results for the most different naked barley cultivars Frealishe (Table 1) and 00/900/5N (Table 2), all seven naked barley cultivars on an average (Table 3), the summer barley cultivar Barke (Table 4) and a comparison of seven naked barley cultivars with summer barley cultivar Barke (Table 5). The detected fungal content expressed as Fusarium toxin (DON and 3-Ac-DON) in the naked barley, and summer barley cultivars showed a significant increase in all the investigated parameters after artificial infection in comparison to the naturally infected cultivars (Tables 1, 2, 3, 4 and 5) except the cultivar 00/900/5N in the location Sattenhausen. This confirms that the tested species and cultivars showed a certain susceptibility to Fusarium spp. depending on the degree of infection [4]. Naked barley showed, in general, a higher content of Fusarium toxin than the investigated summer barley (Table 5). In the seven naked barley cultivars, we found two with a higher degree of susceptibility (Lawina and Linz) and two with nearly the same susceptibility (Frealishe and Taiga) as summer barley cultivar.
The total and extractable protein content of the investigated naked barley cultivars did not changed significantly with respect to the Fusarium infection (Table 5; Fig. 2). These results are in agreement with another study in which no impact on total protein content in F. culmorum-infected wheat samples was also found [11]. In contrast, older results from studies using F. graminearum showed a moderate increase in the total protein content related to the degree of infection [10].
Analysing the difference between the total protein content and the extractable protein content showed that about 62–85% of the total protein content could be extracted by the method used in this investigation (Tables 1, 2, 3, 4 and 5). Non-extractable proteins are membrane-associated or those basic proteins with a high sugar content [26] and also include the non-extractable storage proteins. For a more complete and efficient extraction of the plant proteins, more specialized procedures must be used [27].
Comparing the naked barley cultivars with summer barley, we found 21% higher total and a 15% higher extractable protein content in the naked barley. However, the naked barley showed a high degree of variation of total protein for the seven investigated cultivars and the two growing regions of between 125 and 225 g kg−1 (Tables 1, 2, 3). These values and high variation in the protein content of naked barley are supported by the literature [9], but the cause for the variations between the different cultivars is still unclear.
The influence of Fusarium infection on the Osborne fractions in naked barley and summer barley showed that the albumin and globulin fraction was not significantly changed. The naked barley contained 16% albumins/globulins, while there was a slightly higher content in summer barley of 19% (Table 5).
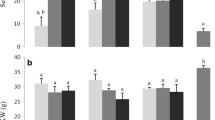
The prolamin (hordeins) and glutelin (hordenins) fractions and types were also not significant influenced by Fusarium infection (Table 5). For hordeins, the artificially infected samples showed a slightly reduced concentration for all types in naked barley and summer barley in comparison to the naturally infected samples (Fig. 2). The hordenins showed also a slight reduction in naked barley, but there was a slightly raised B-hordenin concentration in the summer barley (Fig. 2). However, the ratio hordein/hordenin content either did not change or only to a minor degree.
For the present results, we assumed that these changes were due to a slight degradation of the barley proteins by the fungus as fungal proteases such as trypsin protease or serine protease are part of the exoproteome of Fusarium spp., and they are known to be protein-degrading enzymes [26–28]. The reasons for this lower degree of degradation of barley storage proteins in comparison to that found with wheat [11] may be (1) the localized infection that occurred only on selected single grains in barley [29], or perhaps (2) the occurrence of proteinase inhibition by microbial proteinase inhibitors such as barley Bowman-Birk inhibitor (BBI) or serine protease inhibitor [30, 31], or (3) the synthesis of pathogenesis-related proteins (chitinase), defence-response proteins (oxidative burst response), protein-synthesis-related proteins and proteins involved in the phenylpropanoid biosynthesis (phenole and indole derivates) pathway as a reaction to the Fusarium infection [13–16].
The hordein and hordenin fractions varied in content among the investigated naked barley cultivars with respect to both the location and the cultivar: for hordeins between 54 and 109 g kg−1 and for hordenins between 17 and 32 g kg−1 (Tables 1, 2, 3 not all data shown). The investigated summer barley cultivar, Barke, also varied according to location (Table 3): hordein between 39 and 70 g kg−1 and hordenins between 31 and 32 g kg−1. The comparison between the naked barley and summer barley showed that the hordeins formed a bigger proportion of the extractable protein in naked barley (65%) than in the summer barley (52%; Table 5). In contrast, the proportion of the hordenin fraction was 9% higher in the summer barley than in the naked barley cultivars (Table 5) apart from cvs. Linz and Frealishe that had proportions equivalent to those in the summer barley. These results led to a lower hordein/hordenin ratio for summer barley (1.2–2.3) in comparison to naked barley (2.4–5.2; Tables 1, 2, 3, 4, 5). The reasons for these differences in the storage protein proportions between naked barley and summer barley may include a variation in the rate of synthesis in the developing kernels [20], although it is still unclear which factors cause these differences.
Effect of N supply at two different growing locations on the quantitative protein composition
For both naked barley and summer barley, the total protein content seemed to be significantly connected to the conditions present at the growing location (Tables 1, 2, 3, 4, 5; Fig. 3). For the impact of the location on the extracted protein fractions in naked barley and summer barley, we considered both the naturally and artificially fungal infected samples together. The naked barley showed a significant 23% higher total protein content and a significant 18% higher extractable protein content at RH (higher N supply) in comparison to SH (Table 6; Fig. 3). The summer barley data support this change based on the N supply of the location (Table 6; Fig. 3).
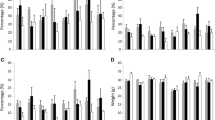
A significant 9% reduction in the albumins and globulins was observed in naked barley at RH in comparison to SH (Fig. 4). This reduction in the albumin and globulin fraction could not be confirmed in the summer barley results as a minor increase in this fraction occurred (Table 6). These results are not supported by N supply studies on wheat as no changes in the albumin and globulin fraction were detected in this cereal [17, 32]. The reasons for this reduction in association with N supply in naked barley are unclear, but there may be a suppression of albumin and globulin synthesis at locations with a high N supply in contrast to the induced storage protein synthesis found in naked barley under these conditions (Table 6) [17–19].
The hordein content was significantly increased by 35% in naked barley and by 63% in summer barley at RH compared to SH (Figs. 4, 5). The C-hordeins were the most affected proteins in both types of barley (Fig. 5; Table 6). It can be, therefore, be postulated that summer barley is more susceptible to a raised N supply with respect to hordeins than naked barley. In contrast, the total amount of hordenins was negatively influenced (Table 6; Figs. 4, 5). At RH, the naked barley showed a reduction of 5% (significant for B- and D-hordenins) and the summer barley a minus of 1% in comparison to SH (Fig. 5). Looking at the hordenin fractions individually, only the D-hordenins showed a higher content (Table 6). The B-hordenins were found in significantly lower concentrations in comparison to the other fractions (Figs. 4, 5). This reduction in B-hordenins was observed in all the investigated naked barley and summer barley cultivars (Tables 1, 2, 3, 4, 5) and is supported by literature results where it is suggested that N has an influence on hordeins [19]. In conclusion, in this study, the C-hordeins, γ-hordeins and D-hordenins increased in content, while the B-hordenins decreased.
So far, little is known about barley protein types. The present results show that B-hordenins synthesis is not connected to the total protein content and the synthesis of the other protein types (Figs. 4, 5). The reason for this irregular synthesis of barley storage protein types is possibly due to the regulation of synthesis. Nitrogen availability and the resulting higher total protein content have been documented as being a factor that can promote gene expression [20]. These upstream factors have been identified as separate motifs E and N in C-hordeins, and at adequate nitrogen levels, these motifs enhanced gene expression and decrease it at low nitrogen levels [20]. Such gene promoters are known also from other cereals, such as wheat or maize, where the base sequence changed in length and in its base sequence or a motif was found to be missing. This could explain why B-hordenins were not enhanced in comparison to the other three barley protein fractions as it is likely that the promoter motifs are missing, so that the protein synthesis cannot be enhanced. An influence of the amino acid composition or synthesis can be excluded because no characteristic features in amino acid composition were found to be apparent for B-hordenins in comparison to the other types [32].
Conclusions
Naked barley, normally grown under organic farming conditions, and summer barley showed no characteristic changes in protein composition as a reaction to Fusarium spp. infection. This is in contrast to wheat, where such changes are known to occur. In addition to these basic findings, new information about the degradation and changes in different protein types was attained. As a consequence, further investigations focussed in general on protein degradation are recommended. The influence of the growing location and nitrogen availability on the protein content has been documented in previous studies on wheat and barley. The present results support and enlarge these findings for naked barley as the naked barley showed characteristic changes in the synthesis of its protein types in response to increased nitrogen. Further studies in this context may help to clarify the mechanisms that lead to the irregular synthesis of barley protein subunits depending on nitrogen availability.
Abbreviations
- ALG:
-
Albumins/globulins
- DM:
-
Dry matter
- LC–MS/MS:
-
Liquid chromatography with coupled mass spectrometry
- P:
-
Significance factor
- RP-HPLC:
-
Reversed-phase high-performance liquid chromatography
- RT:
-
Room temperature
- TFA:
-
Trifluoroacetic acid
References
Bottalico A, Perrone G (2002) Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur J Plant Pathol 108:611–624
Buerstmayr H, Stierschneider M, Steiner B, Lemmens M, Griesser M, Nevo E, Fahima T (2003) Variation for resistance to head blight caused by Fusarium graminearum in wild emmer (Triticum dicoccoides) originating from Israel. Euphytica 130:17–23
Ramirez ML, Reynoso MM, Farnochi MC, Chulze S (2006) Vegetative compatibility and mycotoxin chemotypes among Fusarium graminearum (Gibberella zeae) isolates from wheat in Argentina. Eur J Plant Pathol 115:139–148
Yang L, van der Lee T, Yang X, Yu D, Waalwijk C (2008) Fusarium populations on chinese barley show a dramatic gradient in mycotoxin profiles. Phytopathology 98:719–727
Eriksen GS, Pettersson H (2004) Toxicological evaluation of trichothecenes in animal feed. Anim Feed Sci Technol 114:205–239
Thuvander A, Wikman C, Gadhasson I (1999) In vitro exposure of human lymphocytes to trichothecenes: individual variation in sensitivity and effects of combined exposure on lymphocyte function. Food Chem Toxicol 37:639–648
Tsuda S, Kosaka Y, Murakami M, Matsuo H, Matsusaka N, Taniguchi K, Sasaki YF (1998) Detection of nivalenol genotoxicity in cultured cells and multiple mouse organs by the alkaline single-cell gel electrophoresis assay. Mutat Res Genet Toxicol Environ Mutagen 415:191–200
Yabe T, Hashimoto H, Sekijima M, Degawa M, Hashimoto Y, Tashiro F, Ueno Y (1993) Effects of nivalenol on hepatic drug-metabolizing activity in rats. Food Chem Toxicol 31:573–581
Bhatty RS (1999) The potential of hull-less barley. Cereal Chem 76:589–599
Boyacioglu D, Hettiarachchy NS (1995) Changes in some biochemical-components of wheat-grain that was infected with Fusarium-Graminearum. J Cereal Sci 21:57–62
Wang JH, Wieser H, Pawelzik E, Weinert J, Keutgen AJ, Wolf GA (2005) Impact of the fungal protease produced by Fusarium culmorum on the protein quality and breadmaking properties of winter wheat. Eur Food Res Technol 220:552–559
Abonyi T, Kiraly I, Tomoskozi S, Baticz O, Guoth A, Gergely S, Scholz E, Lasztity D, Lasztity R (2007) Synthesis of gluten-forming polypeptides. 1. Biosynthesis of gliadins and glutenin subunits. J Agric Food Chem 55:3655–3660
Geddes J, Eudes F, Laroche A, Selinger LB (2008) Differential expression of proteins in response to the interaction between the pathogen Fusarium graminearum and its host, Hordeum vulgare. Proteomics 8:545–554
Campo S, Carrascal M, Coca M, Abian J, San Segundo B (2004) The defense response of germinating maize embryos against fungal infection: a proteomics approach. Proteomics 4:383–396
Boddu J, Cho S, Kruger WM, Muehlbauer GJ (2006) Transcriptome analysis of the barley-Fusarium graminearum interaction. Mol Plant Microbe Interact 19:407–417
Piergiovanni AR (2007) Extraction and separation of water-soluble proteins from different wheat species by acidic capillary electrophoresis. J Agric Food Chem 55:3850–3856
Pechanek U, Karger A, Groger S, Charvat B, Schoggl G, Lelley T (1997) Effect of nitrogen fertilization on quantity of flour protein components, dough properties, and breadmaking quality of wheat. Cereal Chem 74:800–805
Benetrix F, Sarrafi A, Autran JC (1994) Effects of genotype and nitrogen nutrition on protein aggregates in barley. Cereal Chem 71:75–82
Wang JM, Chen JX, Dai F, Wu FB, Yang JM, Zhang GP (2007) Protein fractions in barley grains as affected by some agronomic factors and their relationships to malt quality. Cereal Res Commun 35:129–140
Shewry PR, Halford NG (2002) Cereal seed storage proteins: structures, properties and role in grain utilization. J Exp Bot 53:947–958
Eggert K, Wieser H, Pawelzik E (2010) The influence of Fusarium infection and growing location on protein fractions in emmer (Triticum dicoccum). Eur Food Res Technol. doi:10.1007/s00217-010-1229-3
Ndong C, Danyluk J, Wilson KE, Pocock T, Huner NPA, Sarhan F (2002) Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses. Plant Physiol 129:1368–1381
Adejumo TO, Hettwer U, Karlovsky P (2007) Occurrence of Fusarium species and trichothecenes in Nigerian maize. Int J Food Microbiol 116:350–357
Wieser H, Antes S, Seilmeier W (1998) Quantitative determination of gluten protein types in wheat flour by reversed-phase high-performance liquid chromatography. Cereal Chem 75:644–650
van Eckert R, Berghofer E, Ciclitira PJ, Chirdo F, Denery-Papini S, Ellis HJ, Ferranti P, Goodwin P, Immer U, Mamone G, Mendez E, Mothes T, Novalin S, Osman A, Rumbo M, Stern M, Thorell L, Whim A, Wieser H (2006) Towards a new gliadin reference material-isolation and characterisation. J Cereal Sci 43:331–341
Phalip V, Delalande F, Carapito C, Goubet F, Hatsch D, Leize-Wagner E, Dupree P, Van Dorsselaer A, Jeltsch JM (2005) Diversity of the exoproteome of Fusarium graminearum grown on plant cell wall. Curr Genet 48:366–379
Pekkarinen A, Mannonen L, Jones BL, Niku-Paavola ML (2000) Production of proteases by Fusarium species grown on barley grains and in media containing cereal proteins. J Cereal Sci 31:253–261
Pekkarinen AI, Jones BL (2002) Trypsin-like proteinase produced by Fusarium culmorum grown on grain proteins. J Agric Food Chem 50:3849–3855
Jansen C, von Wettstein D, Schafer W, Kogel KH, Felk A, Maier FJ (2005) Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc Natl Acad Sci USA 102:16892–16897
Pekkarinen AI, Jones BL (2003) Purification and identification of barley (Hordeum vulgare L.) proteins that inhibit the alkaline serine proteinases of Fusarium culmorum. J Agric Food Chem 51:1710–1717
Pekkarinen AI, Longstaff C, Jones BL (2007) Kinetics of the inhibition of Fusarium serine proteinases by barley (Hordeum vulgare L.) inhibitors. J Agric Food Chem 55:2736–2742
Johansson E, Prieto-Linde ML, Jonsson JO (2001) Effects of wheat cultivar and nitrogen application on storage protein composition and breadmaking quality. Cereal Chem 78:19–25
Acknowledgments
This work is part of the FAEN Joint Project 3 “Quality-related plant production under modified basic conditions: mycotoxins in the context of production, quality and processing”, financed by the Ministry of Science and Culture of Lower Saxony, Germany. A special thanks is extended to the growers who provided the seeds for this research trial: Dr. Karl-Josef Müller, Getreidezüchtungsforschung Darzau; Eckard Irion, Verein für Pflanzenzucht Hof Grub e.V.; Claus Einfeldt, Saatzucht Ackermann; and Hans-Werner Klein. In addition, we wish also to thank the group of Prof. Petr Karlovsky for the toxin analysis and especially Sasithorn Limsuwan, Molecular Phytopathology and Mycotoxin Research, Department of Crop Science, Georg-August University Goettingen and Alexandra Axthelm for Osborne fractionation and HPLC analysis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Eggert, K., Wieser, H. & Pawelzik, E. The influence of Fusarium infection and growing location on the quantitative protein composition of (Part II) naked barley (Hordeum vulgare nudum). Eur Food Res Technol 230, 893–902 (2010). https://doi.org/10.1007/s00217-010-1234-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-010-1234-6