Abstract
This study aimed to investigate the characteristics, moisture contents, chemical fingerprints changes and aflatoxins accumulation of Atractylodis rhizoma during storage, further to determine the optimum temperature and relative humidity conditions. Based on the suitable temperature (20–40 °C) and relative humidity (80–95%), 13 different temperature and humidity conditions were set up by the central composite design-response surface methodology (CCD-RSM) for Aspergillus flavus. After inoculation with Aspergillus flavus by artificial infection, A. rhizoma samples were stored under normal conditions and 13 different temperature and relative humidity levels. By taking the changes of characteristics, the contents of moisture, chemical fingerprints and aflatoxins as the evaluation indexes for A. rhizoma with or without Aspergillus flavus fungi to optimize the optimal storage conditions. After storage for 10 days, the color of A. rhizoma was deepened, the water content and chemical composition increased, and some unknown components were detected. The susceptible condition for aflatoxins production in A. rhizoma was identified at temperature 22–37 °C and relative humidity over 87.5%. Thus, the suitable storage conditions for A. rhizoma should be controlled at temperature below 20 °C and relative humidity less than 85%. This paper screened out the optimum temperature and humidity for the storage of A. rhizoma. Then, the storage specification for A. rhizoma was proposed, lying technical and data support for the scientific preservation of other food or herbs.
Similar content being viewed by others
Introduction
The quality and safety of foods and traditional Chinese medicines (TCMs) are extremely important for their clinical application, and scientific storage to prevent mildew and mycotoxins contamination is the basis and premise. China has a vast territory and a large span from the north to the south, especially in the south of China, it is warm and humid all the year round. In the rainy season, especially in June and July each year, the air humidity and atmospheric temperature are very high, which are suitable for the growth of most of the toxicogenic fungi in TCMs, leading to many difficulties for their safe storage. In addition, most TCMs are rich in polysaccharides, protein, starch, fat oil and other components, which provide good nutritional conditions for the rapid reproduction and growth of mould including toxicogenic fungi, followed by the mycotoxins contamination (Christensen et al. 2012; Huang et al. 2019; Zhang et al. 2018). Mycotoxins as secondary metabolites produced by many kinds of fungi when the climatic conditions become favorable during the growth, harvest, handling, especially the storage processes of TCMs (Weaver et al. 2020; Liu et al. 2015; Wang et al. 2015; Duarte et al. 2020). Various mycotoxins with strong toxigenic, carcinogenic, mutagenic and immunosuppression properties and serious threats to human health have been found in diverse TCMs all over the world in the past decades (Yang et al. 2020; Ponzilacqua et al. 2019; Nieto et al. 2018). High occurrence of mycotoxins in TCMs, along with their toxic effects, as well as huge economic losses to TCMs industries have resulted in a global concern (Haque et al. 2020; Ashiq et al. 2014). Aflatoxins (AFs) produced by Aspergillus strains, due to their strong liver and kidney toxicity and high incidence in many food and agricultural products, and TCM matrices, have attracted much attention (Ali 2019; Martins et al. 2020). They have been listed as the most dangerous food hazards in nature by the Food and Agriculture Organization (FAO) of the United Nations and the World Health Organization (WHO) (World Health Organization 2007–2015). Of them, AFB1 has strong hepatotoxic and hepatocarcinogenic properties. The International Agency for Research on Cancer has classified AFB1 as Group 1A carcinogen (International Agency for Research on Cancer [IARC] 1999).
Atractylodis rhizoma, a classical TCM, is the dry rhizoma of Atractylodes lancea (Thunb.) DC. (A. lancea) and Atractylodes chinensis (DC.) Koidz. (A. chinensis) and exhibits in 2020th edition of the Chinese Pharmacopeia (Chinese Pharmacopoeia Commission 2020). In addition, A. rhizoma has been prescribed in Chinese, Korean, Japanese and Thai traditional medicine in present paper (Zhang et al. 2021). A. rhizoma is an important crude drug that commonly believed to be used in the treatment of influenza, night blindness, rheumatic dis-eases and several other types of digestive problem. (Kitajima et al. 2003; Jin et al. 2004). Unfortunately, a large amount of starch and volatile components contained in A. rhizoma, in combination of favorable environmental conditions make it easily subject to fungal contamination and mycotoxins residue (Liu et al. 2016a, b). Our previous studies have found that 4 out of 22 batches of A. rhizoma samples contaminated trace levels of mycotoxins (Liu et al. 2019; Hu et al. 2015). High incidence of mycotoxins in A. rhizoma will pose potential threats and influence on the quality and safety of this TCM, which is great importance and necessity, however, has not been clarified from then on.
Therefore, this study aimed to first investigate the toxigenic fungi growth and aflatoxins production in A. rhizoma under various humidity and temperature conditions according to the central composite design-response surface methodology (CCD-RSM) (Kumar et al. 2016; Duangjit et al. 2015; Norioko et al. 2013), then to explore the relationship of mycotoxins accumulation determined by using an ultra-fast liquid chromatography/tandem mass spectrometry (UFLC-MS/MS) method and bioactive components variation measured by introducing a gas chromatography with flame ionization detection (GC-FID) technique, and naturally to elucidate fungal contamination and mycotoxins production affected the internal and external quality of this TCM for screening for the most suitable temperature and humidity conditions for safe storage of A. rhizoma.
Materials and methods
Chemicals and standards
Dried A. rhizoma samples were purchased from the Anguo medicine market in Hebei province, China. All the samples were crushed into powder and passed through a third-sieve, and then stored at − 20 °C until further use.
The lyophilized powder of aflatoxigenic Aspergillus flavus (CGMCC 3.4410) was supplied by the China General Microbiological Culture Collection Center (CGMCC, Beijing, China). Aspergillus flavus dissolved in 0.5 mL of sterile water (121 °C, 20 min) for culture on Salt Czapek Dox Agar medium (Beijing Aobox Biotechnology Co., Ltd., Beijing, China) at constant temperature and humidity (28 °C, 75% relative humidity, RH) under a 12/12 h daylight/dark regime.
Chromatographic-grade methanol, acetonitrile and ethyl acetate were supplied by Fisher Scientific (Fair Lawn, NJ, USA). Tween-20 and formic acid were supplied by Xilong Chemical Corporation (Guangdong, China). Magnesium sulfate anhydrous (MgSO4) were provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Primary secondary amine (PSA) sorbent was purchased from Agela Technologies (Tianjin, China). The water used throughout the experiment was Wahaha purified water (Hangzhou, China).
The stock solution containing four aflatoxins (AFB1, AFB2, AFG1 and AFG2, the chemical structures shown in Fig. 1) was stored in methanol and supplied by SUPELCO (Bellafonte, PA, USA). The reference standards (atractylon, atractydin, atractylenolide I, II and III) were provided by Chengdu Chroma Biotechnology (Chengdu, China) with purity higher than 98.0% and chemical structures in Fig. 1. n-Tridecane as the internal standard was purchased from Dr. Ehrenstorfer (Augsburg, Germany) with purity over 99%.
Preparation of Aspergillus flavus spore suspension
Spores of Aspergillus flavus CGMCC 3.4410 were propagated on Salt Czapek Dox Agar (SCDA) medium for 14 days at 28 oC. Spore culture on SCDA medium from the fungal were collected by suspension in 10 mL water containing 0.1% tween-20. The suspension formed was filtered through 3 layers of cheesecloth. A final spore solutions containing 106 spores/mL was adjusted by sterile water using a haemocytometer (Obata et al. 2019). The inoculation and sporangia images were shown in Additional file 1: Fig. S1.
Sample inoculation with Aspergillus flavus spore suspension
The suitable temperature and humidity for the growth of Aspergillus flavus are 25–40 °C and 80–90%, respectively. The optimal conditions predicted by the Design-Expert V8.0.6 software. Design of experiment analysis utilizes a CCD-RSM to optimize the suitable temperature and humidity for storage of A. rhizoma samples. Create a temperature and humidity chamber in a MJX Intelligent mould culture box (Ningbo Jiangnan Instrument Factory, China).
Surface sterilized culture dishes were irradiated under an UV lamp for 1 h, while the samples were sterilized by irradiation both sides for 30 min by 265 nm ultraviolet rays. 50 g of A. rhizoma sample was placed to each dish. 1 mL of spore suspensions was inoculated onto the surface of each sample for culturing under a 12 h day/night photoperiod under 13 different temperature and humidity conditions for 10 days. After the culture period, the characteristics was observed and the contents of moisture, chemical fingerprints changes and aflatoxins accumulation of A. rhizoma were investigated to determine the optimum temperature and relative humidity conditions.
Establishment of UFLC-MS/MS method for determination of aflatoxins
Equipment and UFLC-MS/MS conditions
The UFLC-MS/MS equipment consisted of Shimadzu ultra-fast liquid chromatography (UFLC) system (Shimadzu, Kyoto, Japan) coupled to an AB SCIEX QTrap® 5500 mass spectrometer (AB SCIEX, Foster City, CA, USA) with electrospray ionization (ESI) source. The chromatographic separation was carried out on a SHISEIDO CAPCELL CORE C18 column (50 mm × 2.1 mm, 2.7 μm, Shiseido). A binary (acetonitrile with 0.1% formic acid (A) and 0.1% (v/v) aqueous formic acid (B)) solvent system was optimized for analysis: 0–2 min, 90–80% B; 2–8 min, 80–30% B; 8–10 min, 30–5% B; 10–12 min, held at 5% B; 12.01–15 min, 90% B. The flow rate was set at 0.3 mL/min and the injection volume was 2 µL.
Quantification was conducted with positive electrospray ionization (ESI+). The ion spray (IS) voltage was 5500 V and the source temperature was 550 °C. Nebulizer gas (GS1), heater gas (GS2) and curtain gas (CUR) were ultrahigh purity nitrogen and the values were adjusted to 50, 50 and 35 psi, respectively, as well as the collision activation dissociation gas (CAD) was medium. The precursor-to-product ion for AFB1, AFB2, AFG1 and AFG2, and the MS/MS parameters including collision energy (CE), collision cell exit potentials (CXP), entrance potential (EP) and declustering potential (DP) were shown in Table 1. The acquired data was processed with Analyst® software version 1.6.2 (AB SCIEX, Foster City, CA, USA).
Preparation of sample solution
Extraction of aflatoxins from A. rhizoma sample was performed by modified QuEChERS (Quick, Easy, Cheap, Effective, Rugged and Safe) procedure. 1 g A. rhizoma powder was accurately weighted into a 30-mL tube, with the addition of 4 mL of 90% aqueous acetonitrile solution, followed by properly vortexing for 1 min and sonication for 5 min in an ultrasonic bath. Afterwards, 180 mg MgSO4 and 60 mg PSA were added, each tube was shaken with a vortex for 1 min and centrifuged for 10 min at 10,000 rpm. After that, 1 mL of the upper organic phase was transferred into a 2-mL centrifugation tube, and concentrated to dryness under a stream of nitrogen at 40 °C. The extracts were reconstituted in 0.5 mL of 50% aqueous methanol solution. Subsequently, the extract solution was shaken vigorously for 30 s and filtered through a 0.22-µm PTEE filter and transferred to an autosampler vial for analysis by the UFLC-MS/MS system.
Matrix-matched calibration curve
Considering matrix effect in aflatoxins determination (Liu et al. 2019), matrix-matched calibration curves were used throughout this study by spiking blank matrix of the extracts of analyte-free sample with aflatoxin standards. Blank matrix was obtained by the preparation of sample as described. The calibration curves were prepared in a blank matrix spiked with a series of concentration at 1, 2, 5, 10, 25, 50 and 100 ng/mL of AFB1 and AFG1, 0.25, 0.5, 1.25, 2.5, 6.25, 12.5 and 25 ng/mL of AFB2 and AFG2 by plotting the peak areas (y) of each analyte against the concentration (x).
Establishment of GC-FID fingerprint
Equipment and GC-FID conditions
GC analyses were carried out with an Agilent 6890N gas chromatograph coupled with a flame ionization detector (Agilent Technologies, Little Falls, DE, USA) and an Agilent 7683 autosampler. Data were collected and processed by a HP ChemStation (Hewlett Packard, Palo Alto, CA, USA). The chromatographic separation was performed on a HP-5 fused silica capillary column of 30 × 0.32 m and 0.25 μm film thickness. High-purity nitrogen (> 99.99% purity) was used as carrier gas. The FID detector was fed by hydrogen (40 mL/min) and air (450 mL/min). The injection volume of sample was 2 µL. The injector and detector temperatures were both 250 °C, and the injection was performed in the splitless mode with a purge time of 0.75 min. The temperature program for GC was as follows: initial temperature 80 °C held for 2 min, increased at rate of 10 °C/min to 120 °C, second increased at 2 °C/min to 150 °C, third increased at 4 °C/min to 170 °C, and finally increased at 10 °C/min to 240 °C and held for 2 min, for a total runtime of 35 min. The content of each component in essential oil was comparing by their areas to the peak areas of IS, expressed as mean ± SD according to relative peak areas.
Preparation of standard solution
Appropriate amount of atractylon, atractydin, atractylenolide I, II and III was weighed accurately, respectively, with the addition of ethyl acetate to prepare a standard solution at the concentration about 1 mg/mL, which were used as qualitative control solutions.
Extraction of essential oils and preparation of sample solution
Essential oil from A. rhizoma sample was obtained using conventional hydrodistillation technique. 30 g A. rhizoma powder was accurately weighed into a flask, followed by the addition of 300 mL of distilled water for mixing evenly. Then, the solution was slowly heated to boiling and kept slightly boiling for about 5 h using specific instrumentation until the content of essential oils in the extractor did not increase. The essential oil was transferred to a close bottle, with the addition of anhydrous sodium sulfate to absorb water, followed by storage at low temperature in the dark. 50 µL of essential oil was accurately transferred to a 10-mL volumetric flask, with the addition of ethyl acetate, as well as n-tridecane as the internal standard (IS). The final concentrations were 1 µL/mL for IS and 5 µL/mL for sample solution.
Results
Characteristics
Following storage for a long incubation period, as shown in Fig. 2, the color of A. rhizoma was gradually deepened. In the certain scope, the majority of Aspergillus flavus increased along with the temperature and humidity change. Fungi appeared on parts of A. rhizoma samples and were clearly visible. Especially, the samples were mildewed most seriously under temperature 30 °C and humidity 95%.
Contents of moisture
Considering more volatile components in the A. rhizoma samples, toluene method was used to determine the water content and calculate the moisture content (%). The moisture content of A. rhizoma was not more than 11% according to Chinese Pharmacopoeia (2020th Edition). Table 2 showed that the set temperature and humidity have obvious influence on the moisture content, which was higher than the required limit, indicating that the sorption and hygroscopicity were serious when storage under different temperature and humidity. Also, the suitable environment provided convenience for the growth of Aspergillus flavus.
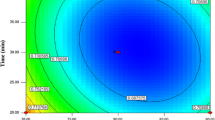
Further, response surface model was used to analyze the changing trend of moisture content under different temperature and humidity conditions (Fig. 3). When the temperature was set at 20 °C and humidity at 80%, the moisture content was the lowest, while at temperature 40 °C and humidity 95%, the moisture content was the highest.
Component changes in chemical fingerprints
Essential oils from 14 batches of A. rhizoma samples extracted by hydrodistillation were analyzed by GC-FID. The GC-FID chromatograms presented all of the volatile components as chromatographic peaks. The chemical fingerprint of volatile oil was established. By taking the ratio of relative retention time (RRT) and relative peak area (RPA) of the each chromatographic peak to n-Tridecane (IS) as the index, the component changes were analyzed in Fig. 4.
First, the percentage of the total volume (mL) of extracted essential oil to the mass (g) of sample powder is considered as the yield (%) of essential oil. Yields in the A. rhizoma sample inoculated with Aspergillus flavus and stored under different temperature and humidity conditions were obviously reduced. Rise of temperature may accelerate the loss of volatile components and Aspergillus flavus may decompose the active component. Secondly, the higher the temperature was, the more complex chemical composition changes with suitable humidity were observer. The contents of atractylone, atractylenolide I, II, and III, especially at 37.07 °C and 92.8% humidity, changed most obviously (Additional file 1: Fig. S2). After a 10-day inoculation with Aspergillus flavus, a new chemical component was measured and identified as aristolene epoxide by GC-MS. However, the specific production mechanism was unclear. The content change and the transformation rule of each component need to be further studied, and the key influencing factors should be clarified.
Aflatoxins accumulation
The concentration of AFG2, AFG1, AFB2 and AFB1 showed a good linear relationship with the peak area. The matrix matching equations were y = 88500x − 19,000 (r = 0.9995), y = 40600x − 4470 (r = 0.9970), y = 48000x − 3240 (r = 0.9982), and y = 16400x − 8580 (r = 0.9987) for AFG2, AFG1, AFB2 and AFB1, respectively. The limits of quantification (LOQs) for AFG2, AFG1, AFB2 and AFB1 were 0.125, 0.1, 0.125 and 0.1 µg/kg, respectively.
The validated UFLC-MS/MS method applied practically to determine AFG2, AFG1, AFB2 and AFB1 in 14 batches of A. rhizoma sample before and after incubation with Aspergillus flavus. The highest concentration of total aflatoxins (AFG1 + AFG2 + AFB1 + AFB2) was 10.26 µg/kg in the sample that was incubated with Aspergillus flavus ans stored at 30 °C and 95% humidity. Also, 3.84 µg/kg of total aflatoxins was detected when the temperature and humidity were set at 37.07 °C and 92.80% humidity. These results were consistent with the mildew on the surface of samples and the content of atractylone, atractylenolide I, II, and III. Accordingly, response surface curve and contour plot in Fig. 5 were given according the experimental results in Table 2 based on CCD-RSM. It showed that the susceptible conditions for aflatoxins production in A. rhizoma was identified as temperature 22–37 °C and humidity over 87.5%.
Exploration of suitable storage conditions for A. rhizoma
In this study, the characteristics, contents of moisture, chemical fingerprints changes and aflatoxins accumulation of A. rhizoma samples inoculation with Aspergillus flavus by artificial infection before and after different temperature and humidity condition were compared. The more serious the mildew of the sample was, the higher the content of aflatoxin in the sample was, and the more complex the change of chemical composition in the sample was. Aflatoxins accumulation seriously affected the chemical components in A. rhizoma, as well as its internal quality. Appropriate environmental conditions, especially environmental temperature and humidity, contributed to the growth of Aspergillus flavus, resulting in the changes in the content and kind of the bioactive components. It was preliminarily determined that the storage environment conditions suitable for the mildew prevention of A. rhizoma was set at lower than 22 °C and humidity less than 87.5%.
Discussion
Aflatoxins are secondary metabolites produced by a wide variety of fungal species and constitute a significant hazard to the TCMs and food chains (Yang et al. 2017; Medina et al. 2017). Zhou et al. (2014) have illustrated that fungal contamination and mycotoxins residue will lead to the content reduction of the active components in TCMs, which might influence the inherent quality and safety of this TCM. Aflatoxins are unavoidable widespread natural contaminants of foods, feeds and TCMs with serious impacts on health, agricultural and livestock productivity, food and medicine safety (Noreddine 2020). The level of development of the country and the availability and degree of enforcement of pertaining regulations also account for the extent of TCMs and foods contamination with aflatoxins (Sirma et al. 2018). The poor level of development of the country exacerbates the risk and the extent of foods contamination due to faulty storage conditions that are usually suitable for mold growth and mycotoxin production: temperature of 22 to 29 °C and water activity of 0.90 to 0.99 (Noreddine 2020).
In this study, based on the trans-culture mode, CCD-RSM technique was used to establish a model to investigate the influence of environmental temperature and relative humidity on the growth of Aspergillus flavus and production of aflatoxins in A. rhizoma through the determination of the characteristics, contents of moisture, chemical fingerprints changes and aflatoxins accumulation of A. rhizoma samples before and after storage under different conditions. First, the color of A. rhizoma after storage had changed, and fungi can be clearly seen on samples. Second, the temperature and humidity had a certain effect on moisture content, and the value was higher than that of Chinese Pharmacopoeia (2020th Edition). Third, temperature had a greater effect on the type and quantity of chemical components than humidity. The contents of atractylone, atractylenolide I, II, and III changed most obviously. Also, a new component that aristolene epoxide identified by GC-MS appeared after a 10-day inoculation with Aspergillus flavus. But the reasons of this component need to be further studied. Lastly, aflatoxins were detected in the samples stored at 30 °C and 95% humidity, as well as 37.07 °C and 92.80% humidity. The results were consistent with the mildew on the surface of samples and the contents of atractylone, atractylenolide I, II, and III. It was found that the mildew of A. rhizoma was the most serious when it was stored in the temperature range of 22–37 °C and the humidity of 87.5%. This study provided a scientific basis for the rational storage and scientific maintenance of mildew prevention of A. rhizoma samples, ensuing the quality and safety of TCMs. Meanwhile, the methods should be applicable to TCMs that are susceptible to mycotoxigenic fungi contamination.
In view of the above-mentioned study, some suggestions were put forward for the safe storage of TCMs. First, the TCMs and relevant products should be stored in a ventilated, dry and cool place. The indoor temperature should be lower than 20 oC, the relative humidity should be set at 45–75% to strictly control the water content of TCMs. It is forbidden to store TCMs at unqualified moisture. Secondly, the warehouse is equipped with cooling and dehumidification facilities, and electronic monitoring system to realize intelligent monitoring. Thirdly, the storage time of TCMs should be not too long. Fourthly, strengthening technical training for administrators to establish and improve a sound storage system for ensuring standardization, scientific and normalization of the storage of TCMs.
Availability of data and materials
The data generated or analyzed during this study are included in this published article and its Additional file.
Abbreviations
- CCD-RSM:
-
Central composite design-response surface methodology
- TCMs:
-
Traditional Chinese medicines
- AFs:
-
Aflatoxins
- FAO:
-
The Food and Agriculture Organization
- WHO:
-
The World Health Organization
- UFLC-MS/MS:
-
Ultra-fast liquid chromatography/tandem mass spectrometry
- GC-FID:
-
Gas chromatography with flame ionization detection
- MgSO4 :
-
Magnesium sulfate anhydrous
- SCDA:
-
Czapek Dox Agar
- ESI:
-
Electrospray ionization
- IS:
-
Ion spray
- CUR:
-
Curtain gas
- CAD:
-
Collision activation dissociation gas
- EP:
-
Entrance potential
- DP:
-
Declustering potential
- IS:
-
Internal standard
- RPA:
-
Relative peak area
- QuEChERS:
-
Quick, Easy, Cheap, Effective, Rugged and Safe
- LOQs:
-
Limits of quantification
References
Ali N (2019) Aflatoxins in rice: Worldwide occurrence and public health perspectives. Toxicol Rep 6:1188–1197
Ashiq S, Hussain M, Ahmad B (2014) Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet Bio 66:1–10
Chinese Pharmacopoeia Commission (2020) Pharmacopeia of the People’s Republic of China, vol I. China Medical Science Press, Beijing
Christensen S, Borrego E, Shim W, Isakeit T, Kolomiets M (2012) Quantification of fungal colonization, sporogenesis, and production of mycotoxins using kernel bioassays. J Vis Exp 62:3727
Duangjit S, Chairat W, Opanasopit P, Rojanarata T, Ngawhirunpat T (2015) Application of Design Expert for the investigation of capsaicin-loaded microemulsions for transdermal delivery. Pharm Dev Technol 21:698–705
Duarte SC, Salvador N, Machado F, Costa E, Almeida A, Silva LJG, Pereira AMPT, Lino C, Pena A (2020) Mycotoxins in teas and medicinal plants destined to prepare infusions in Portugal. Food Control 115:107290
Haque MA, Wang YH, Shen ZQ, Li XH, Saleemi MK, He C (2020) Mycotoxin contamination and control strategy in human, domestic animal and poultry: A review. Microb Pathogenesis 142:104095
Hu YC, Kong WJ, Liu QT, Liu HM, Zhao G, Yang MH (2015) Study on the influence of Aspergillus flavus contamination on the quality of Radix Astragali and its storage condition optimization. Modern Chin Med 17:1133–1138
Huang PX, Liu QT, Wang JB, Ma ZJ, Lu JH, Kong WJ (2019) Development of an economic ultrafast liquid chromatography with tandem mass spectrometry method for trace analysis of multiclass mycotoxins in Polygonum multiflorum. J Sep Sci 42:491–500
International Agency for Research on Cancer [IARC] (1999) Overall evaluations of carcinogenicity to humans. IARC, New York
Jin HZ, Lee JH, Lee D, Lee HS, Hong YS, Kim YH, Lee JJ (2004) Quinolone alkaloids with inhibitory activity against unclear factor of activated T cell from the fruits of Evodia rutaecarpa. Biol Pharm Bull 27:926–928
Kitajima J, Kamoshita A, Ishikawa T, Takano A, Fukuda T, Isoda S, Ida Y (2003) Glycosides of Atractylodes lancea. Chem Pharm Bull 51:673–678
Kumar S, Ail J, Baboota S (2016) Design Expert® supported optimization and predictive analysis of selegiline nanoemulsion via the olfactory region with enhanced behavioural performance in Parkinson’s disease. Nanotechnology 27:435101
Liu QT, K WJ, Yang MH, Guo WY (2015) Review of scientific preservation techniques for traditional Chinese medicine becoming mouldy during storage. China J Chin Mater Med 4:1223–1229
Liu QT, Kong DD, Luo JY, Kong WJ, Yang MH (2016) Quantitative and fingerprinting analysis of Atractylodes rhizome based on gas chromatography with flame ionization detection combined with chemomrtrics. J Sep Sci 39:2517–2526
Liu QT, Zhang SS, Yang XH, Wang RL, Guo WJ, Yang MH (2016) Differentiation of essential oils in Atractylodes lancea and Atractylodes koreana by gas chromatography with mass spectrometry. J Sep Sci 39:4773–4780
Liu QT, Xiao CB, Liu HM, Hu YC, Guo WY, Kong WJ (2019) Sensitive assessment of multi-class mycotoxins residue in Atractylodis rhizoma. Ind Crop Prod 127:1–10
Martins C, Vidal A, De Boevre M, De Saeger S, Nunes C, Torres D, Goios A, Lopes C, Alvito P, Assunção R (2020) Burden of disease associated with dietary exposure to carcinogenic aflatoxins in Portugal using human biomonitoring approach. Food Res Int 134:109210
Medina A, González JM, Sainz MJ (2017) Impact of global warming on mycotoxins. Curr Opin Food Sci 18:76–81
Nieto CHD, Granero AM, Zon MA (2018) Héctor Fernández. Sterigmatocystin: A mycotoxin to be seriously considered. Food Chem Toxicol 118:460–470
Noreddine B (2020) Aflatoxins: Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries. Int J Environ Res Public Health 17:1215
Norioko T, Hayashi Y, Onuki Y, Andou H, Tsunashima D, Yamashita K, Takayama K (2013) A novel approach to establishing the design space for the oral formulation manufacturing process. Chem Pharm Bull 61:39–49
Obata Y, Ashitaka Y, Kikuchi S, Isowa K, Takayama K (2019) A statistical approach to the development of a transdermal delivery system for ondansetron. Int J Pharm 399:87–93
Ponzilacqua B, Rottinghaus GE, Landers BR, Oliveira CAF (2019) Effects of medicinal herb and Brazilian traditional plant extracts on in vitro mycotoxin decontamination. Food Control 100:24–27
Sirma AJ, Lindahl JF, Makita K, Senerwa D, Mtimet N, Kang’ethe EK, Grace D (2018) The impacts of aflatoxin standards on health and nutrition in sub-Saharan Africa: The case of Kenya. Glob Food Secur 18:57–61
Wang LL, Kong WJ, Yang MH, Han JP, Chen SL (2015) Safety issues and new rapid detection methods in traditionsl Chinese medicinal materials. Acta Pharm Sin B 5:38–46
Weaver AC, Adams N, Yiannikouris A (2020) Use of technology to assess and monitor multimycotoxin and emerging mycotoxin challenges in feedstuffs. Appl Anim Sci 36:19–25
World Health Organization (2007-2015) WHO Estimates of the Global Burden of Foodborne Diseases.Foodborne Disease Burden Epidemiology Reference Group
Yang Y, Li GL, Wu D, Liu JH, Li XT, Luo PJ, Hu N, Wang HL, Wu YN (2020) Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trend Food Sci Tech 96:233–252
Yang ZX, Wang HW, Ying GY, Yang MH, Nian YJ, Liu JJ, Kong WJ (2017) Relationship of mycotoxins accumulation and bioactive components variation in ginger after fungal inoculation. Front Pharmacol 8:1–9
Zhang L, Dou XW, Zhang C, Logrieco A, Yang MH (2018) A review of current methods for analysis of mycotoxins in herbal medicines. Toxins 10:65
Zhang WJ, Zhao ZY, Chang LK, Cao Y, Wang S, Kang CZ, Wang HY, Zhou L, Huang LQ, Guo LP (2021) Atractylodis Rhizoma: A review of its traditional uses, phytochemistry, pharmacology, toxicology and quality control. J Ethnopharmacol 266:113415
Zhou SJ, Kong WJ, Cao JL, Logrieco AF, Yang SH, Yang MH (2014) Effect of Aspergillus flavus contamination on the inherent quality of Glycyrrhiza uralensis. World Mycotoxin J 7:83–89
Acknowledgements
Not applicable.
Funding
This research was supported by the Beijing Natural Science Foundation (7192130), and National Natural Science Foundation of China (81973474 and 81673593).
Author information
Authors and Affiliations
Contributions
QTL and WJK conceived the project, QTL and LLJ carried out the experiment and performed literature analysis, QTL wrote the manuscript, LHX and WJK contributed the literature retrieval. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Fig. S1 Three-point inoculation and sporangia under optical microscope (Eyepiece 10×, Objective 400×) of Aspergillus flavus. Fig. S2 Response surface model of contents of atractylone, atractylenolide I, II and III.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, Q., Jiang, L., Xiao, L. et al. Physico-chemical characteristics and aflatoxins production of Atractylodis Rhizoma to different storage temperatures and humidities. AMB Expr 11, 155 (2021). https://doi.org/10.1186/s13568-021-01316-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-021-01316-3