Abstract
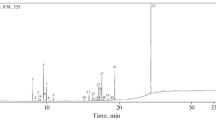
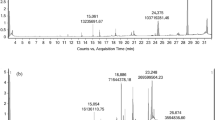
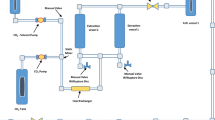
Extraction of laurel leaves by using supercritical carbon dioxide was carried out on a supercritical fluid (SF) pilot-scale plant. The extraction pressure and temperature were set to 250 bar and 60°C, respectively, using a 4% of ethanol as modifier. The employed apparatus, owing to a two-stage separation, allowed us to obtain two different fractions (F1 and F2), whose antioxidant and antimicrobial activities were investigated. Two different methods, β-carotene bleaching test and DPPH• free radical–scavenging assay, were carried out to determine the antioxidant activity. Moreover, antimicrobial activity of laurel fractions was tested against Staphylococcus aureus ATCC 25923, Bacillus subtilis ATCC 6633, Pseudomonas aeruginosa ATCC 10145, Escherichia coli ATCC 11775, Candida albicans ATCC 60193 and Aspergillus niger ATCC 16404. Minimum inhibitory concentration (MIC) and minimal bactericidal and fungicidal concentration (MBC) were obtained. Both fractions showed a similar antioxidant activity, although it was slightly higher for the fraction recovered in separator 2. However, antimicrobial activity against the microorganisms tested was only found when fraction 2 was used. Staphylococcus aureus was the most sensitive microorganism to this fraction, with maximal inhibition zones (25 mm) and the lowest MBC values (1.25 mg/ml), whereas the least susceptible was the fungi Aspergillus niger. In order to determine the compounds responsible for the antimicrobial activity, fraction 2 was analysed by GC–MS; results obtained showed that most of the compounds identified in the supercritical extract have been previously described to show antimicrobial activity; among them, the major compound found in the supercritical extract corresponded to a sesquiterpene lactone of the germacrolide type (6-epi-desacetyllaurenobiolide) previously described in laurel.

Similar content being viewed by others
References
Barlow SM (1990) In: Hudson BJF (ed) Food antioxidants. Elsevier, London, pp 253-307
Namiki M (1990) Crit Rev Food Sci 29:273–300
Hammer KA, Carson C F, Riley TV (1999) J Appl Microbiol 86:985–990
Valero M, Salmerón MC (2002) Int J Food Microbiol 2648:1–9
Dorman HJD, Peltoketo A, Hiltunen R, Tilkkanen MJ (2003) Food Chem 83:255–262
Sahin F, Güllüce M, Daferera D, Sökmen A, Sömen M, Polissiou M, Agar G, Ozer H (2004) Food Control 14:549–557
Tomaino A, Cimino F, Zimbalatti V, Venuti V, Sulfaro V, De Pascale A, Saija A (2005) Food Chem 89:549–554
Bouzouita N, Kachouri F, Hamdi M, Chaabouni MM (2003) Flavour Fragr J 18:380–383
Simic A, Sokovic D, Ristic M, Grujic-Jovanovic S, Vukojevic J, Marin PD (2004) Phytother Res 18:713–717
Simic M, Kundakovic T, Kovacevic N (2003) Fitoterapia 74:613–616
Skerget M, Kotnik P, Hadolin M, Hras AR, Simonic M, Knez Z (2005) Food Chem 89:191–198
Díaz-Maroto M C, Pérez-Coello MS, Cabezudo MD (2002) J Chromatogr A 947:23–29
Ibañez E, Oca A, Murga G, Lopez-Sebastian S, Tabera J, Reglero G (1999) J Agric Food Chem 47:400–1404
Leal PF, Braga MEM, Sato DN, Carvalho JE, Marques MOM, Meireles MAA (2003) J Agric Food Chem 51:2520–2525
Santoyo S, Cavero S, Jaime L, Ibáñez E, Señorans FJ, Reglero G (2005) J Food Protect 68:790–795
Cavero S, Garcia-Risco M R, Marín FR, Jaime L, Santoyo S, Señorans FJ, Reglero G, Ibáñez E (2005) J Supercrit Fluids (in press)
Señorans FJ, Ibáñez E, Cavero S, Tabera J, Reglero G (2000) J Chromatogr A 870:491–499
Brand-Williams W, Cuvelier ME, Berset C (1995) Lebensm-Wiss Technol 28:2530
Velioglu YS, Mazza G, Gao L, Oomah BD (1998). J Agric Food Chem 46:4113–4117
Singleton VL, Orthofer R, Lamuela-Raventós RM (1999) Method Enzymol 299:152–178
NCCLS (National Committee for Clinical Laboratory Standards) (1999) Wayne, PA. M 100-S9
Murray P R, Baron EJ, Pfaller MA, Tenover FC, Yolke RH (1995). Manual of clinical microbiology, 6th edn. Mosby Year Book, London
Shahidi F, Wanasundara RKJPD (1992) Crit Rev Food Sci 32:67–103
Revenchon E (1997) J Supercrit Fluids 10:1–37
Morongiu B, Piras A, Pani F, Proceda S, Barello M (2003) Flavour Fragr J 18:505–509
Caredda A, Marongiu B, Porcedda S, Soro C (2002) Food Chem 50:1492–1496
Fiorini C, Fourasté I, David B, Bessière JM (1997) Flavour Fragr J 12:91–93
Davies NW (1990) J Chromatogr A 503:1–24
Tada H, Takeda K (1971) Chem Comm 12:1391–1392
Bohlmann F, Adler A, King RM, Robinson H (1982) Phytochem 21:1169–1170
Quijano L, Calderon JS, Gomez GF, Lopez PJ, Rios T, Fronczek FR (1984) Phytochem 23:1971–1974
Inoue Y, Shiraishi A, Hada T, Hirose K, Hamashima H, Shimada J (2004) FEMS Microbiol Lett 237:325–331
Sibanda S, Chigwada G, Poole M, Gwebu ET, Noletto JA, Schmidt JM, Rea AI, Setzer WN (2004) J Ethnophram 92:107–111
Scher JM, Speakman J-B, Zapp J, Becker H (2004) Phytochem 65:2583–2588
López-Malo A, Alzamora SM, Palou E (2005) Int J Food Microbiol 99:119–128
Wedge DE, Galindo JCG, Macias FA (2000) Phytochem 53:747–757
Frankel EN (1979) In: Simic MG, Karel M (eds) Autoxidation in food and biological systems. Plenum Press, New York, pp 141–170
Acknowledgements
This work was supported by CICYT projects (AGL2004-C02-01 and AGL2004-C02-02). The authors thank Dr. Jesus Sanz for his kind help in the acquisition of the MS spectra and his helpful discussions. R. Lloría thanks the CSIC for her grant
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santoyo, S., Lloría, R., Jaime, L. et al. Supercritical fluid extraction of antioxidant and antimicrobial compounds from Laurus nobilis L. Chemical and functional characterization. Eur Food Res Technol 222, 565–571 (2006). https://doi.org/10.1007/s00217-005-0027-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-005-0027-9