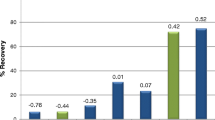
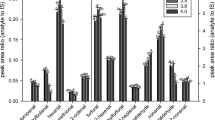
Abstract
γ-Nonalactone is a linear aliphatic lactone ubiquitous in wine, associated with coconut, sweet, and stone fruit aroma descriptors. Little research has been conducted looking at the importance of this compound to New Zealand (NZ) wine aroma. 2H213C2-γ-Nonalactone, a novel isotopologue of γ-nonalactone, was synthesised in this work for use in a stable isotope dilution assay (SIDA) for quantification of γ-nonalactone in NZ Pinot noir wines for the first time. Synthesis was carried out using heptaldehyde as the starting material, and 13C atoms and 2H atoms were introduced via Wittig olefination and deuterogenation steps, respectively. The suitability of this compound as an internal standard was demonstrated by spiking model wine at normal and elevated conditions during sample preparation, with subsequent analysis via mass spectrometry showing stability of 2H213C2-γ-nonalactone. A model wine calibration, with concentrations of γ-nonalactone from 0 to 100 µg L−1, was shown to have excellent linearity (R2 > 0.99), reproducibility (0.72%), and repeatability (0.38%). Twelve NZ Pinot noir wines, representative of a range of NZ Pinot noir–producing regions, prices, and vintages, were analysed by solid-phase extraction–gas chromatography–mass spectrometry (SPE-GC–MS). The concentrations of γ-nonalactone ranged from 8.3 to 22.5 µg L−1, the latter of which was close to the odour detection threshold of this compound. These findings provide a basis for further research into γ-nonalactone and its impact on NZ Pinot noir aroma and provide a robust method for the quantification of this compound in Pinot noir.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aroma profile of grape wines is largely determined by the impact of volatile aroma compounds, formed by the grape, during fermentation and processing, or through maturation and ageing. These aroma compounds contain a wide variety of functional groups and include acids, alcohols, esters, lactones, and thiols to name a few [1]. Lactones are common in wine and generally consist of a cyclic ester, formed by intramolecular cyclisation of a corresponding alcohol and carboxylic acid. Saturated linear aliphatic γ-lactones have historically been overlooked as wine aroma compounds [2]. Coconut, stone fruit, and sweet are among the descriptors attributed to these compounds in wine, and particularly high concentrations have been found in red wines and sweet white wines, such as noble rot wines with the influence of Botrytis cinerea [2-5]. γ-Nonalactone (Fig. 1) is arguably the most ubiquitous and significant of these lactones identified in wine, with an estimated odour detection threshold (ODT) of 30 µg L−1 for the racemic mixture [6]. First identified in wine in 1974 [7], γ-nonalactone has subsequently been found in a range of different wines, with concentrations as high as 971 µg L−1 in a sample of Verdejo wine [8]. γ-Nonalactone has also been quantified well above its ODT in wines made using grape dehydration techniques, including icewines and fortified wines (reported maxima of 179 and 539 µg L−1, respectively) [8, 9]. γ-Nonalactone has been shown to have synergistic interactions with other aroma compounds, including similar saturated linear aliphatic lactones γ-octalactone, γ-decalactone, γ-undecalactone, and γ-dodecalactone, thus potentially contributing to wine aroma even when present below its ODT [10].
Sensory analysis suggested that (R)- and (S)-enantiomers of γ-nonalactone have similar aroma descriptors, with distinct coconut notes [11]. The (S)-enantiomer had a significantly lower ODT in red wine (91 µg L−1) compared to the (R)-enantiomer (284 µg L−1) and is therefore thought to be a more powerful odorant. Cooke et al. [11] analysed a range of wines using chiral gas chromatography–mass spectrometry (GC–MS). These wines had a higher proportion of the (R)-enantiomer, with botrytised white wines showing the greatest proportion (75% (R)-enantiomer), on average [11]. Similar proportions were reported in Bordeaux Merlot and Cabernet Sauvignon wines (ratio of 65:35 (R):(S), on average) [12]. Sensory analysis (gas chromatography–olfactometry) found that γ-nonalactone is strongly associated with prune aroma in aged red wines, and is hypothesised to be a contributor to undesirable prune aroma in prematurely aged red wines, along with another aroma compound, 3-methyl-2,4-nonanedione [4].
The route(s) of biogenesis of γ-nonalactone in grapes and/or wine have only been partially elucidated [2]. Through 18O-labelling experiments in beer, linoleic acid and its 9- and 13-lipoxygenation products 9-hydroxydecadienoic acid (9-HODE) and 13-hydroxydecadienoic acid (13-HODE) were found to be putative precursors to γ-nonalactone during fermentation (Scheme 1) [13]. In another work, grape musts were spiked with 2H-labelled 4-oxononanoic acid prior to fermentation, and analysis of the resulting wine showed that 2H-labelled γ-nonalactone had been produced, confirming 4-oxononanoic acid as a precursor. This result suggests that the ketone functional group of 4-oxononanoic acid undergoes reduction to an alcohol. The resulting 4-hydroxynonanoic acid can then undergo lactone ring-closure, which is thermodynamically favourable under acidic conditions, as found in wine [12]. Despite these key findings, it is not clear how 4-oxononanoic acid is formed, nor how lipoxygenation may be occurring (Scheme 1). Consequently, further work is needed to clarify the formation pathway(s) of γ-nonalactone.
Due to the complex nature of the chiral analysis, most analyses tend to treat these lactones as a racemic mixture [2, 3, 13]. The complexity of the wine matrix means that sophisticated techniques are required for the accurate and precise quantification of specific aroma compounds, particularly those found at low concentrations, including γ-nonalactone. Extraction of γ-nonalactone, along with other low-polarity aroma compounds from the wine matrix, has most commonly been carried out via solid-phase extraction (SPE) [3, 14], headspace solid-phase microextraction (HS-SPME) [11, 15, 16], liquid–liquid extraction (LLE) [9], stir-bar sorptive extraction (SBSE) [17], or variations of these methods [2]. SPE generally involves passing the sample through a cartridge containing solid material, to which the analytes (in this case low-polarity aroma compounds) adsorb. Washing steps remove undesirable interferents in the matrix, while analytes are retained in the solid phase. Finally, a solvent in which the analytes are highly soluble is passed through the cartridge, eluting the analytes. Due to the volatility of γ-nonalactone, GC–MS is the most common means of separation and quantification of this compound [2].
Several internal standards have been used for semi-quantitative or quantitative analysis of γ-nonalactone in wines, including 2-octanol [13, 16], 4-methyl-2-pentanol [9], and 3,3-dimethylphenol [17]. However, for quantitative analysis, an isotopologue of the analyte of interest is the most ideal internal standard for a stable isotope dilution assay (SIDA). SIDA was used for the quantification of saturated linear aliphatic lactones in a range of Australian wines, including γ-octalactone, γ-nonalactone, γ-decalactone, and γ-dodecalactone. 2H7-analogues of these compounds were synthesised, which were used as internal standards during extraction and quantification, using SPE and subsequent analysis through GC–MS [3]. A similar deuterium exchange step was used for the synthesis of 2H7-γ-decalactone, which was subsequently used as an internal standard for the quantification of several linear aliphatic lactones (γ- and δ-nona- to dodecalactones), instead using HS-SPME for sample extraction [18].
In this investigation, when using the aforementioned 2H-labelling route, incomplete deuterium exchange was observed. This observation led to the synthesis of a novel 2H213C2-labelled γ-nonalactone standard in this work, using Wittig olefination and deuterogenation steps for the introduction of 13C and 2H atoms into the molecule, respectively. This novel standard was subsequently used for the robust analysis of 12 commercial New Zealand (NZ) Pinot noir wines via SIDA, using an established SPE-GC–MS method for the accurate and sensitive quantification of γ-nonalactone. Since γ-nonalactone has been measured in concentrations well above its ODT (up to 155 µg L−1) in Pinot noir wines from Oregon, USA [17], this work also aims to determine whether γ-nonalactone may have an important role in the aroma profile of NZ Pinot noir wines for the first time.
Materials and methods
Chemicals and reference compounds
γ-Nonalactone (> 97%), isopropyl bromide, oxalyl chloride (> 98%), diisobutylaluminium hydride (1.0 M in toluene), deuterium chloride (35% in 2H2O), sodium borohydride (98%), heptaldehyde (95%), selenium dioxide, deuterium gas, and hydrobromic acid (48%, aq.) were purchased from Sigma-Aldrich (MO, USA). Benzoic acid (AR), triphenylphosphine (97%), and Pd on activated carbon (10%) were purchased from AK Scientific (CA, USA). 13C2-bromoacetic acid, deuterium oxide, and deuterochloroform were purchased from Cambridge Isotope Laboratories (MA, USA). Magnesium sulfate, sodium hydroxide, potassium hydroxide, tartaric acid, and all organic solvents were purchased from ECP Ltd (Auckland, New Zealand). A Sartorius (Göttingen, Germany) Arium Pro Ultrapure Water System was used for Type 1 water production. Chemicals were used as purchased, unless otherwise specified.
General synthetic details
Thin-layer chromatography (TLC) was performed using Merck silica gel 60 F254 aluminium sheets. Short-wave ultraviolet (UV) fluorescence and vanillin and potassium permanganate (KMnO4) stains were used for TLC spot visualisation. Flash chromatography was performed using Chem-Supply silica gel 60 (particle size 0.04–0.06 mm).
Proton (1H) and carbon (13C) NMR were recorded using a Bruker AVIII400 spectrometer (MA, USA), at frequencies of 400 and 100 MHz, respectively. Deuterochloroform (C2HCl3) or deuterium oxide (2H2O) were used as sample solvents and internal references. 1H NMR data were reported as follows: chemical shift (δ, ppm), relative integral, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, qn = quintet, dd = doublet of doublets, dq = doublet of quartets, ddquin = doublet of doublet of quintets, qd = quartet of doublets, ddq = doublet of doublet of quartets, sept = septet). High-resolution mass spectra (HRMS) were recorded using a Bruker MicrOToF-QII mass spectrometer, coupled to an electrospray ionisation source (ESI), and analysis performed in positive ionisation mode. Infrared (IR) spectra were recorded using a PerkinElmer (MA, USA) FT-IR Spectrum Two Spectrophotometer, equipped with a UATR Two attachment.
General analytical details
Bond Elut-ENV 200 mg and 3 mL SPE cartridges were purchased from Agilent Technologies (CA, USA). Analyses of wine samples were conducted using an Agilent Technologies 6890N Network GC coupled to a Single Quadrupole Agilent Technologies 5973 inert mass selective detector. SCAN mode was used for all samples. The GC column used was Agilent Technologies HP-INNOWax (polyethylene glycol stationary phase, 60 m length, 0.25 mm inner diameter, 0.25 µm film thickness, and temperature limits 40–260 °C). Samples (1 µL) were injected with an Agilent Technologies 7683B Series Splitless Injector. The carrier gas used was He, with a flow rate of 1 mL min−1. A temperature gradient was utilised for all samples; beginning at 50 °C, the temperature was increased to 60°C at a rate of 1°C min−1, then to 250°C at a rate of 10°C min−1, after which it was held at 25°C for 25 min, for a total run time of 54 min.
Alcohol levels (v/v) in commercial wine samples were analysed using an Alcolyzer Wine M from Anton Paar (Graz, Austria). pH and titratable acidity (TA) of commercial wine samples were determined using a Hanna Instruments (RI, USA) Wine Titrator, equipped with a Hanna Instruments pH meter. Residual sugar (RS) and free and total sulfite levels were determined in commercial wine samples using the Megazyme (Bray, Ireland) D-Fructose/D-Glucose Assay Kit and Total and Free Sulfite Assay Kit, respectively.
Wine samples and model wine
Dry red cask wine for calibration curve construction and 12 commercial NZ Pinot noir wines were purchased from retailers in Auckland, NZ. Cask wine was non-vintage with unspecified grape varieties. Pinot noir wines were selected from three key NZ Pinot noir–producing regions (Central Otago, Marlborough, and Waipara Valley), with a range of recent vintages (2018, 2019, 2020, and 2021) and a wide price range (NZ$18.99–NZ$99.99), to provide a representative set of NZ Pinot noir wines. Measured technical data for these wines are given in Table S1. Model wine was comprised of tartaric acid (5 g) dissolved in a solution of 12% (v/v) ethanol in type 1 water (1 L), with the pH adjusted to 3.2 using NaOH (aq., 3 M then 1 M).
2H6-γ-Nonalactone synthesis
Synthesis of a deuterated (2H6) internal standard was initially attempted, based on the synthesis of 2H7-γ-nonalactone by Cooke et al. [3]. The scale was reduced from that used in literature, and in the final step, NaBH4 was used as a reducing agent instead of NaB2H4, resulting in a 2H6-labelled γ-nonalactone analogue rather than a 2H7-labelled analogue (Scheme 2 and see SI for further details of this investigation). Despite hydrogen–deuterium exchange being carried out under reported conditions (reflux in 2HCl/2H2O), and extraction, then replacement of the 2HCl/2H2O 1H-2H exchange mixture after 7 days, and reaction for a further 7 days, complete 1H-2H exchange was not observed and thus the deuterated internal standard was not deemed appropriate for use for accurate quantitative analysis.
1H-2H exchange of α-protons of isopropyl 4-oxononanoate 4 during the synthesis of 2H6-γ-nonalactone 6. In this work, NaBH4 was used instead of NaB2H4 for the reduction of the ketone group [3]
2H2 13C2-γ-Nonalactone synthesis
13C2-Ethyl bromoacetate 8: to a solution of 13C2-bromoacetic acid 7 (0.33 g, 2.34 mmol) in dry ethanol (1.5 mL), under an atmosphere of nitrogen, H2SO4 (conc., 2 drops) was added, and the resulting mixture was heated at 60 °C for 6 h, with stirring. The mixture was then cooled to room temperature, and water (10 mL) was added. The mixture was extracted with CH2Cl2 (3 × 10 mL portions); then, the combined organic extracts were dried (MgSO4) and concentrated in vacuo to give 13C2-ethyl bromoacetate 8 as a colourless oil (0.396 g, quantitative), which was used in the next reaction without further purification.
-
1H NMR (400 MHz, C2HCl3) 1.30 (t, 3H, H-2′, J = 7.1 Hz), 3.82 (dd, 2H, H-2, J = 4.6, 153.2 Hz), 4.24 (dq, 2H, H-1′, J = 3.1, 7.1 Hz). 1H NMR was in agreement with literature values [19, 20].
13C2-(Ethoxycarbonylmethyl)triphenylphosphonium bromide 9: a solution of 13C2-ethyl bromoacetate 8 (0.390 g, 2.31 mmol) in ethyl acetate (2 mL) was slowly added to a solution of triphenylphosphine (0.700 g, 2.67 mmol) in ethyl acetate (2 mL) and the mixture stirred overnight at room temperature. The resulting white precipitate was filtered, washed with diethyl ether (3 × 2 mL portions), and dried in vacuo to give 13C2-(ethoxycarbonylmethyl)triphenylphosphonium bromide 9 (0.909 g, 91%) as a white crystalline powder, which was used in the next reaction without further purification.
-
1H NMR (400 MHz, C2HCl3) 1.08 (t, 3H, H-2′, J = 7.1 Hz), 4.05 (dq, 2H, H-1′, J = 3.1, 7.1 Hz), 5.39–5.86 (m, 2H, H-2), 7.64–7.72 (m, 6H, H-Ar), 7.75–7.82 (m, 3H, H-Ar), 7.87–7.96 (m, 6H, H-Ar). 1H NMR was in agreement with literature values [21].
13C2-(Carbethoxymethylene)triphenylphosphorane 10: to a solution of 13C2-(ethoxycarbonylmethyl)triphenylphosphonium bromide 9 (0.900 g, 2.09 mmol) in CH2Cl2 (5 mL) NaOH (aq., 1.0 M, 5 mL) was added and the mixture stirred vigorously for 15 min. The organic layer was collected, and the aqueous layer was extracted with CH2Cl2 (3 × 5 mL portions). The combined organic extracts were dried (MgSO4) and concentrated in vacuo to give 13C2-(carbethoxymethylene)triphenylphosphorane 10 (0.722 g, 99%) as a white powder which was used in the next reaction without further purification.
-
1H NMR (400 MHz, C2HCl3) 1.04 (m, H-2′), 3.96 (m, 2H, H-1′), 7.41–7.49 (m, 6H, H-Ar), 7.51–7.58 (m, 3H, H-Ar), 7.61–7.70 (m, 6H, H-Ar). 1H NMR was in agreement with literature values [21].
13C2-Ethyl non-2-enoate 12: to a solution of heptaldehyde 11 (0.312 g, 0.422 mL, 2.73 mmol) in CH2Cl2 (5 mL) was added a solution of 13C2-(carbethoxymethylene)triphenylphosphorane 10 (1.00 g, 2.85 mmol) in CH2Cl2 (5 mL), and the mixture was stirred under an atmosphere of nitrogen, at room temperature, overnight. The solvent was removed in vacuo and pentane (10 mL) was added. The resulting suspension was stirred for 1 h, then filtered through a short pad of Celite. The solvent was removed in vacuo to give the crude product, which was purified by flash chromatography (98:2 pentane/Et2O) to give 13C2-ethyl non-2-enoate 12 (0.447 g, 88%) as a colourless oil.
-
Rf (98:2 pentane/Et2O) 0.32
-
1H NMR (400 MHz, C2HCl3) 0.88 (t, 3H, H-9, J = 7.0 Hz), 1.29 (t, 3H, H-2′, J = 7.1 Hz), 1.31–1.37 (m, 2H, H-5), 1.40–1.50 (m, 6H, H-6, H-7, H-8), 2.14–2.24 (m, 2H, H-4), 4.18 (dq, 2H, H-1′, J = 3.0, 4.1 Hz), 5.81 (ddquin, 1H, H-2, J = 1.6, 15.6, 161.6 Hz), 6.91–7.01 (m, 1H, H-3))
-
13C NMR (100 MHz, C2HCl3) 14.1 (C-9), 14.3 (C-2′), 22.6 (C-4, C-5, C-6, or C-7), 22.7(C-4, C-5, C-6, or C-7), 29.0 (C-4, C-5, C-6, or C-7), 29.1 (C-4, C-5, C-6, or C-7), 31.7 (C-4, C-5, C-6, or C-7), 59.8 (C-1′), 119.6 (d, C-2), 150.6 (C-3), 166.6 (d, C-1)
-
IR: υmax (film)/cm−1 2958, 2928, 2858, 1677, 1626, 1231, 1153, 975
-
m/z (ESI+) 209 (MNa+, 40%), 187 (MH.+, 100), 159 (99), 149 (4), 141 (6)
-
HRMS Found (Mna+): 209.1412, expect 209.1409 for 13C2C9H20O2Na+
13C2-Ethyl 4-hydroxy-non-2-enoate 13: to a solution of 13C2-ethyl non-2-enoate 12 (0.43 g, 2.31 mmol) in a mixture of dioxane (3 mL) and water (0.33 mL) selenium dioxide (0.52 g, 4.69 mmol) was added, and the resulting mixture was heated at reflux overnight, under an atmosphere of nitrogen. The mixture was then cooled to room temperature, and water (3 mL) was added. The resulting solid was filtered, then the filtrate was extracted with CH2Cl2 (3 × 10 mL portions). The combined organic layers were dried (MgSO4) and concentrated in vacuo to give the crude product, which was subsequently purified by flash chromatography (85:15 pentane/EtOAc) to give 13C2-ethyl 4-hydroxy-non-2-enoate 13 (0.170 g, 36%) as a pale-yellow oil.
-
Rf (85:5 pentane/EtOAc) 0.39
-
1H NMR (400 MHz, C2HCl3) 0.88 (t, 3H, H-9, J = 6.7 Hz), 1.25–1.67 (m, 11H, H-2′, H-5, H-6, H-7, and H-8), 4.20 (qd, 2H, H-1′, J = 3.1, 7.2 Hz), 4.27–4.35 (m, 1H, H-4), 6.02 (ddq, 1H, H-2, JH-C = 163.4 Hz, J = 1.6, 15.7 Hz), 6.90–6.99 (m, 1H, H-3)
-
13C NMR (100 MHz, C2HCl3) 14.1 (C-9), 14.2 (C-2′) 21.1 (C-5, C-6, C-7, or C-8), 22.8 (C-5, C-6, C-7, or C-8), 26.0 (C-5, C-6, C-7, or C-8), 31.6 (C-5, C-6, C-7, or C-8), 35.2 (C-5, C-6, C-7, or C-8), 60.4 (C-1′), 67.1 (C-4), 120.2 (d, C-2, JC-C = 119.8 Hz), 145.7 (C-3), 166.5 (d, C-1, JC-C = 75.2 Hz)
-
IR: υmax (film)/cm−1 3441, 2957, 2860, 1677, 1661, 1242, 1151, 1035, 979
-
m/z (ESI+) 225 (MNa+, 100%), 199 (10), 153 (10), 137 (9), 121 (8)
-
HRMS Found (MNa+) 225.1365, expect 225.1358 for 13C2C9H20O3Na+
2H213C2-Ethyl 4-hydroxynonanoate 14: to a solution of 13C2-ethyl 4-hydroxy-non-2-enoate 13 (0.160 g, 0.79 mmol) in ethanol (10 mL), palladium on activated carbon (10% loading, 20 mg) was added. This mixture was placed under an atmosphere of deuterium gas (99.7% purity) and stirred for 2 h, at room temperature. The mixture was then filtered through a short pad of Celite, which was then washed with ethanol (2 × 50 mL portions). The solvent was removed in vacuo to give 2H2-13C2-ethyl 4-hydroxynonanoate 14 (0.120 g, 74%) as a pale-yellow oil, which was used in the following reaction without further purification.
2H213C2-γ-Nonalactone (2H2-13C2-pentyldihydrofuran-2(3H)-one) 15: to a solution of 2H213C2-ethyl 4-hydroxynonanoate 14 (0.120 g, 0.58 mmol) in a mixture of THF (2 mL) and water (1 mL) was added NaOH (0.13 g, 3.25 mmol). The resulting mixture was stirred for 2 h, then HCl (1.0 M) was added dropwise until the pH was 1. The mixture was then extracted with CH2Cl2 (3 × 5 mL portions). The combined organic extracts were dried (Na2SO4) and the solvent removed in vacuo to give the crude product, which was purified by flash chromatography (85:15 pentane/EtOAc) to give 2H213C2-γ-nonalactone 15 (0.035 g, 38%) as a colourless oil.
-
Rf (85:15 pentane/EtOAc) 0.45
-
1H NMR (400 MHz, C2HCl3) 0.89 (t, 3H, H-9, J = 6.9 Hz), 1.22–1.51 (m, 6H, H-6, H-7, and H-8), 1.54–1.64 (m, 2H, H-5), 1.68–1.78 (m, 1.23*, H-5), 1.79–1.89 (m, 0.72H*, H-2, and/or H-3) 2.26–2.38 (m, 1.28H*, H-2 and/or H-3), 2.62–2.72 (m, 0.75H*, H-2, and/or H-3) 4.44–4.52 (m, 1H, H-4)
-
13C NMR (100 MHz, C2HCl3) 14.0 (C-9), 22.5 (C-5, C-6, C-7, or C-8), 24.9 (C-5, C-6, C-7, or C-8), 27.9–29.3 (m, C-2, and C-3), 31.6 (C-5, C-6, C-7, or C-8), 35.6 (C-5, C-6, C-7, or C-8), 81.1 (C-4), 177.3 (d, C-1, JC-C = 49.0)
-
IR: υmax (film)/cm−1 2955, 2931, 2860, 1724, 1460, 1157, 1114, 947
-
m/z (ESI+) 199 (5%), 183 (MNa+, 100), 143 (5)
-
HRMS Found (MNa+) 183.1096, expect 183.1242 for 13C2C72H2H14O2Na+
-
*Non-integer integrals (approximations) are provided where deuterium labelling has been used.
Sample preparation: solid-phase extraction
SPE cartridges (Bond Elut-ENV 200 mg, 3 mL) were conditioned with methanol (2 mL), then water (4 mL). Wine samples (50 mL) spiked with internal standard 15 (100 µL of 10,000 µg L−1 in ethanol) were then passed through the SPE cartridges, which were subsequently washed with water (5 mL), then a solution of methanol (40% v/v) and sodium bicarbonate in type 1 water (1% w/v, 20 mL). The cartridges were allowed to dry for 30 min, by passing air through them. Finally, analytes were eluted using CH2Cl2 (2.5 mL, giving a sample enrichment factor of 20), which was collected and dried with Na2SO4, before being filtered and concentrated at 30 °C under a stream of nitrogen, to an approximate final volume of 100 µL, before analysis by GC–MS [15].
Verification of suitable SIDA internal standard
The stability of 2H213C2-γ-nonalactone 15 during spiking and extraction was verified by comparing more standard spiking conditions to more elevated conditions (higher temperatures and/or longer time periods) and determining whether any changes in relative abundances of isotopologues were observed. Model wine samples (50 mL) were spiked with internal standard (100 µL of 10,000 µg L−1 in ethanol). After spiking, the samples were subjected to the following conditions, in triplicate: 30 min at room temperature (standard conditions), and 30 min at 40 °C, 3 h at room temperature or 3 h at 40 °C (extreme conditions). Subsequently, extraction and analysis by SPE-GC–MS were conducted as stated above. Additionally, samples of 2H213C2-γ-nonalactone dissolved in CH2Cl2 were analysed by GC–MS, for comparison. Samples were compared to 2H213C2-γ-nonalactone 15 dissolved in CH2Cl2, by observing the relative peak areas of isotopically labelled γ-nonalactone 15 (base peak m/z 89), and the peaks which represented the loss of one or two mass units (m/z 88 and 87), which would correspond to the exchange of one or two deuterium atoms with hydrogen atoms, respectively.
Calibration curves and method validation
Calibration curves were constructed in two different matrices: dry red cask wine and model wine. For each matrix, 50 mL samples were spiked with internal standard (100 µL of 10,000 µg L−1 2H213C2-γ-nonalactone in ethanol) to give a concentration of 20 µg L−1, and a range of volumes of unlabelled γ-nonalactone (10,000 µg L−1 in ethanol) to give concentrations of 0, 0.5, 1, 2, 5, 10, 25, 50, and 100 µg L−1, each in triplicate, while 5 µg L−1 and 25 µg L−1 were each repeated seven times for initial assessment of the repeatability of this method. This data was included in the final calibration curves for completeness, but further repeatability and reproducibility assessments were carried out, as described below. Calibration samples were extracted and analysed as described above, using SPE-GC–MS. The order of analysis was randomised.γ-Nonalactone 1 and 2H213C2-γ-nonalactone 15 had retention times of 27.96 and 27.94 min, respectively (Fig. S1). Relative responses of γ-nonalactone 1 and 2H213C2-γ-nonalactone 15 were measured using areas of their respective base peaks; m/z 85 and 89. The hypothesised fragmentation pathway from their respective parent ions is shown in Scheme S1. Qualifier ions used were m/z 99 and 114 for γ-nonalactone, and m/z 103 and 118 for 2H213C2-γ-nonalactone 15. Calibration curves were constructed by plotting relative responses of γ-nonalactone and 2H213C2-γ-nonalactone 15 against known concentrations of γ-nonalactone 1. In the dry red wine calibration, the relative response at 0 µg L−1 added γ-nonalactone 1 was significantly higher than expected, indicating that γ-nonalactone 1 was already present in the matrix. Standard addition analysis revealed a γ-nonalactone 1 concentration of 12 µg L−1 in the dry red wine matrix. It was therefore necessary to further verify the relationship between relative response and γ-nonalactone 1 concentration, particularly below 12 µg L−1, to avoid extrapolation when analysing unknown samples if possible. Model wine was selected for this purpose, as it was known that no γ-nonalactone 1 was already present in this matrix. The resulting calibration curve was constructed in the same manner as for the dry red wine. Calibration curve slopes were shown not to be statistically different at the 95% confidence level (see SI for details of the calibration curves, including the 95% confidence interval for the slope estimates, showing that they overlap). Thus, it could be concluded that the model wine calibration curve accurately reflected the relationship between γ-nonalactone 1 concentration and the relative response of γ-nonalactone 1 and the internal standard 15 within the dry red wine matrix and was therefore used for quantification in the Pinot noir wines.
In order to verify the repeatability and reproducibility of this analytical method, instrumental and experimental repeats were carried out, respectively, according to recommended analytical best practice [22]. Instrumental repeats were carried out using one model wine sample spiked with 5 µg L−1 γ-nonalactone 1 and 10 µg L−1 2H213C2-γ-nonalactone 15, and repeating the sample analysis seven times in as short a period of time as possible, i.e. consecutive analyses of the sample within the same day. Experimental repeats were conducted with the same concentrations of analyte and internal standard in model wine, with the full experimental method (sample preparation and extraction) being repeated in triplicate, on five consecutive days. Instrumental and experimental repeatability metrics for the method were obtained; 0.38 and 0.72%, respectively). Percentage recovery was calculated using the experimental repeats and was 104%.
Finally, the limit of detection (LOD) and limit of quantitation (LOQ) were calculated for the model wine calibration curve, using the following formulae (where a = estimate of the y intercept and s = standard error of the calibration curve) [23]:
Values for LOD and LOQ were 0.4 and 1.1 µg L−1, respectively.
Quantification of γ-nonalactone in 12 NZ Pinot noir samples
Samples of 12 commercial NZ Pinot noir wines (50 mL) were spiked with 2H213C2-γ-nonalactone 15 (100 µL of 10,000 µg L−1 in ethanol solution), each in triplicate. Samples were extracted and analysed according to the SPE-GC–MS method described above. The order of sample analysis was randomised. The relative responses of γ-nonalactone 1 and 2H213C2-γ-nonalactone 15 were used to calculate the concentrations of γ-nonalactone 1 present in each sample using the calibration curve and stated γ-nonalactone 1 concentrations calculated for each wine were the average of the three experimental replicates. Any outliers were detected using Grubbs’ test, and subsequently removed.
Relationships between the variables of vintage, region of production, and price with γ-nonalactone 1 concentration for each wine were investigated. To assess if there were any significant effects from any of the factors, multiple linear regression (stats package) was used [24]. R software was used for data analysis, and the ggplot2 package in R was used to visualise the data.
Results and discussion
SIDA was selected for quantification of γ-nonalactone 1 in wine samples, as isotopically labelled internal standards are expected to behave in an equivalent way as their natural analogues during sample extraction and analysis. Since a method for SIDA of γ-nonalactone 1 in wine was already established [3], synthesis of 2H6-γ-nonalactone was initially attempted, adapting the reported method. Starting with commercially available unlabelled γ-nonalactone 1, lactone ring opening, followed by isopropyl ester formation, was carried out. Next, Swern oxidation of the 4-hydroxyl group was performed, to give isopropyl 4-oxononanoate 4. Regrettably, problems were encountered during the deuterium labelling step. Isopropyl 4-oxononanoate 4 was heated at reflux with deuterium oxide (2H2O) and deuterium chloride (35% w/v in 2H2O) for 7 days, following the literature procedure (Scheme 2) [3]. The reaction mixture was then extracted with diethyl ether, and the product, 4-oxononanoic acid 5, was obtained and characterised via 1H NMR and HRMS. Mixtures of deuterium-labelled isotopologues of this compound were observed, evidenced by peaks remaining in the 1H NMR spectrum corresponding to protons which should have been completely replaced by deuterium atoms. This result was corroborated by the HRMS spectrum, with mixtures of m/z base peaks observed, indicating predominance of 2H4- and 2H5-4-oxononanoic acid isotopologues, rather than the intended 2H6-4-oxononanoic acid 5.
Consequently, heating of this compound at reflux in 2HCl/2H2O was continued for a further 7 days, before extraction and characterisation were repeated. A mixture of isotopologues was again observed via 1H NMR and HRMS (Figs. S2 and S3). No mention of this phenomenon was given by the authors; however, a similar deuterium exchange step, using isopropyl 4-oxodecanoate, was carried out in another work, for the synthesis of a deuterated γ-decalactone analogue. The final deuterated γ-decalactone analogue was reported to have reduced deuterium incorporation, and purity of 2H7-γ-decalactone was 70%, relative to a pure sample of unlabelled γ-decalactone [18]. Despite reportedly successful quantification of desired lactones in wine by Cooke et al. [3] and Langen et al. [18], this mixture of isotopologues was considered unacceptable for the purposes of SIDA in this work; thus, the synthesis of an alternative isotopically labelled internal standard was pursued for the quantification of γ-nonalactone 1.
Labelling of the lactone ring, rather than the alkyl chain, was desirable as during ionisation, the major fragmentation product from γ-nonalactone 1 results from the loss of the alkyl chain (Scheme S1). It was decided to follow a literature method for the synthesis of 13C2-γ-nonalactone [25], and then adapt it to introduce two 2H atoms, to give a difference in m/z- ratio of 4 units between the natural analogue and the isotopically labelled internal standard. Scheme 3 shows the reaction pathway employed for the synthesis of 13C2-2H2-γ-nonalactone 15. For the introduction of two 13C atoms, a single Wittig olefination step was utilised. A stabilised Wittig reagent 10 was synthesised from commercially available 13C2-bromoacetic acid 7 over three steps [19]. Subsequently, Wittig olefination was carried out with commercially available heptaldehyde 11. A hydroxyl group was then introduced at the 4-position of 12 via allylic hydroxylation, using SeO2. Deuterogenation was used to saturate the double bond in 13 using 2H2 gas. Finally, the ester group in 13 was hydrolysed to liberate the 4-hydroxyacid, which underwent thermodynamically favourable lactone ring-closure under acidic conditions to give the desired target 13C2-2H2-γ-nonalactone 15.
Synthesis of 13C2-2H2-γ-nonalactone 15 in this work based on a previously reported synthetic route [25]
As this isotopically labelled standard 15 is novel, it was necessary to ensure that it is stable during the sample extraction and analysis processes and thus generally suitable for use as an internal standard. Previous literature has reported the occurrence of hydrogen–deuterium scrambling during deuterogenation reactions (hydrogenation using deuterium gas), due to the mechanism of the reaction as it interacts with the palladium catalyst [26]. This phenomenon was observed in this work, with the product of the deuterogenation reaction (2H213C2-ethyl 4-hydroxynonanoate 13) being incompletely labelled, as demonstrated by analysis via both 1H NMR and HRMS. This was not ideal, as a small portion of the internal standard would not have the expected mass-to-charge ratio during analysis by GC–MS. However, if it could be demonstrated that the levels of the different internal standard isotopologues were consistent between samples, the fragmentation of the internal standard was consistent, and the internal standard isotopologues did not interfere with the mass-to-charge ratio of the analyte being monitored; this could still be an appropriate internal standard for SIDA. Additionally, the presence of a deuterium atom adjacent to a carbonyl group meant that deuterium exchange could be possible in acidic media, such as wine, which would decrease the mass-to-charge ratio of the internal standard; thus, it would not fulfil the requirement of being stable for SIDA. It was therefore necessary to ensure that no significant deuterium exchange was occurring.
The stability of the internal standard 15 was evaluated by replicating the spiking method in model wine at more elevated conditions (high temperature of 40 °C, long period of time in wine matrix of 3 h) compared to the more standard conditions (room temperature, 30 min in wine matrix). Each condition was trialled in triplicate. Samples were subsequently extracted following the typical SPE procedure, then analysed by GC–MS. The relative intensities corresponding to the base peak of 2H213C2-γ-nonalactone (m/z 89) and its partially labelled isotopologues 2H113C2-γ-nonalactone (m/z 88) and 13C2-γ-nonalactone (m/z 87) were calculated for each of the samples. No significant trends were observed in the relative peak areas of these isotopologues, due to neither time nor temperature (Table S2). Consequently, 2H213C2-γ-nonalactone 15 was considered sufficiently stable as an internal standard under standard spiking conditions. For quantification of the analyte in wine samples, the peak area of 2H213C2-γ-nonalactone 15 (m/z 89) only was used in calculations, as this isotopologue was shown via mass spectrometry to be the most abundant.
Initially, dry red cask wine was used as the calibration matrix, to closely mimic the matrices of the samples to be analysed. Known concentrations of γ-nonalactone 1 (ranging from 0 to 100 µg L−1) and internal standard 15 (10 µg L−1) were used to spike the red wine. The resulting calibration curve had excellent linearity (R2 = 0.997) (Fig. S4, Table S3). However, the intercept of the linear regression showed that the red wine matrix already had γ-nonalactone 1 present (12.0 µg L−1), prior to spiking. This was undesirable, as it could not be determined that the relationship between the relative response of γ-nonalactone 1 and the internal standard 15 remained linear below the concentration of γ-nonalactone 1 present in the red wine. Thus, an additional calibration curve was constructed in an identical manner, using model wine. Although this matrix does not very accurately resemble that of the samples to be analysed, it was known that no γ-nonalactone 1 was present in the matrix prior to spiking. As SIDA was being used, it was also anticipated that the matrix should not significantly impact the relative responses of γ-nonalactone 1 and the internal standard.
The resulting model wine calibration curve (Fig. 2) also had excellent linearity (R2 = 0.9985) (Table S4). It was determined that at the 95% confidence level, the gradients of the red wine and model wine calibration curves were not different; thus, the model wine calibration curve was deemed appropriate for NZ Pinot noir wine analysis. Experimental repeatability was assessed by repeating model wine spiking (5 µg L−1) and SPE-GC–MS extraction and analysis in triplicate, on five different days. Similarly, the reproducibility of the GC–MS instrument was assessed by analysing the same sample (5 µg L−1) seven consecutive times [22]. Repeatability and reproducibility were found to be 0.72% and 0.38% (Tables S5 and S6), respectively. The LOD and LOQ were 0.4 and 1.1 µg L−1, respectively (Table S4), which were also considered appropriate for analysis, as they are well below the ODT of γ-nonalactone. These values are similar to those reported in previous literature for the quantification of γ-nonalactone 1 in wine (Table 1) [3, 5, 15, 16, 18, 27-30]. The percentage recovery of γ-nonalactone 1 for this method was estimated at 104%, which is either on par with or an improvement on previous methods [15].
Twelve NZ Pinot noir samples were subsequently analysed in triplicate using the above method and concentrations of γ-nonalactone 1 in each sample were calculated. The results of these analyses (means of triplicates and their standard) are shown in Table 2. The data collected for each wine concerning their vintage, region of production, and price are provided in Table S7. All wines had concentrations of 1 well above the LOD and LOQ for this analytical method. Interestingly, however, none of the samples exceeded the reported ODT of γ-nonalactone 1 (30 µg L−1). The highest concentration was measured in PN_4, with 22.5 µg L−1. The range of concentrations reported in the current work (8.30–22.5 µg L−1) is on par with concentrations measured in other studies of Pinot noir aroma; 10–18 µg L−1 γ-nonalactone 1 was reported in Oregon Pinot noir wines made from grapes of different maturities, from two consecutive vintages [31]; 6.9–14.8 µg L−1 in Pinot noir made with grapes subjected to different levels of dehydration [32]; and < 0.1–34.8 µg L−1 in Australian Pinot noir samples [3].
Very high concentrations of γ-nonalactone exceeding 150 µg L−1 were reported in Oregon Pinot noir, five times the ODT of this compound. This study assessed the impacts of different levels of vine fruit zone leaf removal, in two consecutive vintages. γ-Nonalactone concentration was not associated with leaf removal [17]. The reason for these contrasting results is unclear, and merits further investigation, particularly considering that other studies looking at Oregon Pinot noir have reported similar concentrations to those reported in the current work [26, 27].
Although below the ODT, it has been reported that there are synergistic interactions between γ-nonalactone and other linear aliphatic lactones. Even when present below their respective ODTs, a strong synergism was shown to exist between γ-octalactone, γ-nonalactone, γ-decalactone, γ-undecalactone, and γ-dodecalactone, resulting in a significantly higher odour activity than the addition of their concentrations relative to respective ODTs would predict [10]. A similar phenomenon was discovered in reconstitution studies of Australian Viognier, where lactones including γ-nonalactone were shown to significantly increase the perception of apricot aroma, when monoterpenes were present. This suggests that some additional synergy exists between γ-nonalactone and other aroma compounds [33]. Clearly, the impact of aroma compounds in wine is more complex than simply assessing their ODT, and despite γ-nonalactone being present below its ODT in all Pinot noir samples analysed in this work, their impact on NZ Pinot noir aroma could still be significant.
The effect of vintage, region of origin, and price on the concentration of γ-nonalactone 1 for these samples was explored. There was no observable trend for price and vintage, nor difference depending on region (Fig. 3a–c). These factors were formally assessed by multiple regression analysis, with sequential removal of non-significant variables (p-value > 0.05) to find any significant associations (p-value < 0.05). No significant associations were found between any of these variables and γ-nonalactone 1 concentration, suggesting that differences in γ-nonalactone 1 concentrations are largely due to other factors. Based on the knowledge of variables that influence γ-nonalactone 1, such factors could include viticultural factors, the yeast strain used for fermentation, or the type/extent of oak influence utilised in winemaking [2, 29-31]. Additionally, as this set of data is relatively small, significant associations between price, region, vintage, and γ-nonalactone 1 concentration may be uncovered with the use of a larger dataset.
Investigation of any contribution of γ-nonalactone to undesirable prune aroma in NZ Pinot noir aroma also merits further investigation. This work did not involve any sensory analysis; however, it would be useful to determine if there were any links between higher concentrations of γ-nonalactone 1 and increased prune aroma, indicative of premature ageing of red wines [4]. This work also did not investigate Pinot noir wines older than 2018; it would be interesting to investigate whether older examples of NZ Pinot noir wines exhibited higher concentrations of γ-nonalactone 1.
In conclusion, the synthesised novel 2H213C2-γ-nonalactone 15 is a suitable internal standard for the accurate and precise quantification of γ-nonalactone 1 in red wines through the SIDA-SPE-GC–MS method reported herein, as it is stable under wine spiking and SPE conditions. Although similar LODs for γ-nonalactone 1 in wine have been reported in previous literature (Table 1), using an isotopically labelled internal standard as opposed to a surrogate internal standard is beneficial as it is anticipated that an isotopically labelled standard will behave similarly to the analyte of interest, even when used in a variety of matrices. The results of this study suggest that the concentration of γ-nonalactone 1 in NZ Pinot noir wines is not correlated with vintage and price or influenced by grape growing region, and instead other factor/s are likely to be involved in the modulation of γ-nonalactone 1 concentration. The concentrations of γ-nonalactone 1 in the samples in this study are in agreement with those found previously in Pinot noir wines, except for those found in one study looking at Oregon Pinot noir, which reported much higher concentrations. Investigation into the biogenesis of γ-nonalactone 1 during wine production may help to shed light on the origins of this compound, and the drivers of variability in concentration between different wines.
References
Robinson AL, Boss PK, Solomon PS, Trengove RD, Heymann H, Ebeler SE. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am J Enol Vitic. 2014;65(1):1–24. https://doi.org/10.5344/ajev.2013.12070.
Miller GC, Pilkington LI, Barker D, Deed RC. Saturated linear aliphatic γ- and δ-lactones in wine: a review. J Agric Food Chem. 2022;70(49):15325–46. https://doi.org/10.1021/acs.jafc.2c04527.
Cooke RC, Capone DL, van Leeuwen KA, Elsey GM, Sefton MA. Quantification of several 4-alkyl substituted γ-lactones in Australian wines, J Agric Food Chem, 2009; 57(2):Art. no. 2. https://doi.org/10.1021/jf8026974.
Pons A, Lavigne V, Eric F, Darriet P, Dubourdieu D. Identification of volatile compounds responsible for prune aroma in prematurely aged red wines. J Agric Food Chem. 2008;56(13):5285–90. https://doi.org/10.1021/jf073513z.
Qian X, et al. Comprehensive investigation of lactones and furanones in icewines and dry wines using gas chromatography-triple quadrupole mass spectrometry. Food Res Int. 2020;137:109650. https://doi.org/10.1016/j.foodres.2020.109650.
Nakamura S, Crowell EA, Ough CS, Totsuka A. Quantitative analysis of γ-nonalactone in wines and its threshold determination. J Food Sci. 1988;53(4):Art. no. 4. https://doi.org/10.1111/j.1365-2621.1988.tb13578.x.
Schreier P, Drawert F. Gas chromatographic mass-spectrometric investigation of volatile constituents of wine. V. Alcohols, hydroxy esters, lactones, and other polar compounds of wine flavour. Chem Mikrobiol Technol Lebensm. 1974;3:154–60.
González-Rompinelli EM et al. A winery-scale trial of the use of antimicrobial plant phenolic extracts as preservatives during wine ageing in barrels. Food Control. 2013;33(2):Art. no. 2. https://doi.org/10.1016/j.foodcont.2013.03.026.
Lan Y-B, et al. Characterization and differentiation of key odor-active compounds of ‘Beibinghong’ icewine and dry wine by gas chromatography-olfactometry and aroma reconstitution. Food Chem. 2019;287:186–96. https://doi.org/10.1016/j.foodchem.2019.02.074.
Jarauta I, Ferreira V, Cacho JF. Synergic, additive and antagonistic effects between odorants with similar odour properties, in Developments in Food Science, Bredie WLP and Petersen MA, Eds., in Flavour Science. 2006;43: 205–208. Elsevier. https://doi.org/10.1016/S0167-4501(06)80049-X.
Cooke RC (née Brown), van Leeuwen KA, Capone DL, Gawel R, Elsey GM, Sefton MA. Odor detection thresholds and enantiomeric distributions of several 4-alkyl substituted γ-lactones in Australian red wine. J Agric Food Chem. 2009;57(6): Art. no. 6. https://doi.org/10.1021/jf802866f.
de Ferron P, Thibon C, Shinkaruk S, Darriet P, Allamy L, Pons A. Aromatic potential of Bordeaux grape cultivars: identification and assays on 4-oxononanoic acid, a γ-nonalactone precursor. J Agric Food Chem. 2020;68(47):Art. no. 47. https://doi.org/10.1021/acs.jafc.0c04171.
Garbe L-A, Lange H, Tressl R. Biosynthesis of γ-nonalactone in yeast, in Aroma Active Compounds in Foods, in ACS Symposium Series, 794(794). American Chemical Society. 2001; 176–182. https://doi.org/10.1021/bk-2001-0794.ch014.
Ferreira V, López R, Cacho JF. Quantitative determination of the odorants of young red wines from different grape varieties. J Sci Food Agric. 2000;80(11):Art. no. 11. https://doi.org/10.1002/1097-0010(20000901)80:11<1659::AID-JSFA693>3.0.CO;2-6.
Ferreira V, Jarauta I, Ortega L, Cacho J. Simple strategy for the optimization of solid-phase extraction procedures through the use of solid–liquid distribution coefficients: application to the determination of aliphatic lactones in wine. J Chromatogr A. 2004;1025(2):147–56. https://doi.org/10.1016/j.chroma.2003.10.086.
Pérez-Olivero SJ, Pérez-Pont ML, Conde JE, Pérez-Trujillo JP. Determination of lactones in wines by headspace solid-phase microextraction and gas chromatography coupled with mass spectrometry. J Anal Methods Chem. 2014;1–10. https://doi.org/10.1155/2014/863019.
Feng H, Skinkis PA, Qian MC. Pinot noir wine volatile and anthocyanin composition under different levels of vine fruit zone leaf removal. Food Chem. 2017;214:736–44. https://doi.org/10.1016/j.foodchem.2016.07.110.
Langen J, Wang C-Y, Slabizki P, Wall K, Schmarr H-G. Quantitative analysis of γ- and δ-lactones in wines using gas chromatography with selective tandem mass spectrometric detection. Rapid Commun Mass Spectrom. 2013;27(24):Art. no. 24. https://doi.org/10.1002/rcm.6736.
Han Y, Alexander TE, Tochtrop GP. Design, synthesis, and evaluation of an isotopic labeling strategy for studying fatty acid– protein binding by NMR. Mol Biosyst. 2008;4(6):Art. no. 6. https://doi.org/10.1039/B800471D.
Iida K, Tokiwa S, Ishii T, Kajiwara M. Synthesis of 5-[4,5–13C2]- and 5-[1,5–13C2]aminolevulinic acid. J Label Compd Radiopharm. 2002;45(7):569–76. https://doi.org/10.1002/jlcr.583.
Sadhukhan S, Han Y, Zhang G-F, Brunengraber H, Tochtrop GP. Using isotopic tools to dissect and quantitate parallel metabolic pathways. J Am Chem Soc. 2010;132(18):Art. no. 18. https://doi.org/10.1021/ja100399m.
A. M. Committee and A. No. 70. An analyst’s guide to precision. Anal Methods. 2015;7(20):8508–10. https://doi.org/10.1039/C5AY90071A.
Capone DL, Sefton MA, Hayasaka Y, Jeffery DW. Analysis of precursors to wine odorant 3-mercaptohexan-1-ol using HPLC-MS/MS: resolution and quantitation of diastereomers of 3-S-cysteinylhexan-1-ol and 3-S-glutathionylhexan-1-ol. J Agric Food Chem. 2010;58(3):1390–5. https://doi.org/10.1021/jf903720w.
Wickham H. ggplot2: elegant graphics for data analysis. Springer-Verlag New York, 2016 [updated 2023 Feb 10]. Available: https://ggplot2.tidyverse.org. Accessed 17 Mar 2023
Sadhukhan S, Han Y, Zhang G-F, Brunengraber H, Tochtrop GP. Using isotopic tools to dissect and quantitate parallel metabolic pathways. J Am Chem Soc. 2010;132(18):6309–11. https://doi.org/10.1021/ja100399m.
Felder S, Rowan DD. Synthesis of deuterated C-6 and C-9 flavour volatiles. J Label Compd Radiopharm. 1999;42(1):83–92. https://doi.org/10.1002/(SICI)1099-1344(199901)42:1%3c83::AID-JLCR169%3e3.0.CO;2-F.
Ferreira V, López R, Escudero A, Cacho JF. Quantitative determination of trace and ultratrace flavour active compounds in red wines through gas chromatographic–ion trap mass spectrometric analysis of microextracts. J Chromatogr A. 1998;806(2):349–54. https://doi.org/10.1016/S0021-9673(98)00070-3.
López R, Aznar M, Cacho J, Ferreira V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J Chromatogr A. 2002;966(1):167–77. https://doi.org/10.1016/S0021-9673(02)00696-9.
Genovese A, Dimaggio R, Lisanti MT, Piombino P, Moio L. Aroma composition of red wines by different extraction methods and gas chromatography-SIM/mass spectrometry analysis. Ann Chim. 2005;95(6):383–94. https://doi.org/10.1002/adic.200590045.
Carrillo JD, Garrido-López Á, Tena MT. Determination of volatile oak compounds in wine by headspace solid-phase microextraction and gas chromatography–mass spectrometry. J Chromatogr A. 2006;1102(1):25–36. https://doi.org/10.1016/j.chroma.2005.10.038.
Fang Y, Qian MC. Quantification of selected aroma-active compounds in Pinot noir wines from different grape maturities. J Agric Food Chem. 2006;54(22):8567–73. https://doi.org/10.1021/jf061396m.
Moreno JJ, Cerpa-Calderón F, Cohen SD, Fang Y, Qian M, Kennedy JA. Effect of postharvest dehydration on the composition of Pinot noir grapes (Vitis vinifera L.) and wine. Food Chem. 2008;109(4):755–62. https://doi.org/10.1016/j.foodchem.2008.01.035.
Espinase Nandorfy D, Siebert T, Watson F, Keast R, Francis Il. Understanding the interactive effects of volatile compounds contributing to ‘stone fruit’ aroma nuances in white wines. Aust J Grape Wine Res. 2022;n/a(n/a):Art. no. n/a. https://doi.org/10.1111/ajgw.12540.
Acknowledgements
The authors are grateful to Dr Shaun Rees for providing assistance in synthesis of the isotopically labelled internal standard. Stuart Morrow and Dr. Githal Porawakara Arachchige also provided assistance with mass spectrometry analysis throughout this research.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions This work was made possible by funding from the University of Auckland Doctoral Scholarship and the Frederick Douglas Brown Memorial Scholarship (G. C. M.). The Faulty Research Development Fund (FRDF) (University of Auckland) was also received for this work (R. C. D.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Miller, G.C., Barker, D., Pilkington, L.I. et al. Synthesis of a novel isotopically labelled standard for quantification of γ-nonalactone in New Zealand Pinot noir via SIDA-SPE-GC–MS. Anal Bioanal Chem 415, 5035–5047 (2023). https://doi.org/10.1007/s00216-023-04789-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-04789-2