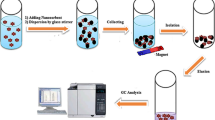
Abstract
Aflatoxins (AFs), an important category of pollutants, are formed in many foods and adversely affect human health. Therefore, their determination is critical to ensuring human food health. An efficient dispersive solid-phase microextraction technique was developed as a simple and straightforward sample preparation technique for determination of four aflatoxins using a high-performance liquid chromatography (HPLC) fluorescence detector. A novel efficient, green sorbent for extracting AFs was synthesized based on hydrothermal and chemical strategies. The amounts of three sorbent components were optimized using a mixture design (simplex lattice design), including 14 experiments. The optimal amount of amino-bimetallic Fe/Ni-MIL-53 nanospheres, chitosan, and magnetic Fe3O4 nanoparticles as sorbent components was 0.87, 0.67, and 0.47 g, respectively. Also, various factors affecting the process of AF determination were studied and optimized in two successive experimental designs, including the definitive screening design and the Box–Behnken design. Under optimal conditions, the linear ranges for measuring aflatoxin B1, aflatoxin B2, aflatoxin G1, and aflatoxin G2 were 0.05–82.6, 0.07–86.4, 0.08–85.7, and 0.07–89.5 ng mL−1, respectively. The relative standard deviations under inter-day and intra-day conditions for measuring AFs at three analyte concentrations were determined in triplicate analysis and were in the ranges of 3.7–4.6% and 4.9–6.1% for water sample analysis, respectively. The qualitative detection limits for determining AFs were between 0.01 and 0.05 ng mL−1. The pre-concentration factor of the method for measuring AFs ranged from 739.7 to 802.1. The proposed method was used for determining AFs in several real samples, including herbal distillate, black tea, corn, and real water samples. The relative recovery and standard deviation were 87.8–97.8% and 4.10–6.82%, respectively.
Graphical abstract





Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
References
Mahato DK, Lee KE, Kamle M, Devi S, Dewangan KN, Kumar P, et al. Aflatoxins in food and feed: an overview on prevalence, detection and control strategies. Front Microbiol. 2019;10:2266.
Chain EPoCitF, Schrenk D, Bignami M, Bodin L, Chipman JK, del Mazo J, et al. Risk assessment of aflatoxins in food. EFSA J. 2020;18(3):e06040.
Liu Q, Jiang L, Xiao L, Kong W. Physico-chemical characteristics and aflatoxins production of Atractylodis Rhizoma to different storage temperatures and humidities. AMB Express. 2021;11(1):1–10.
Wangia RN, Tang L, Wang J-S. Occupational exposure to aflatoxins and health outcomes: a review. J Environ Sci Health, Part C. 2019;37(4):215–34.
Dhakal A, Sbar E, Aflatoxin toxicity. StatPearls. [Internet]: StatPearls Publishing; 2021.
Aicha Y, Cisse H, Oumarou Z, Nikiema F, Traore Y, Savadogo A. Reduction of aflatoxins and microorganisms in the koura-koura produced in Burkina Faso with spices and aromatic leaves. J Food Technol. 2021;8(1):9–17.
Negash D. A review of aflatoxin: occurrence, prevention, and gaps in both food and feed safety. J Appl Microbiol Res. 2018;1(1):35–43.
Dai Y, Huang K, Zhang B, Zhu L, Xu W. Aflatoxin B1-induced epigenetic alterations: an overview. Food Chem Toxicol. 2017;109:683–9.
Gu Y-X, Yan T-C, Yue Z-X, Liu F-M, Cao J, Ye L-H. Recent developments and applications in the microextraction and separation technology of harmful substances in a complex matrix. Microchem J. 2022;107241
Câmara JS, Perestrelo R, Berenguer CV, Andrade CF, Gomes TM, Olayanju B, et al. Green extraction techniques as advanced sample preparation approaches in biological, food, and environmental matrices: a review. Molecules. 2022;27(9):2953.
Ghorbani M, Mohammadi P, Keshavarzi M, Ziroohi A, Mohammadi M, Aghamohammadhasan M, et al. Developments of microextraction (extraction) procedures for sample preparation of antidepressants in biological and water samples, a review. Crit Rev Anal Chem. 2021;1–28
Alhmaunde A, Masrournia M, Javid A. Facile synthesis of new magnetic sorbent based on MOF-on-MOF for simultaneous extraction and determination of three benzodiazepines in various environmental water samples using dispersive micro solid-phase extraction and HPLC. Microchem J. 2022;181:107802.
Ghorbani M, Mohammadi P, Keshavarzi M, Saghi MH, Mohammadi M, Shams A, et al. Simultaneous determination of organophosphorus pesticides residues in vegetable, fruit juice, and milk samples with magnetic dispersive micro solid-phase extraction and chromatographic method; recruitment of simplex lattice mixture design for optimization of novel sorbent composites. Anal Chim Acta. 2021;338802
Ghorbani M, Aghamohammadhassan M, Ghorbani H, Zabihi A. Trends in sorbent development for dispersive micro-solid phase extraction. Microchem J. 2020;158:105250.
Safaei M, Foroughi MM, Ebrahimpoor N, Jahani S, Omidi A, Khatami M. A review on metal-organic frameworks: synthesis and applications. TrAC Trends Anal Chem. 2019;118:401–25.
Razavi SAA, Morsali A. Linker functionalized metal-organic frameworks. Coord Chem Rev. 2019;399:213023.
Gordi Z, Ghorbani M, Ahmadian KM. Adsorptive removal of enrofloxacin with magnetic functionalized graphene oxide@ metal-organic frameworks employing D-optimal mixture design. Water Environment Research. 2020;92(11):1935–47.
Pérez-Cejuela HM, Herrero-Martínez JM, Simó-Alfonso EF. Recent advances in affinity MOF-based sorbents with sample preparation purposes. Molecules. 2020;25(18):4216.
Mohammadi P, Ghorbani M, Mohammadi P, Keshavarzi M, Rastegar A, Aghamohammadhassan M, et al. Dispersive micro solid-phase extraction with gas chromatography for determination of Diazinon and Ethion residues in biological, vegetables and cereal grain samples, employing D-optimal mixture design. Microchem J. 2021;160:105680.
Uzun I, Güzel F. Kinetics and thermodynamics of the adsorption of some dyestuffs and p-nitrophenol by chitosan and MCM-chitosan from aqueous solution. J Colloid Interface Sci. 2004;274(2):398–412.
Zheng S, Yang Z, Park YH. Removal of chlorophenols from groundwater by chitosan sorption. Water Res. 2004;38(9):2315–22.
Ngah WW, Endud C, Mayanar R. Removal of copper (II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym. 2002;50(2):181–90.
Wan Ngah W, Ab Ghani S, Hoon L. Comparative adsorption of Lead (II) on flake and bead-types of chitosan. J Chin Chem Soc. 2002;49(4):625–8.
Ngah WW, Kamari A, Koay Y. Equilibrium and kinetics studies of adsorption of copper (II) on chitosan and chitosan/PVA beads. Int J Biol Macromol. 2004;34(3):155–61.
Rabea EI, Badawy ME-T, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457–65.
Kabanov VL, Novinyuk LV. Chitosan application in food technology: a review of rescent advances. Food Syst. 2020;3(1):10–5.
Ng CH, Ng KKS, Lee SL, Suwa R, Lee CT, Tnah LH. Growth performance and scale insect infestation of Shorea leprosula in a common garden experimental plot. J For Res. 2023;34(3):781–92.
Snyder L. Classification off the solvent properties of common liquids. J Chromatogr Sci. 1978;16(6):223–34.
Nazhand A, Durazzo A, Lucarini M, Souto EB, Santini A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods. 2020;9(5):644.
Karami-Osboo R, Maham M. Pre-concentration and extraction of aflatoxins from rice using air-assisted dispersive liquid–liquid microextraction. Food Anal Methods. 2018;11(10):2816–21.
Hamed AM, Abdel-Hamid M, Gámiz-Gracia L, García-Campaña AM, Arroyo-Manzanares N. Determination of aflatoxins in plant-based milk and dairy products by dispersive liquid–liquid microextraction and high-performance liquid chromatography with fluorescence detection. Anal Lett. 2019;52(2):363–72.
Asadi M. Separation and quantification of aflatoxins in grains using modified dispersive liquid–liquid microextraction combined with high-performance liquid chromatography. J Food Measurement Characterization. 2020;14(2):925–30.
Feizy J, Es’haghi Z, Lakshmipathy R. Aflatoxins’ clean-up in food samples by graphene oxide–polyvinyl poly pyrrolidone—hollow fiber solid-phase microextraction. Chromatographia. 2020;83(3):385–95.
Safavizadeh V, Arabkhani P, Mojkar M, Shyrina D, Nemati M. Application of dispersive liquid-liquid microextraction to determine Aflatoxin B1 in tomato paste samples. J Nutrit Food Security. 2021;6(1):24–30.
Chmangui A, Jayasinghe GTM, Driss MR, Touil S, Bermejo-Barrera P, Bouabdallah S, et al. Assessment of trace levels of aflatoxins AFB1 and AFB2 in non-dairy beverages by molecularly imprinted polymer based micro solid-phase extraction and liquid chromatography-tandem mass spectrometry. Anal Methods. 2021;13(30):3433–43.
Zhu A, Jiao T, Ali S, Xu Y, Ouyang Q, Chen Q. Dispersive micro solid phase extraction based ionic liquid functionalized ZnO nanoflowers couple with chromatographic methods for rapid determination of aflatoxins in wheat and peanut samples. Food Chem. 2022;391:133277.
Karami-Osboo R, Ahmadpoor F, Nasrollahzadeh M, Maham M. Polydopamine-coated magnetic spirulina nanocomposite for efficient magnetic dispersive solid-phase extraction of aflatoxins in pistachio. Food Chem. 2022;377:131967.
Rezaei F, Masrournia M, Pordel M. Simultaneous determination of four aflatoxins using dispersive micro solid phase extraction with magnetic bimetallic MOFs composite as a sorbent and high-performance liquid chromatography with fluorescence detection. Microchem J. 2023;189:108506.
Acknowledgments
The authors gratefully acknowledge this research’s support from the Razi Research Center, Khorasan Razavi Education, Mashhad, Iran; Mashhad University of Medical Sciences, Mashhad, Iran; Near East University, Nicosia, Cyprus; Islamic Azad University of Mashhad, Iran; and Nanjing University of Information Science & Technology (NUIST), China.
Author information
Authors and Affiliations
Contributions
Mahdi Ghorbani: conceptualization, methodology, formal analysis, writing—review & editing.
Majid Keshavarzi: writing—original draft, validation.
Maryam Pakseresht: investigation, resources.
Parisa Mohammadi: writing—original draft, investigation.
Alireza Shams: writing and visualization.
Abouzar Mehraban: resources.
Amir Ismailzadeh: resources, data curation.
Corresponding author
Ethics declarations
Conflicts of interest
The authors confirm that there are no known conflicts of interest related to this publication, and this work has not received any significant financial support to influence its outcome.
Ethical approval
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 273 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghorbani, M., Keshavarzi, M., Pakseresht, M. et al. Optimization and synthesis of a novel sorbent composite based on magnetic chitosan-amine-functionalized bimetallic MOF for the simultaneous dispersive solid-phase microextraction of four aflatoxins in real water, herbal distillate, and food samples. Anal Bioanal Chem 415, 5681–5694 (2023). https://doi.org/10.1007/s00216-023-04842-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04842-0