Abstract
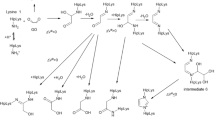
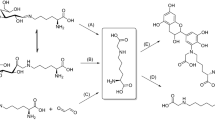
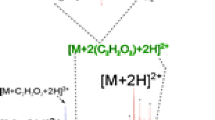
The reaction of arginine and arginine derivatives with glyoxal under mild conditions revealed the formation of a previously unknown amino acid, designated as “Glarg”. 1H-, 15N- and 13C-NMR analysis of the new compound elucidated its structure to be 1-(4-amino-4-carboxybutyl)-2-imino-5-oxo-imidazolidine. Experiments with solutions containing N α-acetylarginine and glyoxal showed that “Glarg” is formed quickly under physiological conditions, but is labile at higher temperatures as well as at low pH values. After incubation of β-casein with glyoxal, the formation of protein-bound “Glarg” in enzymatic hydrolysates via amino acid analysis could be demonstrated. Due to the fast reaction of glyoxal with arginine residues, under physiological conditions, proteins may act as scavengers for glyoxal.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 8 November 1996 / Revised version: 10 January 1997
Rights and permissions
About this article
Cite this article
Schwarzenbolz, U., Henle, T., Haeßner, R. et al. On the reaction of glyoxal with proteins. Z Lebensm Unters Forsch 205, 121–124 (1997). https://doi.org/10.1007/s002170050137
Issue Date:
DOI: https://doi.org/10.1007/s002170050137