Abstract
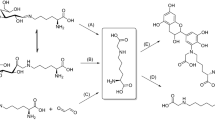
After heating N-α-hippuryl-L-arginine (Hip-Arg) with varying amounts of lactose for 1–4 h at 100 °C, a previously unknown arginine derivative could be detected by RP-HPLC and UV-detection. Following semi-preparative isolation, the compound was unequivocally identified as 2-(2-benzoylamino-acetylamino)-5-[5-(3-hydroxypropyl)-4-oxo-imidazolon-2-yl]-L-ornithine (Hippuryl-PIO, Hip-PIO) by electrospray-time of flight-mass spectroscopy as well as one- and two-dimensional 1H- and 13C-nuclear magnetic resonance. Hip-PIO was exclusively formed during incubation of Hip-Arg with disaccharides containing a 1,4-glycosidic linkage. As reference for amino acid analysis, free PIO was synthesized starting from N-(tert-butoxycarbonyl)-L-arginine (t-Boc-Arg) via t-Boc-PIO and final hydrolysis with acetic acid. Preliminary evidence for the formation of protein-bound PIO, which proved to be acid-labile, was obtained using amino acid analysis with ninhydrin detection for enzymatic hydrolysates of casein samples which had been heated in the presence of lactose at 100 °C. The ornithinoimidazolinone PIO represents a new type of post-translational protein modification formed during food processing, which might be responsible for the major part of arginine derivatisation in disaccharide-containing foods like milk.





Similar content being viewed by others
References
Henle T, Schwarzenbolz U, Walter AW, Klostermeyer H (1998) The Maillard reaction in foods and medicine. In: O’Brien J et al (eds) Proceedings of the 6th International Symposium on the Maillard Reaction. The Royal Society of Chemistry, Cambridge, pp 178–183
Hartkopf J, Erbersdobler H F (1995) Z Lebensm Unters Forsch 201:27–29
Mohamad A, Fraenkel-Conrad H, Olcott HS (1949) Arch Biochem 24:157–178
Henle T, Walter AW, Klostermeyer H (1994) Z Lebensm Unters Forsch 199:55–58
Glomb M, Lang G (2001) J Agric Food Chem 49:1493–1501
Schwarzenbolz U, Henle T, Haeßner R, Klostermeyer H (1997) Z Lebensm Unters Forsch 205:121–124
Hayase F, Koyama T, Konishi Y (1997) J Agric Food Chem 45:1137–1143
Glomb MA, Lang G (2001) J Agric Food Chem 49:1493–1501
Sopio R, Lederer M (1995) Z Lebensm Unters Forsch 201:381–386
Sell DR, Monnier VM (1989a) J Biol Chem 264:21597–21602
Henle T, Schwarzenbolz U, Klostermeyer H (1997) Z Lebensm Unters Forsch 204:95–98
Biemel KM, Bühler HP, Reihl O, Lederer MO (2001) Nahrung 45:210–214
Henle T, Walter H, Krause I, Klostermeyer H (1991) Int Dairy J 1:125–135
Henle T, Walter H, Klostermeyer H (1991) Z Lebensm Unters Forsch 193:119–122
Troyano E, Olano A, Jimeno ML, Sanz J, Martinez-Castro I (1992) J Dairy Res 59:507–515
Hollnagel A, Kroh WL (2002) J Agric Food Chem 50:1659–1664
Acknowledgements
We thank Mrs. Karla Schlosser for excellent technical assistance with amino acid analysis. Further thanks are due to Dr. Uwe Schwarzenbolz for LC-MS measurements. This study was supported by a research grant of Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mavric, E., Kumpf, Y., Schuster, K. et al. A new imidazolinone resulting from the reaction of peptide-bound arginine and oligosacccharides with 1,4-glycosidic linkages. Eur Food Res Technol 218, 213–218 (2004). https://doi.org/10.1007/s00217-003-0817-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-003-0817-x